Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system (CNS) caused by the human neurotropic polyomavirus JC (JCV). The disease occurs virtually exclusively in immunocompromised individuals, and, prior to the introduction of antiretroviral therapy, was seen most commonly in the setting of HIV/AIDS. More recently, however, the incidence of PML in HIV-uninfected persons has increased with broader use of immunosuppressive and immunomodulatory medications utilized in a variety of systemic and neurologic autoimmune disorders. In this review, we discuss the epidemiology and clinical characteristics of PML in HIV-uninfected individuals, as well as diagnostic modalities and the limited treatment options. Moreover, we describe recent findings regarding the neuropathogenesis of PML, with specific focus on the unique association between PML and natalizumab, a monoclonal antibody that prevents trafficking of activated leukocytes into the CNS that is used for the treatment of multiple sclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive multifocal leukoencephalopathy (PML) is an opportunistic demyelinating infection of the central nervous system (CNS) caused by the JC virus (JCV), a ubiquitous polyoma virus which is found in approximately 50–86 % of the adult population worldwide [1–3]. In immunocompetent individuals, JCV infection remains a latent asymptomatic infection of the kidneys [1, 4]. However, in immunocompromised individuals, it can cause a rapidly progressive and often fatal CNS infection which was first described in the setting of Hodgkin’s disease and chronic lymphocytic leukemia in 1958 [5]. PML remained rare until the human immunodeficiency virus (HIV) epidemic when it was seen in the context of acquired immunodeficiency syndrome (AIDS) with 1.3 cases per 1000 HIV+ person-years [6]. In recent years, PML has also increased in incidence in HIV-uninfected (HIV−) persons with the rise of immunomodulatory and immunosuppressive agents, including chemotherapies, rheumatologic disease-modifying therapies, and multiple sclerosis (MS) treatments, which result in decreased immune surveillance in the CNS and increased risk of PML. Here, we review emerging evidence regarding the neuropathogenesis, epidemiology, clinical characteristics, diagnostic strategies, and empiric therapies for PML with a focus on HIV− persons. We also review the particular association of natalizumab treatment with increased risk of PML among patients with MS.
Neuropathogenesis of PML
The pathological hallmarks of PML include the triad of multifocal areas of demyelination, oligodendrocytes containing inclusion bodies, and bizarre-appearing astrocytes. Both oligodendrocytes and astrocytes are immunopositive for the JCV capsid protein VP1 and contain virions that can be detected via electron microscopy, indicating that JCV is capable of replicating in both glial cell types [7, 8]. Evidence acquired over the past several decades has suggested a model of neuropathogenesis in which (1) virions from the environment enter via the oropharynx or the gastrointestintestinal tract; (2) establishment of a primary viremia ensues, with subsequent spread to the kidneys resulting in persistent infection; (3) following persistence, the virus enters the brain, potentially via hematogenous routes; and (4) reactivation of the virus in glial cells results in PML [7]. However, the molecular and cell biology of many of these events, including JCV persistence and reactivation, entry into the central nervous system (CNS), and factors that result in initiation of glial infection remains poorly understood. While a thorough review of JCV neuropathogenesis is beyond the scope of the current review, we will briefly describe several recent advances in our knowledge of the pathogenesis of non-HIV PML, and in the “Natalizumab-Associated PML” section, we will address specific mechanisms by which the drug natalizumab is postulated to predispose to PML.
Development of a Neurotropic Strain
The JCV genome encodes early and late proteins that are transcribed in opposite directions from a common noncoding control region (NCCR), which contains the origin of viral DNA replication as well as promoter and enhancer elements for transcription [9]. Production of the early proteins, small t and large T antigens, results in initiation of viral DNA replication and a transcriptional switch from early to late gene expression, while the late region encodes the structural proteins VP1, VP2, and VP3 along with the agnoprotein which has been found to play multiple functions that contribute to a productive viral life cycle [10, 11]. The structure of the NCCR has received considerable scrutiny, as it appears that two different forms exist: the highly conserved archetype form and the variable prototype form. Several lines of evidence suggest that a switch from the archetype to the prototype form within an individual may play a role in initiation of PML. First, the prototype forms have numerous deletions, duplications, and rearrangements compared to the archetype, suggesting that they might arise from the conserved archetype form. Second, the archetype form has been shown to predominate in the environment and in the urine of normal non-immunocompromised individuals, while the prototype form appears to be enriched in the brains of individuals with PML [12, 13]. Interestingly, a recent study that reported on deep sequencing of JCV DNA from the urine, plasma, and cerebrospinal fluid (CSF) of patients with non-HIV PML demonstrated that the JCV composition in the CSF and plasma represents a highly complex mixture comprised of multiple rearranged viral variants that differs markedly from the predominantly archetypal JCV composition in the urine [14••]. These results support the notion that the rearrangement of archetype JCV is associated with neurotropism and are in line with in vitro studies demonstrating that rearrangements enhance replication rates in glial cells [15]. However, the study demonstrated that archetype JCV can also rarely be detected in the plasma and CSF, and thus, NCCR rearrangement may not be absolutely required for neurotropism [14••].
Mechanisms of Immune Evasion
An intact cellular immune response has long been recognized to play a role in preventing PML, while reconstitution of an impaired cellular response has been shown to enhance resolution of JCV infection in the brain. While earlier studies had emphasized a major role for JCV-specific CD8+ T cells in viral clearance and survival after PML [16–18], a recent study that included individuals with persistent JCV CNS infection in the setting of natalizumab demonstrated that mutations in VP1 can result in reduced CD4+ T cell responses, thereby impacting CD8+ T cell-mediated viral clearance [19•]. Thus, both CD4+ and CD8+ T cell responses may be important for viral control, and JCV neuropathogenesis may involve the acquisition of mutations that enable evasion of these responses. Moreover, VP1 mutations have been found to be associated with deficient CSF antibody responses against the protein which, upon immune reconstitution, are improved, suggesting an additional role for evasion of humoral immunity by JCV in the pathogenesis of PML [20•, 21].
