Abstract
It has been recognized for many years that leukotrienes play an important role in mediating various effects of the allergic reaction. Recent evidence has shown that they play a role in other diseases including chronic sinusitis, particularly those sub-types involving eosinophils. Leukotrienes can be separated into the fairly well characterized cysteinyl leukotrienes and less well characterized leukotriene B4. Effects of the leukotrienes are mediated through receptors that are expressed on a variety of cell types and can be modulated based on the inflammatory environment present. The pharmaceutical industry has long been interested in blocking leukotriene action and as such, two approaches have been developed that led to drugs approved for treatment of allergic disease. The most widely used class is the cysteinyl type 1 receptor antagonists, which block binding of the cysteinyl leukotrienes to the cell. The second class is an inhibitor of the 5-lipoxygenase enzyme that prevents synthesis of both the cysteinyl leukotrienes and leukotriene B4. This review will focus on the role that leukotrienes play in chronic sinusitis and consider the rationale for choosing either a leukotriene antagonist or synthesis inhibitor as a treatment option. We will also discuss off-label uses for other medications that might be useful in these diseases as they relate to their ability to modulate leukotriene action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diseases within the sinuses produce one of the most common health care problems, affecting ~16% of the population and having a significant adverse impact on quality of life and daily functioning [1]. Historically, chronic sinusitis (CS) was considered a singular disease and as such all patients received the same treatment options. Current practice parameters suggest the presence of two subtypes of CS, CS with nasal polyps (NP) and CS without NP [2, 3]. In recent years, this idea has been challenged, and it is now recognized that there exist multiple variants of CS including non-eosinophilic sinusitis (NES), chronic hypereosinophilic sinusitis (CHES), aspirin exacerbated respiratory disease (AERD), allergic fungal sinusitis (AFS), and cystic fibrosis (CF), each with specific disease processes and pathways requiring unique approaches to management [4••].
In the late 1930s, the slow-reacting substance of anaphylaxis was described based on the identification of a biochemical that, in an antihistamine-resistant fashion, mediated the slow onset, but sustained contraction of guinea-pig ileum smooth muscle. It was not until 1979 that this chemical messenger was successfully identified as a group of compounds known as the leukotrienes (LT); further divided into the cysteinyl leukotrienes (cysLTs: LTC4, LTD4 and LTE4) and LTB4. These compounds are secreted by many cell types with mast cells, basophils and eosinophils being the most important. LTs have been studied in patients with asthma and allergic disease including effects on bronchoconstriction and delayed allergic responses, and, for these reasons, leukotriene modifiers and antagonists have been manufactured. For other diseases including CS variants like CHES and AERD, the shear volume of cells that produce CysLTs, largely the eosinophil, leads us to believe they are important in the pathogenesis of disease and that medications altering CysLT production or activity may be important to consider as a potential therapeutic options. This review examines the role of LT synthesis inhibitors and LT receptor antagonists in the treatment of chronic sinusitis.
Leukotriene Synthesis
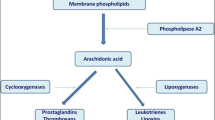
Leukotrienes are generated from the metabolic breakdown of arachidonic acid and consist of the CysLTs (LTC4, LTD4 and LTE4) and LTB4 (Fig. 1). Biosynthesis of LTs begins with activation of a family of enzymes termed the phospholipases, with phospholipase A2 being the most important, resulting in the conversion of membrane phospholipids into arachidonic acid. Following activation, the enzyme 5-lipoxygenase (5-LO) translocates from the cytosol to the perinuclear envelope where in association with 5-lipoxygenase-activating protein (FLAP), arachidonic acid is oxygenated to form 5-hydroperoxy-eicosatetraenoic acid (5-HPETE) and then it is dehydrated to generate LTA4 [5]. 5-LO is a calcium dependant enzyme that is active in a variety of cell types including mononuclear phagocytes, B lymphocytes, granulocytes, and mast cells [6]. FLAP forms a trimeric unit that serves as a membrane anchor to which 5-LO binds and is the initial docking site for arachidonic acid before transfer to 5-LO [7]. LTA4 is unstable and is either converted to LTB4 by LTA4 hydrolase or, alternatively, LTA4 can be metabolized into the cysteinyl leukotriene LTC4 by the enzyme LTC4 synthase (LTC4S) (or the related enzyme microsomal glutathione transferase II [MSGT-II]) via conjugation with glutathione. Like FLAP, LTC4S also exists as a trimer. Glutathione binds between adjacent monomers of LTC4S creating a hydrophobic environment in which LTA4 can dock following generation by 5-LO/FLAP [8]. LTC4 is exported from the cell via the multi-drug transporter ATP-binding cassette (Abc)c1 where hydrolysis and removal of the amino acid glutaminate occurs by action of serum γ-glutamyl transferase giving rise to LTD4. LTE4 is produced following cleavage of a glycine residue from LTD4 by the enzyme dipeptidase, leaving behind the single amino acid cysteine from which this family derives its name [9]. LTE4 is the final end product of CysLT synthesis and is excreted in the urine without further modification. LTs have been implicated in the pathogenesis of many diseases and as such there has been an intense effort by the pharmaceutical industry to develop compounds that antagonize their action.
Leukotriene Receptors
Response to CysLTs is mediated through high affinity interactions with two cloned receptors that are referred to as the CysLT1 and CysLT2 receptors. While sharing only 38% amino acid identity, both receptors are seven transmembrane domain G protein-coupled receptors that utilize calcium as a second messenger [10, 11]. The two receptors can be distinguished by their relative potency for binding the CysLTs: CysLT1 receptor LTD4>LTC4>>LTE4 and CysLT2 receptor LTD4 = LTC4>>LTE4. Thus, the CysLT1 receptor has an approximately 10-fold higher affinity for LTD4 than that for LTC4. At the time of the cloning of the two CysLT receptors, there was pharmacological evidence for a third CysLT receptor as human pulmonary artery stimulated with LTC4 was resistant to inhibition with all known receptor antagonists [12]. It was also known that subjects with AERD had a unique sensitivity to LTE4, which also led to the suggestion that additional CysLT receptors must exist. To date, at least two selective leukotriene E4 receptors have been described (P2Y12 and CysLTER) with their binding affinity being LTE4>LTC4 = LTD4 [13, 14, 15•]. Based on phylogenetic similarity, the GPR17 orphan receptor has been shown to respond to both uracil nucleotides and CysLTs. Expression is found in the heart, brain and kidney undergoing ischemic damage and may represent another LT receptor [16], however several groups have been unable to replicate this finding and even suggest that it may be a negative regulator of CysLT action [17, 18].
The distribution of the CysLT receptors on peripheral blood leukocytes is shown in Table 1 [11, 19, 20]. High levels of expression of both the CysLT1 and CysLT2 receptors have been observed on eosinophils and mast cells whereas only low levels of the CysLT1 receptor are expressed on neutrophils. Few circulating T lymphocytes express either class of receptor (~4% to 8%), however higher levels are seen in inflamed tissue [11, 21]. In addition to these immune cells, the CysLT1 and 2 receptor has been found on smooth muscle cells and the CysLT2 receptor is expressed on heart Purkinjie fiber cells, adrenal chromaffin cells, brain and human umbilical vein endothelial cells (HUVEC) [19, 22]. In contrast to lung fibroblasts that can express the CysLT1 receptor [23], nasal polyp derived fibroblasts do not express either the CysLT1 or 2 receptors [24].