Epidemiology of PML
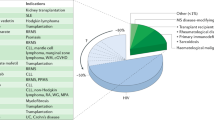
Prior to the introduction of combination antiretroviral therapy (ART), PML was observed in 5–10 % of people with AIDS, but this rate has declined significantly with the advent of ART [22, 23]. However, the incidence of PML in HIV− persons has increased significantly with broader use of immunosuppressive and immunomodulatory medications. PML has now been reported in association with a variety of such medications, including alemtuzumab [24, 25], belatacept [26], dimethyl fumarate [27–30], eculizumab [31], brentuximab [32], fingolimod [33], fludaribine [34, 35], infliximab [36, 37], leflunomide [38], mycophenolate mofetil [39, 40], natalizumab (NTZ) [41–43], and rituximab [44, 45], among others [46]. In addition, hematologic malignancies, immunodeficiency disorders, idiopathic lymphopenia, and autoimmune rheumatologic disorders seem to lead to an increased risk of PML even in the absence of pharmacologic therapies, likely due to the aberrant immune function associated with these conditions. Based on these two distinct risk groups, a classification system has been proposed to group drugs into three classes based on conferred PML risk as follows: (1) Class 1—drugs with a high PML risk, long latency to PML onset and use in conditions that do not predispose to PML; (2) Class 2—drugs used to treat conditions that predispose to PML and likely confer additional PML risk; (3) Class 3—sporadic cases of PML reported with the use of these agents but associated risk is very low or not able to be quantified [47, 48].
Among autoimmune diseases, systemic lupus erythematosus (SLE), in particular, is associated with an increased risk of PML even in the absence of immunosuppression [49••, 50]. A retrospective review of a national admissions database found that rates of PML among SLE patients were 4 per 100,000 admissions as opposed to rates of 0.4 for rheumatoid arthritis patients and a rate of 2 for all other connective tissue disorders [51]. Furthermore, 40 % of reported cases occurred in the setting of minimal or no immunosuppressive medication use raising the possibility that SLE-related lymphopenia may be the major risk factor for PML in many of these cases [40, 52, 53]. Therefore, clinical suspicion for PML should be high in SLE patients presenting with neurologic symptoms even in the absence of significant immunosuppressive medications.
Clinical Characteristics of PML
The clinical presentation of PML in both HIV+ and HIV− individuals is quite variable as it is a multifocal process that can affect almost any area of the CNS and, thus, can cause a variety of neurological symptoms. One quarter to one half of patients develop visual field deficits, often at the time of initial presentation; deficits are primarily due to involvement of visual pathways within the brain rather than to optic neuritis [54]. Seizures are also common in PML. Approximately one third of patients develop seizures during their acute presentation, nearly half of those who survive 1 year after diagnosis have seizures, and management often requires multiple anti-epileptic drugs to obtain adequate seizure control [55•, 56]. In long-term PML survivors, seizure onset occurs an average of 5.4 months after initial presentation, and seizures are nearly all focal with the majority originating from the frontal lobes [56]. Finally, patients with juxtacortical lesions and lesions associated with hyperintense cortical signal on T1-weighted pre-contrast magnetic resonance imaging (MRI) were at higher risk of seizures than those without lesions with these characteristics [55•, 56]. Other common symptoms of PML include difficulty with ambulation, weakness, sensory symptoms, cognitive impairment, and headaches [57].
As the populations at risk for PML have expanded in recent decades, so has the clinical spectrum of PML. As a result, clinicians have become increasingly aware that the term “PML” can be misleading as the clinical presentation is not always progressive, multifocal, or limited to white matter [3]. Initially thought to be exclusively a white matter disease caused by infection of oligodendrocytes and astrocytes, it is now recognized that JCV can cause several other clinical syndromes due to infection of neurons and meningeal cells, including granule cell neuronopathy (GCN) [58–60], encephalopathy [61], and meningitis [62•]. GCN results in cerebellar atrophy from loss of cerebellar granule cells without MRI or pathologic evidence of demyelination [60]. Clinically, it presents with a cerebellar syndrome often consisting of dysarthria, dysdiadochokinesia, and gait and appendicular ataxia [59, 60]. While initially reported in the context of HIV infection, GCN has now also been reported in patients with sarcoidosis [63], rituximab-treated non-Hodgkin’s lymphoma [64], and NTZ-treated MS [65, 66]. JCV encephalopathy has been reported in only one patient who presented with aphasia, cognitive decline, and seizures and was found to have lesions restricted to the cortical gray matter on MRI. Cerebrospinal fluid (CSF) and biopsy both confirmed the presence of JCV [61]. JCV meningitis, presenting with headaches, stiff neck, and altered mental status without concomitant MRI abnormalities, has been reported in several patients with detectable CSF JCV titers and in one patient who presented clinically with the classic triad of normal pressure hydrocephalus and had pathologically confirmed JCV infection of meningeal and choroid plexus cells [62•, 67, 68]. The true prevalence of JCV meningitis is likely under-appreciated as JCV is rarely tested in presentations of aseptic meningitis.
Furthermore, PML has recently been reported to present as an extrapyramidal syndrome due to bilateral periventricular and basal ganglia involvement [69], to present acutely with symptoms mirroring a cerebellar stroke [70], and to present with multiple small round contrast-enhancing lesions mimicking military tuberculosis [71]. Demyelinating lesions of the spinal cord have also been reported on MRI and confirmed to be related to JCV infection on post-mortem pathology [72, 73]. Mounting reports of varied clinical entities associated with JCV suggests that PML-spectrum disorders and JCV-related disease should be included in the differential diagnosis of a wide array of clinical presentations in immunosuppressed patients.