For response to LTB4, two G protein-coupled receptors, BLT1 and BLT2, have been identified as mediating action. The receptors share 45% amino acid identity and it is believed they arose due to gene duplication as BLT2 uses the BLT1 promoter and its sequence overlaps a 5′ untranslated region of a BLT1 splice variant. The BLT1 receptor displays higher affinity and specificity for LTB4 than the BLT2 receptor [25]. The expression pattern for the receptors in peripheral blood leukocytes is shown in Table 1. In general, BLT1 expression is highest in peripheral blood leukocytes with lower levels found on spleen, heart and brain and lung fibroblasts [23, 25]. Of the leukocytes, neutrophils and monocytes have the highest BLT1 levels. BLT2 receptors are ubiquitously expressed with high levels observed in spleen, liver, ovaries and peripheral blood leukocytes. Within the leukocyte population, it is interesting to note that high levels are found on both CD4+ and CD8+ T cell populations, B cells and dendritic cells [26].
LT receptor expression can be modulated depending on cell type and stimulus. This has obvious implications for drug targeting, as many of these cells and cytokines are present in the tissue of CS patients. Interleukin (IL)-4 increases cell surface expression of both the type 1 and 2 CysLT receptors on mast cells without altering mRNA or protein levels; presumably by stimulating vesicles with preassembled CysLT receptors to fuse with the outer cellular membrane [27, 28]. In a more traditional manner, IL-4 stimulates mRNA and cell surface expression of the CysLT1 and CysLT2 receptors in human monocytes, eosinophils, T cells and B cells [29, 30]. Similar to IL-4, IFN-γ increases CysLT2 receptor mRNA expression on human monocytes, T cells and B cells and eosinophils [30, 31]. On human endothelial cells, both IL-4 and IFN-γ stimulate CysLT2 receptor mRNA production [32, 33]. IL-5 can increase CysLT1 receptor mRNA and cell surface expression on human eosinophils [34]. Few studies exist on the modulation of the LTB4 receptors. Of the cytokines, IFN-γ and IL-1β have been shown to increase mRNA and protein expression of the BLT1 receptor and TNF-α and IL-1β lead to increases in the BLT2 receptor [35]. Non-cytokine stimulation of BLT1 receptor expression can be produced by lipopolysaccharide, dexamethasone and LTB4.
The Role of Leukotrienes in Chronic Sinusitis
The extreme example for the role of CysLTs in chronic sinusitis is AERD. AERD was originally defined by the “triad” of nasal polyps, asthma, and aspirin sensitivity (Samter’s triad) [36]. Aspirin intolerance occurs in as many as 20% of adult asthmatics and up to 30% of asthmatics with chronic sinusitis or nasal polyposis [37]. The underlying respiratory disease activates an intense infiltration of mast cells and eosinophils into the respiratory mucosa that synthesize and secrete high levels of CysLTs [38, 39]. Mast cells also release histamine, tryptase and PGD2; vasodilatory and bronchoconstricting agents that augment the LT response. AERD subjects display dramatic upregulation of the two essential enzymes involved in CysLT synthesis, 5-LO and LTC4S [40, 41]. This over-expression drives both the constitutive over-production of CysLTs and the life-threatening surge in CysLTs that occurs with ingestion of aspirin and other non-selective COX inhibitors [42]. CysLTs have important pro-inflammatory and pro-fibrotic effects that contribute to the extensive hyperplastic sinusitis and nasal polyposis that characterize this disorder. In addition to their over-production, these patients display greatly enhanced sensitivity to the CysLTs, reflecting over-expression of the CysLT receptors [21, 43], including both the two well characterized receptors (CysLT1 and CysLT2) and newly described selective LTE4 receptors [13, 14, 15•].