Diagnosis of PML
Diagnosis of PML requires clinical, radiographic and virologic evidence compatible with JCV infection [74]. Virologic evidence consists either of laboratory data or histopathology. Laboratory diagnosis can be established with a positive cerebrospinal fluid (CSF) JCV polymerase chain reaction (PCR). Viable JCV genomes contain a region within the T protein coding nucleotide sequence that is conserved for JC viruses but not other human polyomaviruses [75]. CSF PCR assays developed to recognize this region have been shown to be highly specific, though sensitivity can vary between labs and there are a number of reports of false-negative CSF PCR in the setting of confirmed PML [76]. For a definitive histopathologic diagnosis, two of three of the following are required: (1) brain biopsy specimen demonstrating the classic histopathologic triad of demyelination, bizarre astrocytes, and enlarged oligodendroglial nuclei; (2) immunohistochemistry or electron microscopy revealing JCV; or (3) tissue PCR positive for JCV. However, a recent review of pathologically confirmed PML cases found that biopsy specimens are often small and that relying on only the typical morphologic features of PML in combination with immunohistochemistry can result in high rates of false-negative results [77]. These authors found that the addition of in situ hybridization and/or real-time PCR to the standard approach led to increased sensitivity of histopathologic diagnoses. These results suggest that further testing should be completed for pathologic specimens that do not show typical morphologic or immunohistochemical features if clinical suspicion for PML is high. In addition, with the rapidly expanding use of metagenomic deep sequencing in microbiological diagnosis of CNS infections, these techniques may also be useful in improving the sensitivity of JCV-related disease diagnoses [78–80].
Classic MRI findings of PML include hyperintense lesions on T2-weighted and fluid-attenuated inversion recovery (FLAIR)-weighted imaging with corresponding hypointense lesions on T1-weighted imaging. PML lesions tend to predominantly and asymmetrically affect subcortical and periventricular white matter in the frontal and parieto-occipital regions, often involve the subcortical U-fibers, and usually have no associated mass effect [81, 82]. However, cases of PML limited to the brainstem and cerebellum have also been reported, and this may be even more common in patients with lupus as an underlying risk factor for PML [83–86]. While the classical teaching is that PML lesions do not show gadolinium enhancement, retrospective studies have identified enhancement in 5–10 % of AIDS-related PML cases, 40–50 % of NTZ-associated PML cases, and ≥50 % of PML-immune reconstitution inflammatory syndrome (IRIS) cases [57, 82, 87, 88]. Similarly, PML lesions are classically described as hypometabolic on positron emission topography (PET) imaging but, in practice, are often hypermetabolic [89, 90].
Additional MRI characteristics may be useful in distinguishing PML from other demyelinating disorders. For example, Miyagawa et al. reported that susceptibility weighted imaging (SWI) reveals low signal intensity of the U-fibers in juxtacortical PML lesions, a finding which was corroborated by several other groups [91•, 92, 93]. However, this same group performed a retrospective review of other MRI studies with FLAIR-hyperintense juxtacortical lesions due to etiologies other than PML and found that 7 % of these lesions had an associated low signal intensity rim involving the U-fibers on SWI sequences. All of the identified MRI studies were obtained from patients with either stroke or encephalitis [94]. These findings suggest that SWI low signal intensity may be a sensitive but non-specific radiographic finding in PML.
Treatment of PML
To date, no curative treatment for JCV is known, and no treatment has been shown to improve PML survival in a randomized controlled trial [95]. Therefore, the current standard of care is to reconstitute the immune system as quickly as possible. In the setting of HIV infection, this is achieved with prompt initiation of ART. In cases associated with immunosuppressive medications, measures include cessation of the offending drug and plasma exchange to remove any remaining drug in the circulation (if the medication is amenable to clearance via plasma exchange), in addition to supportive care [96]. However, anecdotal reports purporting improved PML outcomes associated with off-label use of several different medications have been published. For example, mirtazapine has been shown in vitro to inhibit JC virus entry into glial cells by blocking the serotonin 2A receptor [97]. It has been associated with improved outcomes in individual cases of PML in both HIV+ and HIV− individuals but has never been systematically evaluated in a clinical trial [98, 99]. In vitro studies also suggested that the anti-malarial drug mefloquine may inhibit JCV replication within glial cells [100]. However, a randomized double-blind placebo-controlled trial of mefloquine in addition to ART in HIV+ patients with PML failed to show a reduction in CSF JCV DNA copy number and was terminated early [101]. The topoisomerase inhibitor, topotecan, was recently reported to inhibit JCV replication in in vitro studies [102], and a phase 2 trial of 12 HIV+ patients showed a trend toward decreased PML lesion size and increased survival [103]. However, a phase 3 trial has not been performed to confirm these findings.
Other off-label therapeutic approaches to treat PML have aimed to facilitate immune restoration. Interleukin-7 (IL-7), an important cytokine in T cell function and homeostasis, has been proposed as a potential treatment for PML that may augment the immune response to JCV. Several cases of favorable outcomes in HIV− patients treated with recombinant human IL-7 have now been published [104–106]. Recently, maraviroc, an approved HIV ART medication that acts by blocking chemokine receptor type 5 (CCR5), was associated with better than expected outcomes among three HIV− patients with PML (two with sarcoidosis and one with idiopathic lymphopenia) [107•]. However, neither of these agents have been tested in prospective randomized controlled trials, and multiple drugs which were reported to be effective in case reports of HIV-associated PML later failed to show any effect in prospective clinical trials, including cytarabine [108], cidofovir [109], and alpha-interferon [110]. As a result, these findings should be interpreted with caution until stronger evidence is available.
Natalizumab-Associated PML
NTZ, a monoclonal antibody against alpha-4-integrin used in the treatment of MS, is likely the most well-known medication associated with an increased PML risk and one of two Class 1 drugs in the classification scheme described above [47]. NTZ received initial Food and Drug Administration approval in 2004 but was withdrawn from the market in 2005 after several cases of PML were reported. It was re-approved in 2006 at which time the risk of PML was estimated at 1:1000 over 18 months of treatment [111].