CHES is an inflammatory disease characterized by the prominent accumulation of eosinophils in the sinuses and, when present, associated NP tissue [38, 44, 45]. While NPs frequently occur with CF, AFS and AERD, in the absence of one of these conditions, the presence of nasal polyposis (especially in the concomitant presence of asthma) has been proposed as presumptive evidence for CHES [46, 47]. However, CHES can only be unambiguously diagnosed upon histochemical staining of tissue for eosinophils or via quantification of eosinophil-derived mediators (such as eosinophil cationic protein or major basic protein). In CHES, the sinus tissue demonstrates a marked increase in cells that express cytokines (IL-5, GM-CSF, etc.), chemokines (CCL5, CCL11, CCL24, etc.), and pro-inflammatory lipid mediators (e.g., cysteinyl leukotrienes (CysLTs)) that are responsible for the differentiation, survival, and activation of eosinophils [38, 48, 49]. As eosinophils are a prominent source of many of these cytokines and lipid mediators, this suggests that CHES is a disease of unrestrained inflammation and that once eosinophils are recruited, they provide the growth factors necessary for their further recruitment, proliferation, activation, and survival [44, 48, 49]. Thus, CHES behaves as a self-perpetuating syndrome and, as such, does not respond well to surgery alone [4••].
In patients with CHES and NPs, the role of LTs and CysLT receptors has not been extensively studied. We have demonstrated increased levels of CysLTs in polyp tissue from patients with CHES as compared to tissue from patients with NES or healthy sinus tissue, which was subsequently confirmed in a follow-up study [38, 39]. Our study also found CHES patients had increased mRNA transcripts for the proteins involved in the metabolic pathway of LT synthesis. As mentioned above, in AERD there is upregulation of CysLT synthesis pathways that leads to a life-threatening surge in CysLT secretion following ingestion of aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) in AERD which can be measured in bronchoalveolar lavage samples or through quantification of urinary LTE4 [50–52]. CysLTs are capable of inducing vascular leakage, mucous secretion, myofibroblast proliferation, and eosinophil recruitment, adhesion, and survival. Since CHES and AERD are characterized by CysLT overproduction, enhanced CysLT responsiveness [53], and CysLT receptor over-expression [21], it appears that CysLTs in a feed-forward manner contribute to the ongoing and repeated hyperplastic inflammation and fibrosis of CHES [38].
Pharmacological Inhibition of Leukotriene Action
To antagonize or to inhibit LTs, that is the question. There has been an intense effort put forth by the pharmaceutical industry to develop molecules that block LT action. To date, only four have been approved for use in treating asthma or allergic rhinitis. The structures of these compounds are shown in Fig. 2 and their location of action in the LT pathway is indicated in Fig. 1. Zileuton is the only 5-LOX inhibitor on the market and as such has the capacity to prevent synthesis of both the CysLTs and LTB4. These attributes would seemingly make this the drug of choice, however, even with the advent of a sustained release version, zileuton suffers from a lack of potency and potential hepatic toxicity in some individuals necessitating periodic liver enzyme testing. Montelukast, zafirlukast and pranlukast all act by antagonizing the CysLT1 receptor. The benefits of these drugs are that they can be taken once to twice daily and are generally well tolerated with few side effects reported. The downside is that only the CysLT1 receptor is blocked and they have no effect on actions mediated through the CysLT2 receptor, LTE4 receptors or by LTB4. A few LTB4 receptor antagonists have been developed, but none have reached market at this time. One compound, LY293111 was in Phase II clinical trials for pancreatic cancer [54]. A LTA4 hydrolase inhibitor (DG031) was in Phase I trials for the prevention of heart attacks, but this trial has been suspended for unknown reasons. Neither compound will be discussed further.
Reflecting the important role of the CysLTs in AERD, these patients are often therapeutically responsive to leukotriene modifiers. Although reported to improve asthma and upper airway symptoms, and appropriate as first line therapy, these patients seem less responsive to the leukotriene receptor antagonists (eg, montelukast and zafirlukast). In contrast, the leukotriene synthesis inhibitor zileuton has been shown in placebo-controlled clinical trials to improve asthma, reduce corticosteroid requirements, reduce nasal polyps, and restore anosmia [55•]. The greater beneficial response to this agent is poorly understood, but could reflect either a role for other leukotrienes (eg, LTB4 and 5(S)HETE) or for the CysLTs primarily acting through other CysLT receptors (such as the putative LTE4 receptor(s)) [13, 14, 15•].