Epidemiology
Post-marketing surveillance following reintroduction of NTZ revealed three primary risk factors for NTZ-associated PML: (1) duration of therapy >24 months, (2) history of prior immunosuppression, and (3) JCV antibody seropositivity. Based on these factors, in 2012, PML risk ranged from <1:50,000 in JCV antibody seronegative persons with no prior history of immunosuppression and <24 months of NTZ treatment to 1:85 in JCV antibody seropositive persons with a history of prior immunosuppression treated with NTZ for more than 24 months [112]. However, many new cases of NTZ-associated PML have occurred since the development of these initial estimates. A total of 566 cases of PML were reported to the manufacturer as of July 2015, and estimates of the overall risk of PML have increased from 2.13 per 1000 patients in 2011 to 3.96 per 1000 patients [113, 114]. The more recent analysis revealed that estimates for PML risk within the first 2 years of NTZ treatment have remained essentially unchanged. However, in persons treated for >24 months, estimates have nearly doubled from 3.85 per 1000 patients to 6.22 per 1000 patients overall and from 1:85 to 1:44 in the highest risk group [115].
Pathogenic Mechanisms
By binding to alpha-4-integrin, NTZ prevents trafficking of activated leukocytes across the blood-brain barrier into the CNS. One mechanism by which NTZ may facilitate development of PML is via its effects on blocking extravasation of T cells into the CNS, thus limiting immune surveillance in the brain [116]. However, this would not specifically account for the increased association of PML with NTZ compared to other immunosuppressants, as well as the relative lack of other CNS opportunistic infections seen in the setting of NTZ treatment. Thus, it appears that there may be additional direct mechanisms by which the drug interacts with JCV. The well-recognized ability of NTZ to promote the peripheral mobilization of certain mononuclear populations from the bone marrow [117] has raised the question as to whether such mobilization may contribute to PML. Serial sampling of peripheral blood cells from MS patients treated with NTZ demonstrated substantial numbers of patients who were viremic in CD34+ cells and fewer in CD19+ cells, with JCV copy numbers correlating positively with duration of treatment [118]. Another study of 32 JCV-seropositive MS patients treated with NTZ also found that JCV DNA viral load was higher in CD34+ cells compared to other subpopulations [65]. These and other studies suggest that CD34+ cells from the bone marrow may be latently infected with JCV and mobilize to the periphery during treatment with NTZ, thus potentially representing a route of entry of the virus into the CNS.
Treatment with NTZ has also been associated with altered gene expression in peripheral mononuclear cells [119]. Increased expression of the transcription factor Spi-B [120], a protein required for B cell receptor maturation and signaling, has received considerable attention since it can bind to target sites in neurotropic JCV NCCR but not archetype NCCR and increase viral gene expression [121, 122]. Moreover, in individuals with MS treated with NTZ, Spi-B is markedly upregulated in CD34+ cells [123••]. Thus, NTZ treatment may both result in increased JCV DNA-harboring CD34+ cells in the periphery and result in upregulation of transcription factors that promote viral gene expression within these cells. Despite these provocative data, however, there has been no direct evidence of a productive infection or presence of viral gene expression products in blood cells, raising questions as to the role of blood cells in PML pathogenesis.
Risk Stratification in Clinical Practice
JCV antibody testing has become a routine part of clinical care in those MS patients treated with NTZ as seropositivity has been shown to increase risk of PML by nearly 40-fold [114]. Furthermore, JCV antibody titers seem to correlate with PML risk with index values >1.5 associated with a significantly higher risk of PML than lower index values [124]. However, whereas JCV antibody seropositivity was once thought to be 100 % sensitive for PML risk, 1 % of cases with JCV antibody testing within the 6 months prior to PML diagnosis were JCV antibody seronegative, including one case in which JCV antibody testing was negative just 2 weeks prior to PML diagnosis [113]. Delbue et al. also recently reported that 3/42 NTZ-treated MS patients who were JCV antibody seronegative had detectable JCV DNA in their urine, potentially suggesting that the serological test is not 100 % sensitive [125]. A recent cohort study of French and German NTZ-treated MS patients also revealed high rates of JCV antibody seroconversion—up to 10 % per year—and increasing JCV antibody index values of nearly 13 % per year in seropositive patients [126]. As such, current recommendations are to obtain JCV antibody serology every 6 months for the duration of NTZ treatment in order to facilitate discussions of a patient’s ongoing and possibly changing PML risk [47, 113]. However, it should also be emphasized that a negative JCV antibody testing does not eliminate the possibility of developing PML in the future.
New Biomarkers
The need to improve PML risk stratification among NTZ-treated MS patients has led to research to identify new biomarkers of PML risk in this patient group. One emerging candidate is L-selectin, which is also known as CD62-ligand, and is an adhesion molecule on CD4+ T lymphocytes. Low levels of L-selectin expression have been associated with increased PML risk in the setting of both HIV infection and NTZ treatment [127, 128]. Retrospective analysis of international multi-center cohorts of NTZ-treated MS patients found that low L-selectin levels demonstrated 86 % sensitivity and 91 % specificity for PML while JCV antibody seropositivity was 100 % sensitive and 59 % specific [129]. The authors suggest that incorporating both JCV antibody and L-selectin into a risk stratification and treatment decision algorithm could improve risk stratification and ultimately reduce PML incidence among NTZ-treated MS patients up to tenfold [129]. However, a recent well-controlled study of 21 NTZ-treated patients with MS who developed PML and 104 matched treated patients who did not develop PML did not find the percentage of L-selectin-positive cells in peripheral blood mononuclear cells to be a useful biomarker of PML risk [130]. Thus, there continues to be a need to develop biomarkers in those MS patients whose PML risk is intermediate and who desire further information before making a decision regarding NTZ therapy.
Diagnosis
Diagnosis of NTZ-associated PML does not differ from diagnosis of PML in other risk groups. However, several recent reports have found additional MRI characteristics that may be suggestive of NTZ-associated PML. In addition to low signal intensity on SWI sequences, Hodel and colleagues also identified low signal intensity on T2* sequences in NTZ-associated PML [92]. A punctate pattern, which refers to multiple T2-hyperintense punctate brain lesions, was recently studied in the context of NTZ-associated PML and found to be 78 % sensitive and 100 % specific in these cases [131•]. In addition, the punctate pattern was often present in the pre-symptomatic stage of PML, suggesting that it might be one of the earliest imaging findings that can be identified, and punctate pattern with contrast enhancement highly correlated to PML-IRIS. Because identification of PML in asymptomatic stages may be associated with improved outcomes, routine MRI surveillance is recommended in all NTZ-treated MS patients with the frequency of MRI based on JCV antibody serostatus. In seronegative patients, MRI is recommended on an annual basis with the interval decreasing to at least every 6 months in JCV antibody seropositive patients with an index of <1.5 and to every 3–4 months in seropositive patients with an index value >1.5 [113].
Treatment
After PML is diagnosed in the setting of NTZ therapy, NTZ infusions should be discontinued, and plasma exchange should be initiated in order to facilitate clearance of NTZ from circulation and hasten recovery of immune function [132]. However, even with this standard of care, mortality remains quite high, reaching approximately 20 %, with two thirds of survivors experiencing moderate to severe permanent disability [96, 133•]. Improved outcomes are associated with younger age, higher pre-PML functional status, lower CSF JC viral loads at the time of diagnosis, and lower PML MRI lesion volumes [133•]. Of note, the empirical rationale for maraviroc use has been extended by Stork, et al. to include its use in NTZ-associated PML-IRIS after histopathologic examination of NTZ-associated PML-IRIS lesions showed very high numbers of CCR5+ lymphocytes within inflammatory PML lesions [134]. This suggests that CCR5 blockade using maraviroc may at least partially decrease the inflammatory component of PML-IRIS in these patients.
Conclusions
The incidence of non-HIV PML is likely to continue to increase with more widespread adoption of newer immunosuppressive treatments that impact leukocyte trafficking and function. Clinicians will need to be familiar with the changing epidemiology and the clinical characteristics of PML in order to remain vigilant for emergence of the disease in patients. An active area of investigation is the development of better biomarkers of disease emergence and activity, which would in turn help clinicians manage affected individuals. Treatment options are limited, in part because fundamental questions regarding the neuropathogenesis of PML remain unanswered. Although progress has been made in characterizing different viral forms that inhabit distinct in vivo environments, a broader understanding of the JCV life cycle and the impact of immunosuppressive medications on aspects such as establishment of viral persistence, entry into the CNS, reactivation, and lytic infection of glial cells is needed in order to develop immunosuppressive medications with limited potential to lead to PML and, if PML develops, specific therapies for the disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy—revisited. J Infect Dis. 2011;203:578–86.
Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B. Anti-JC virus (JCV) antibody prevalence in the JCV epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21:299–304.
Miskin DP, Koralnik IJ. Novel syndromes associated with JC virus infection of neurons and meningeal cells: no longer a gray area. Curr Opin Neurol. 2015;28:288–94.
Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–46.
Astrom K, Mancall E, Richardson E. Progressive multifocal luekoencephalopathy: a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958;81:93–111.
Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis. 2009;199:77–83.
Wollebo HS, White MK, Gordon J, Berger JR, Khalili K. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–70.
Watanabe I, Preskorn SH. Virus-cell interaction in oligodendroglia, astroglia and phagocyte in progressive multifocal leukoencephalopathy. An electron microscopic study. Acta Neuropathol. 1976;36:101–15.
Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–69.
Khalili K, Gordon J, White MK. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–87.
Saribas AS, Coric P, Hamazaspyan A, et al. Emerging from the unknown: structural and functional features of agnoprotein of polyomaviruses. J Cell Physiol. 2016.
Yogo Y, Kitamura T, Sugimoto C, et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–43.
Bofill-Mas S, Clemente-Casares P, Major EO, Curfman B, Girones R. Analysis of the excreted JC virus strains and their potential oral transmission. J Neurovirol. 2003;9:498–507.
Van Loy T, Thys K, Ryschkewitsch C, et al. JC virus quasispecies analysis reveals a complex viral population underlying progressive multifocal leukoencephalopathy and supports viral dissemination via the hematogenous route. J Virol. 2015;89:1340–7. The first analysis of the JCV population in different body compartments of individuals with PML. In addition to demonstrating complex viral populations in the CSF and plasma that are distinct from those found in the urine, the study demonstrates that archetype virus can be present in the CNS.
Gosert R, Kardas P, Major EO, Hirsch HH. Rearranged JC virus noncoding control regions found in progressive multifocal leukoencephalopathy patient samples increase virus early gene expression and replication rate. J Virol. 2010;84:10448–56.
Martin-Blondel G, Bauer J, Cuvinciuc V, et al. In situ evidence of JC virus control by CD8+ T cells in PML-IRIS during HIV infection. Neurology. 2013;81:964–70.
Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–8.
Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011;85:7256–63.
Jelcic I, Kempf C, Largey F, et al. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann Neurol. 2016;79:404–18. This study demonstrated that despite strong specificity of CD8+ T cells for JCV antigens in an individual with CNS JCV persistence, mutations in the major capsid protein VP1 resulted in reduced CD4+ T cell responses which impacted CD8+ responses. Thus, efficient CD4+ T cell recognition of viral antigens is necessary to support CD8+ T cell function in combating JCV infection.
Ray U, Cinque P, Gerevini S, et al. JC polyomavirus mutants escape antibody-mediated neutralization. Sci Transl Med. 2015;7:306ra151. This provocative study found that mutations in the JCV capsid protein VP1 allow the virus to evade antibody-mediated neutralization, and suggests a role for the humoral response in containment of JCV infection.
Jelcic I, Combaluzier B, Faigle W, et al. Broadly neutralizing human monoclonal JC polyomavirus VP1-specific antibodies as candidate therapeutics for progressive multifocal leukoencephalopathy. Sci Transl Med. 2015;7:306ra150.
Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68.
Antinori A, Ammassari A, Giancola ML, et al. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol. 2001;7:323–8.
Isidoro L, Pires P, Rito L, Cordeiro G. Progressive multifocal leukoencephalopathy in a patient with chronic lymphocytic leukaemia treated with alemtuzumab. BMJ Case Rep. 2014;2014.
Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16–24.
Klintmalm GB, Feng S, Lake JR, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817–27.
Dammeier N, Schubert V, Hauser TK, Bornemann A, Bischof F. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108.
Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–8.
Nieuwkamp DJ, Murk JL, van Oosten BW, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med. 2015;372:1474–6.
Bartsch T, Rempe T, Wrede A, et al. Progressive neurologic dysfunction in a psoriasis patient treated with dimethyl fumarate. Ann Neurol. 2015;78:501–14.
Gomez-Cibeira E, Ivanovic-Barbeito Y, Gutierrez-Martinez E, et al. Eculizumab-related progressive multifocal leukoencephalopathy. Neurology. 2016;86:399–400.
Carson KR, Newsome SD, Kim EJ, et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: a report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer. 2014;120:2464–71.
Gyang TV, Hamel J, Goodman AD, Gross RA, Samkoff L. Fingolimod-associated PML in a patient with prior immunosuppression. Neurology. 2016;86:1843–5.
Kiewe P, Seyfert S, Korper S, Rieger K, Thiel E, Knauf W. Progressive multifocal leukoencephalopathy with detection of JC virus in a patient with chronic lymphocytic leukemia parallel to onset of fludarabine therapy. Leuk Lymphoma. 2003;44:1815–8.
Lejniece S, Murovska M, Chapenko S, et al. Progressive multifocal leukoencephalopathy following fludarabine treatment in a chronic lymphocytic leukemia patient. Exp Oncol. 2011;33:239–41.
Jarand J, Zochodne DW, Martin LO, Voll C. Neurological complications of infliximab. J Rheumatol. 2006;33:1018–20.
Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum. 2010;62:3191–5.
Rahmlow M, Shuster EA, Dominik J, et al. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65:1538–9.
Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86:1474–8.
Pavlovic AM, Bonaci-Nikolic B, Kozic D, et al. Progressive multifocal leukoencephalopathy associated with mycophenolate mofetil treatment in a woman with lupus and CD4+ T-lymphocyte deficiency. Lupus. 2012;21:100–2.
Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81.
Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74.
Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–8.
Al-Tawfiq JA, Banda RW, Daabil RA, Dawamneh MF. Progressive multifocal leukoencephalopathy (PML) in a patient with lymphoma treated with rituximab: a case report and literature review. J Infect Public Health. 2015;8:493–7.
Sano Y, Nakano Y, Omoto M, et al. Rituximab-associated progressive multifocal leukoencephalopathy derived from non-Hodgkin lymphoma: neuropathological findings and results of mefloquine treatment. Intern Med. 2015;54:965–70.
Melis M, Biagi C, Smabrekke L, et al. Drug-induced progressive multifocal leukoencephalopathy: a comprehensive analysis of the WHO adverse drug reaction database. CNS Drugs. 2015;29:879–91.
Chahin S, Berger JR. A risk classification for immunosuppressive treatment-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21:623–31.
Zaheer F, Berger JR. Treatment-related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther Adv Drug Saf. 2012;3:227–39.
Henegar CE, Eudy AM, Kharat V, Hill DD, Bennett D, Haight B. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus: a systematic literature review. Lupus. 2016;25:617–26. Systematic review of previously published cases of PML and systemic lupus erythematosus suggesting that PML risk is increased in SLE patients compared to patients with other rheumatologic conditions and can occur even in patients on minimal or no immunosuppressive therapy.
Itoh K, Kano T, Nagashio C, Mimori A, Kinoshita M, Sumiya M. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:1020–2.
Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 2009;60:3761–5.
Brandao M, Damasio J, Marinho A, et al. Systemic lupus erythematosus, progressive multifocal leukoencephalopathy, and T-CD4+ lymphopenia. Clin Rev Allergy Immunol. 2012;43:302–7.
Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev. 2008;8:144–6.
Sudhakar P, Bachman DM, Mark AS, Berger JR, Kedar S. Progressive multifocal leukoencephalopathy: recent advances and a neuro-ophthalmological review. J Neuroophthalmol. 2015;35:296–305.
Khoury MN, Alsop DC, Agnihotri SP, et al. Hyperintense cortical signal on magnetic resonance imaging reflects focal leukocortical encephalitis and seizure risk in progressive multifocal leukoencephalopathy. Ann Neurol. 2014;75:659–69. This study reviews imaging findings which are associated with increased risk of seizures in patients with PML.
Miskin DP, Herman ST, Ngo LH, Koralnik IJ. Predictors and characteristics of seizures in survivors of progressive multifocal leukoencephalopathy. J Neurovirol. 2015.
Berger JR. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2014;123:357–76.
Henry C, Jouan F, De Broucker T. JC virus granule cell neuronopathy: a cause of infectious cerebellar degeneration. J Neurol Sci. 2015;354:86–90.
Koralnik IJ, Wuthrich C, Dang X, et al. JC virus granule cell neuronopathy: a novel clinical syndrome distinct from progressive multifocal leukoencephalopathy. Ann Neurol. 2005;57:576–80.
Du Pasquier RA, Corey S, Margolin DH, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61:775–82.
Wuthrich C, Dang X, Westmoreland S, et al. Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons. Ann Neurol. 2009;65:742–8.
Agnihotri SP, Wuthrich C, Dang X, et al. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann Neurol. 2014;76:140–7. This case report describes a patient who presented with a clinical syndrome consistent with normal pressure hydrocephalus and was later found to have JCV in both meningeal and choroid plexus cells. It provides a pertinent example of the ever expanding spectrum of JCV-related disease.
Keith J, Bilbao J, Baskind R. JC virus granular neuronopathy and rhombencephalic progressive multifocal leukoencephalopathy: case report and review of the literature. Neuropathology. 2012;32:280–4.
Dang L, Dang X, Koralnik IJ, Todd PK. JC polyomavirus granule cell neuronopathy in a patient treated with rituximab. JAMA Neurol. 2014;71:487–9.