There are very few well performed controlled studies of leukotriene modifiers in CHES and most of the published studies with leukotriene modifiers were conducted as add-on therapy to intranasal corticosteroids, making them even more difficult to interpret. In one study, 24 patients who were actively treated with intranasal steroids were followed for 3 months after starting montelukast. Nasal polyp symptom scores improved in 17 of the subjects and eosinophil polyp counts dropped when compared to pretreatment values [56]. Similar results in terms of improvements in nasal symptom scores were observed in a single-blind placebo-controlled crossover study of patients with asthma and nasal polyps [57]. In addition, this group also documented a reduction in nasal fluid levels of CysLTs, ECP, neurokinin A and substance P. An open-label study performed by Wilson, et al., showed improvement in nasal symptom scores, but not in objective parameters in 32 patients with CRS treated with montelukast [58]. LT modifiers are likely to provide benefit in CHES through both direct reduction of eosinophil recruitment and activation in the sinuses and through their ability to diminish eosinophilopoiesis and promote apoptosis. CysLT1 receptor antagonists (zafirlukast and montelukast) have been suggested to have efficacy in CHES/NP in uncontrolled trials [59]. Recently, this group has reported results of a double-blind placebo-controlled trial with montelukast. Postoperative CT scans of patients on montelukast showed either improvement or no change in comparison to the perioperative scans while 30% of patients in the placebo group had CT scans that worsened following surgery. Endoscopic evaluation revealed that nasal polyps recurred in 60% of the placebo patients compared to only 25% in the montelukast group [60]. These findings are supported by two groups, who were able to demonstrate that the recurrence rate of nasal polyps in aspirin sensitive asthmatics was reduced when given montelukast [61, 62].
Long-term double-blind placebo controlled trials are needed to determine the efficacy of these drugs on CHES and AERD. However, leukotriene modifiers are indicated for use in asthmatics, and it has long been understood that a subset of these patients have significant sinusitis. Therefore, it may fall on clinicians to evaluate the effectiveness of leukotriene modifiers in sinusitis when they place asthmatics with sinus disease on these medications.
Indirect Inhibition of Leukotriene Activity by Other Pharmacological Agents
As discussed above, the vast majority of CysLTs and LTs in sinus disease are derived from one cell, the eosinophil and, to a lesser extent, the mast cell. Therefore, it stands to reason that interfering with the eosinophil’s ability survive or to synthesize these biochemicals will lead to improvement of symptoms in patients with sinus disease.
In work done by Boyce et al., mast cells treated with IL-4 had increased expression of LTC4S and enhanced calcium flux and ERK phosphorylation through the CysLT1 receptor [63–65]. Steinke et al. demonstrated that IL-4 could increase CysLT1 and 2 receptor expression on eosinophils, monocytes, and lymphocytes [30]. As such, one could speculate that treatment with anti-IL-4 will improve symptoms in eosinophilic sinusitis by decreasing the effects CysLTs can have on target cells via down-regulation of receptors and the enzyme involved in synthesis. Several IL-4 antagonists are currently in development for the treatment of asthma.
There has been renewed interest in the past several years in anti-IL-5 drugs, particularly in eosinophilic asthma. Evidence suggests that IL-5 is elevated in nasal polyps of patients with CHES. In 2006, a double-blind placebo-controlled trial of 24 subjects with nasal polyps randomized to receive 3 mg/kg, 1 mg/kg, or placebo of humanized anti-IL-5 was published [66]. Reduced peripheral blood eosinophils and ECP levels were found in treated patients. Unfortunately, only half of these patients displayed decreased polyp burden. In post-hoc analysis, the responders were found to have increased IL-5 in their polyps prior to treatment, suggesting that with proper screening a subset of subjects may be candidates for treatment [66]. Mepolizumab, another anti-IL-5 drug, reduces steroid dosages in patients who suffer from Churg Strauss Syndrome (CSS), an eosinophilic vasculitic process that leads to severe asthma, sinusitis, peripheral eosinophilia, pulmonary infiltrates, and tissue infiltration by eosinophils. In a pilot study by Kim, et al., seven patients with CSS were treated with 4 monthly injections of mepolizumab. At the end of 4 months, all seven subjects had decreased steroid requirements and improved symptoms. After cessation of mepolizumab, all symptoms including sinus problems returned [67••].