Chalkias S, Dang X, Bord E, et al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014;75:925–34.
Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology. 2014;83:727–32.
Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Bonington A. BKV-DNA and JCV-DNA in CSF of patients with suspected meningitis or encephalitis. Infection. 2003;31:374–8.
Viallard JF, Ellie E, Lazaro E, Lafon ME, Pellegrin JL. JC virus meningitis in a patient with systemic lupus erythematosus. Lupus. 2005;14:964–6.
Avellaneda-Gomez C, Torres Iglesias R, Puente Periz V, Guerri Fernandez RC. Extrapyramidal syndrome with generalized chorea as an atypical presentation of progressive multifocal leukoencephalopathy. Neurologia 2016.
Willott RH, Sunman W, Munshi SK. Progressive multifocal leukoencephalopathy masquerading as cerebellar infarction. Age Ageing. 2016.
Corral I, Quereda C, Dronda F, et al. Progressive multifocal leukoencephalopathy mimicking milliary CNS tuberculosis. J Neurovirol. 2015;21:691–3.
Takeda S, Yamazaki K, Miyakawa T, Takahashi H, Ikuta F, Arai H. Progressive multifocal leukoencephalopathy showing extensive spinal cord involvement in a patient with lymphocytopenia. Neuropathology. 2009;29:485–93.
Murayi R, Schmitt J, Woo JH, Berger JR. Spinal cord progressive multifocal leukoencephalopathy detected premortem by MRI. J Neurovirol. 2015;21:688–90.
Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–8.
Ryschkewitsch CF, Jensen PN, Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57:243–8.
White MK, Sariyer IK, Gordon J, et al. Diagnostic assays for polyomavirus JC and progressive multifocal leukoencephalopathy. Rev Med Virol. 2016;26:102–14.
Zivanovic M, Savsek L, Poljak M, Popovic M. Possible pitfalls in the diagnostic of progressive multifocal leukoencephalopathy. Clin Neuropathol. 2016;35:66–71.
Dunne Jr WM, Westblade LF, Ford B. Next-generation and whole-genome sequencing in the diagnostic clinical microbiology laboratory. Eur J Clin Microbiol Infect Dis. 2012;31:1719–26.
Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370:2408–17.
Wilson MR, Shanbhag NM, Reid MJ, et al. Diagnosing balamuthia mandrillaris encephalitis with metagenomic deep sequencing. Ann Neurol. 2015;78:722–30.
Garrels K, Kucharczyk W, Wortzman G, Shandling M. Progressive multifocal leukoencephalopathy: clinical and MR response to treatment. AJNR Am J Neuroradiol. 1996;17:597–600.
Shah R, Bag AK, Chapman PR, Cure JK. Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol. 2010;65:431–9.
Goncalves FG, Lamb L, Del Carpio-O’Donovan R. Progressive multifocal leukoencephalopathy restricted to the posterior fossa in a patient with systemic lupus erythematosus. Braz J Infect Dis. 2011;15:609–12.
Jones Jr HR, Hedley-Whyte ET, Freidberg SR, Kelleher Jr JE, Krolikowski J. Primary cerebellopontine progressive multifocal leukoencephalopathy diagnosed premortem by cerebellar biopsy. Ann Neurol. 1982;11:199–202.
Svensson PA, Larsson EM. Infratentorial progressive multifocal leucoencephalopathy (PML) in a patient with SLE (2008: 4b). Eur Radiol. 2008;18:1526–8.
White RP, Abraham S, Singhal S, Manji H, Clarke CR. Progressive multifocal leucoencephalopathy isolated to the posterior fossa in a patient with systemic lupus erythematosus. Rheumatology (Oxford). 2002;41:826–7.
Arbusow V, Strupp M, Pfister HW, Seelos KC, Bruckmann H, Brandt T. Contrast enhancement in progressive multifocal leukoencephalopathy: a predictive factor for long-term survival? J Neurol. 2000;247:306–8.
Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72:1458–64.
Heald AE, Hoffman JM, Bartlett JA, Waskin HA. Differentiation of central nervous system lesions in AIDS patients using positron emission tomography (PET). Int J STD AIDS. 1996;7:337–346.90.
Mertens K, Acou M, Van den Broecke C, et al. Progressive multifocal leukoencephalopathy (PML) mimicking high-grade glioma on delayed F-18 FDG PET imaging. J Clin Neurosci. 2012;19:1167–9.
Miyagawa M, Maeda M, Umino M, et al. Low signal intensity in U-fiber identified by susceptibility-weighted imaging in two cases of progressive multifocal leukoencephalopathy. J Neurol Sci. 2014;344:198–202. The authors describe an MRI SWI pattern of juxtacortical lesions with low signal intensity of the U fibers which was present in two cases of PML. This finding was later replicated in NTZ-associated PML but has also been found to be non-specific for PML (present in cases of cerebral infarcts and encephalitis as well). However, it likely represents a sensitive but non-specific imaging finding associated with PML.
Hodel J, Outteryck O, Verclytte S, et al. Brain magnetic susceptibility changes in patients with natalizumab-associated progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol. 2015;36:2296–302.
Carra-Dalliere C, Menjot de Champfleur N, Ayrignac X, Deverdun J, Labauge P. Quantitative susceptibility mapping suggests a paramagnetic effect in PML. Neurology. 2015;84:1501–2.
Umino M, Maeda M, Ii Y, Tomimoto H, Sakuma H. Low-signal-intensity rim on susceptibility-weighted imaging is not a specific finding to progressive multifocal leukoencephalopathy. J Neurol Sci. 2016;362:155–9.
Adang L, Berger J. Progressive multifocal leukoencephalopathy. F1000Res 2015;4.
Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76:1697–704.
Elphick GF, Querbes W, Jordan JA, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306:1380–3.
Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Arch Neurol. 2009;66:255–8.
Epperla N, Medina-Flores R, Mazza JJ, Yale SH. Mirtazapine and mefloquine therapy for non-AIDS-related progressive multifocal leukoencephalopathy. WMJ. 2014;113:242–5.