Imatinib is a 2-phenylaminopyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It is an effective treatment in chronic myelogenous leukemia and gastrointestinal stromal tumors (GIST), and, more recently, there is evidence that it is effective in certain types of hypereosinophilic syndromes and mastocytosis [68, 69]. It has been shown to have anti-eosinophil and mast cell properties particularly through its inhibition of tyrosine kinase, and, as such, it should be beneficial in patients particularly with CHES and AERD. An open-label trial of eight patients with CHES was performed and showed decreased peripheral eosinophils in seven patients and improved symptoms in four [70].
Macrolides possess anti-inflammatory effects likely secondary to their ability to inhibit inflammatory mediators including IL-1β, IL-8, and ICAM-1. Recent in vitro studies of clarithromycin have shown suppression of IL-5, IL-8, and GM-CSF equal to that of prednisolone in nasal biopsy samples of patients with CRS [71]. Suppression of IL-5 and GM-CSF in this manner could lead to decreased peripheral blood eosinophilia and, potentially, decreased tissue eosinophilia leading to improvement in patients with CRS. In a study from Japan, 56 subjects with CRS and nasal polyps were treated with roxithromycin daily for 3 months. A total of 53.6% had overall improvement based on subjective and objective criteria [72].
Phospholipase A2 inhibitors have been marketed for various diseases including atherosclerosis, rheumatoid arthritis, Crohn’s disease, and colitis. Unfortunately, these medications have met with several problems leaving most terminated after phase II trials. GSK has one PLA2 inhibitor (darapladib) that is still in phase II trials for its ability to stabilize atherosclerotic plaques in coronary artery disease. Another secreted PLA2 inhibitor created by Eli Lilly has completed phase I and II trials for its ability to improve survival during sepsis [73, 74]. One could speculate that a PLA2 inhibitor in sinusitis could lead to complete block of leukotriene production in those with eosinophilic disease. Interestingly, the ashwagandha plant (Withania somnifera) used in Ayurvedic medicine is a PLA2 inhibitor, but no clinical trials have been performed to prove its efficacy.
Conclusions
LTs play an important role in the pathogenesis of allergic and non-allergic diseases. This led to the development of drugs that either blocked LT action or inhibited LT synthesis. Despite the recognized morbidity and financial expense incurred by those who suffer CS and the documented high levels of LTs and cells that respond to LTs in CS, few clinical studies have addressed the benefits of modifying LT action in these diseases. In order to lessen the impact of CS, studies are needed for the approved LT modifiers currently on the market in order to expand their clinical usefulness. In addition, as new drugs are developed, clinical trials should include sinusitis as an indication. With this, we may finally get new drugs in the arsenal that will help combat this often-overlooked disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32.
Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clincal research and patient care. J Allergy Clin Immunol. 2004;114:S155–212.
Slavin RG, Spector SL, Berstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S13–47.
•• Payne SC, Borish L, Steinke JW. Genetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol. 2011;in press. Rather than lumping all forms of sinusitis together, this paper discusses the various subtypes of sinusitis and different approaches to treatment.
Yopp AC, Randolph GJ, Bromberg JS. Leukotrienes, sphingolipids, and leukocyte trafficking. J Immuol. 2003;171:5–10.
Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487:323–6.
Ferguson AD, McKeever BM, Xu S, et al. Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein. Science. 2007;317:510–2.
Ago H, Kanaoka Y, Irikura D, et al. Crystal structure of a human membrane protein involved in cysteinyl leukotriene biosynthesis. Nature. 2007;448:609–12.
Christmas P, Weber BM, McKee M, et al. Membrane localization and topology of leukotriene C4 synthase. J Biol Chem. 2002;277:28902–8.
Lynch KR, O’Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–93.
Heise CE, O’Dowd BF, Figueroa DJ, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–6.
Walch L, Norel X, Back M, et al. Pharmacological evidence for a novel cysteinyl-leukotriene receptor subtype in human pulmonary artery smooth muscle. Br J Pharmacol. 2002;137:1339–45.
Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–8.
•• Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. This is one of the first papers that describes a receptor for LTE 4 , providing functional evidence in support of previous pharmacological data.
• Maekawa A, Kanaoka Y, Xing W, et al. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. Using knock-out mice this papers demonstrates that a receptor for LTE 4 exists based on responsiveness to LTE 4 in the absence of other known CysLT receptors.
Ciana P, Fumagalli M, Trincavelli ML, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–27.
Maekawa A, Balestrieri B, Austen KF, et al. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci U S A. 2009;106:11685–90.
Wunder F, Tinel H, Kast R, et al. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT(2)) receptor. Br J Pharmacol. 160:399–409.
Figueroa DJ, Breyer R, Defoe S, et al. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Crit Care Med. 2001;163:226–33.
Figueroa DJ, Borish L, Baramki D, et al. Expression of cysteinyl leukotriene synthetic and signaling proteins in inflammatory cells in active seasonal allergic rhinitis. Clin Exp Allergy. 2003;33:1380–8.
Sousa AR, Parikh A, Scadding G, et al. Leukotriene-receptor expression on nasal mucosal inflammatory cells in asprin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–9.
Sjostrom M, Johansson AS, Schroder O, et al. Dominant expression of the CysLT2 receptor accounts for calcium signaling by cysteinyl leukotrienes in human umbilical vein endotheilial cells. Arterioscler Thromb Vasc Biol. 2003;23:E37–41.
Yoshisue H, Kirkham-Brown J, Healy E, et al. Cysteinyl leukotrienes synergize with growth factors too induce proliferation of human bronchial fibroblasts. J Allergy Clin Immunol. 2007;119:132–40.
Steinke JW, Crouse CD, Bradley D, et al. Characterization of interleukin-4 stimulated nasal polyp fibroblasts. Am J Respir Cell Mol Biol. 2004;30:212–9.
Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prost Leukot Essent Fatty Acids. 2003;69:123–34.
Yokomizo T, Izumi T, Shimizu T. Co-expression of two LTB4 receptors in human mononuclear cells. Life Sci. 2001;68:2207–12.
Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583–92.
Mellor EA, Frank N, Soler D, et al. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: Functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100:11589–93.
Thivierge M, Stankova J, Rola-Pleszczynski M. IL-13 and IL-4 up-regulate cysteinyl leukotriene 1 receptor expression in human monocytes and macrophages. J Immunol. 2001;167:2855–60.
Early SB, Barekzi E, Negri J, et al. Concordant modulation of cysteinyl leukotriene receptor expression by IL-4 and IFN-γ on peripheral immune cells. Am J Respir Cell Mol Biol. 2007;36:715–20.
Fujii M, Tanaka H, Abe S. Interferon-gamma up-regulates expression of cysteinyl leukotriene type 2 receptors on eosinophils in asthmatic patients. Chest. 2005;128:3148–55.
Lotzer K, Spanbroek R, Hildner M, et al. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:e32–6.
Woszczek G, Chen LY, Nagineni S, et al. IFN-gamma induces cysteinyl leukotriene receptor 2 expression and enhances the responsiveness of human endothelial cells to cysteinyl leukotrienes. J Immunol. 2007;178:5262–70.
Thivierge M, Doty M, Johnson J, et al. IL-5 up-regulates cysteinyl leukotriene 1 receptor expression in HL-60 cells differentiated into eosinophils. J Immunol. 2000;165:5221–6.
Qiu H, Johansson AS, Sjostrom M, et al. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc Natl Acad Sci U S A. 2006;103:6913–8.
Samter M, Beers Jr RF. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–83.
Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax. 2000;55:S42–6.
Steinke JW, Bradley D, Arango P, et al. Cytseinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–9.