Brickelmaier M, Lugovskoy A, Kartikeyan R, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother. 2009;53:1840–9.
Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19:351–8.
Nukuzuma S, Nakamichi K, Kameoka M, et al. Suppressive effect of topoisomerase inhibitors on JC polyomavirus propagation in human neuroblastoma cells. Microbiol Immunol. 2016;60:253–60.
Royal 3rd W, Dupont B, McGuire D, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. J Neurovirol. 2003;9:411–9.
Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71:1030–5.
Sospedra M, Schippling S, Yousef S, et al. Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin Infect Dis. 2014;59:1588–92.
Gasnault J, de Goer de Herve MG, Michot JM, et al. Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis. 2014;1:ofu074.
Middel A, Arends JE, van Lelyveld SF, et al. Clinical and immunologic effects of maraviroc in progressive multifocal leukoencephalopathy. Neurology. 2015;85:104–6. This article describes the rationale for maraviroc therapy in cases of PML and describes several cases in which there was anecdoctal benefit. It provides a basis upon which future clinical trials of maraviroc therapy in the treatment of both HIV+ and HIV- PML cases could be based.
Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–51.
De Luca A, Ammassari A, Pezzotti P, et al. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS. 2008;22:1759–67.
Geschwind MD, Skolasky RI, Royal WS, McArthur JC. The relative contributions of HAART and alpha-interferon for therapy of progressive multifocal leukoencephalopathy in AIDS. J Neurovirol. 2001;7:353–7.
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–33.
Fox RJ, Rudick RA. Risk stratification and patient counseling for natalizumab in multiple sclerosis. Neurology. 2012;78:436–7.
McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87:117–25.
Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80.
Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol. 2016.
Warnke C, Mausberg AK, Stettner M, et al. Natalizumab affects the T-cell receptor repertoire in patients with multiple sclerosis. Neurology. 2013;81:1400–8.
Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–41.
Frohman EM, Monaco MC, Remington G, et al. JC virus in CD34+ and CD19+ cells in patients with multiple sclerosis treated with natalizumab. JAMA Neurol. 2014;71:596–602.
Lindberg RL, Achtnichts L, Hoffmann F, Kuhle J, Kappos L. Natalizumab alters transcriptional expression profiles of blood cell subpopulations of multiple sclerosis patients. J Neuroimmunol. 2008;194:153–64.
Meira M, Sievers C, Hoffmann F, et al. Natalizumab-induced POU2AF1/Spi-B upregulation: a possible route for PML development. Neurol Neuroimmunol Neuroinflamm. 2016;3:e223.
Marshall LJ, Dunham L, Major EO. Transcription factor Spi-B binds unique sequences present in the tandem repeat promoter/enhancer of JC virus and supports viral activity. J Gen Virol. 2010;91:3042–52.
Marshall LJ, Moore LD, Mirsky MM, Major EO. JC virus promoter/enhancers contain TATA box-associated Spi-B-binding sites that support early viral gene expression in primary astrocytes. J Gen Virol. 2012;93:651–61.
Marshall LJ, Ferenczy MW, Daley EL, Jensen PN, Ryschkewitsch CF, Major EO. Lymphocyte gene expression and JC virus noncoding control region sequences are linked with the risk of progressive multifocal leukoencephalopathy. J Virol. 2014;88:5177–83. This study provides evidence that the transcription factor SpiB is markedly upregulated in CD34+ cells from natalizumab-treated individuals. In light of previous evidence that CD34+ cells are increased in the periphery following natalizumab treatment, and that SpiB activates JCV gene expression, these findings suggest that natalizumab may potentiate PML by several complementary mechanisms.
Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–12.
Delbue S, Elia F, Carloni C, et al. JC virus urinary excretion and seroprevalence in natalizumab-treated multiple sclerosis patients. J Neurovirol. 2015;21:645–52.
Schwab N, Schneider-Hohendorf T, Pignolet B, et al. Therapy with natalizumab is associated with high JCV seroconversion and rising JCV index values. Neurol Neuroimmunol Neuroinflamm. 2016;3:e195.
Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–71.
Schneider-Hohendorf T, Philipp K, Husstedt IW, Wiendl H, Schwab N. Specific loss of cellular L-selectin on CD4(+) T cells is associated with progressive multifocal leukoencephalopathy development during HIV infection. AIDS. 2014;28:793–5.
Schwab N, Schneider-Hohendorf T, Pignolet B, et al. PML risk stratification using anti-JCV antibody index and L-selectin. Mult Scler. 2015.
Lieberman LA, Zeng W, Singh C, et al. CD62L is not a reliable biomarker for predicting PML risk in natalizumab-treated R-MS patients. Neurology. 2016;86:375–81.
Hodel J, Darchis C, Outteryck O, et al. Punctate pattern: a promising imaging marker for the diagnosis of natalizumab-associated PML. Neurology. 2016;86:1516–23. This study suggests that the MRI finding of a punctate pattern is a very sensitivity and specific indicator of PML. Their findings also suggested that a punctate pattern may be an early imaging finding of NTZ-associated PML and may allow identification of PML in the asymptomatic stage of the disease which is important as earlier identification and treatment likely leads to better clinical outcomes.
Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–9.
Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21:637–44. This study presents demographic and clinical characteristics which predict better outcomes in cases of NTZ-associated PML.
Stork L, Bruck W, Bar-Or A, Metz I. High CCR5 expression in natalizumab-associated progressive multifocal leukoencephalopathy immune reconstitution inflammatory syndrome supports treatment with the CCR5 inhibitor maraviroc. Acta Neuropathol. 2015;129:467–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Saylor and Venkatesan declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Central Nervous System Infections
Rights and permissions
About this article
Cite this article
Saylor, D., Venkatesan, A. Progressive Multifocal Leukoencephalopathy in HIV-Uninfected Individuals. Curr Infect Dis Rep 18, 33 (2016). https://doi.org/10.1007/s11908-016-0543-8
Published:
DOI: https://doi.org/10.1007/s11908-016-0543-8