Perez-Novo CA, Watelet JB, Claeys C, et al. Prostaglandin, leukotiene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–96.
Cowburn AS, Sladek K, Soja J, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–46.
Adamjee J, Suh YJ, Park HS, et al. Expression of 5-lipoxygenase and cyclooxygenase pathway enzymes in nasal polyps of patients with aspirin-intolerant asthma. J Pathol. 2006;209:392–9.
Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–9.
Corrigan C, Mallett K, Ying S, et al. Expression of the cysteinyl leukotriene receptors cysLT(1) and cysLT(2) in aspirin-sensitive and aspirin-tolerant chronic rhinosinusitis. J Allergy Clin Immunol. 2005;115:316–22.
Bachert C, Wagenmann M, Hauser U, et al. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42.
Bachert C, Gevaert P, van Cauwenberge P. Nasal polyposis- a new concept on the formation of polyps. ACI International. 1999;11:130–5.
Elovic A, Wong DT, Weller PF, et al. Expression of transforming growth factors-alpha and beta 1 messenger RNA and product by eosinophils in nasal polyps. J Allergy Clin Immunol. 1994;93:864–9.
Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9.
Minshall EM, Cameron L, Lavigne F, et al. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Amer J Resp Cell Mol Biol. 1997;17:683–90.
Hamilos DL, Leung DYM, Huston DP, et al. GM-CSF, IL-5, and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis. Clin Exp Allergy. 1998;28:1145–52.
Israel E, Fischer AR, Rosenberg MA, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Amer Rev Respir Dis. 1993;148:1447–51.
Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirn-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–9.
Daffern PJ, Muilenburg D, Hugli TE, et al. Association of urinary leukotriene E4 excretion during aspirin challenges with severity of resiratory responses. J Allergy Clin Immunol. 1999;104:559–64.
Arm JP, O’Hickey S, Spur BW, et al. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–53.
Ding XZ, Talamonti MS, Bell Jr RH, et al. A novel anti-pancreatic cancer agent, LY293111. Anti Canc Drugs. 2005;16:467–73.
Dahlen B, Nizankowska E, Szczeklik A. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Critc Care Med. 1998;157:1187–94.
Kieff DA, Busaba NY. Efficacy of montelukast in the treatment of nasal polyposis. Ann Otol Rhinol Laryngol. 2005;114:941–5.
• Schaper C, Noga O, Koch B, et al. Anti-inflammatory properties of montelukast, a leukotriene receptor antagonist in patients with asthma and nasal polyposis. J Investig Allergol Clin Immunol. 2011;21:51–58. Monteluekast was shown to mediate slight improvements in chronic sinusitis symptoms and reduction in nasal polyps.
Wilson AM, White PS, Gardiner Q, et al. Effects of leukotriene receptor antagonist therapy in patients with chronic rhinosinusitis in a real life rhinology clinic setting. Rhinology. 2001;39:142–6.
Parnes SM, Churna AV. Acute effects of antileukotrienes on sinonasal polyposis and sinusitis. ENT Journal. 2000;79:18–21.
Parnes SM. The role of leukotriene inhibitors in patients with paranasal sinus disease. Curr Opin Otolaryngol Head Neck Surg. 2003;11:184–91.
Di Rienzo L, Artuso A, Cerqua N. Antileukotrienes in the prevention of postoperative recurrence of nasal polyposis in ASA syndrome. Acta Otorhinolaryngol Ital. 2000;20:336–42.
Grundmann T, Topfner M. Treatment of ASS-Associated Polyposis (ASSAP) with a cysteinyl leukotriene receptor antagonist - a prospective drug study on its antiinflammatory effects. Laryngorhinootologie. 2001;80:576–82.
Hsieh FH, Lam BK, Penrose JF, et al. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4. J Exp Med. 2001;193:123–33.
Mellor EA, Maekawa A, Austen KF, et al. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci USA. 2001;98:7964–9.
Lin DA, Boyce JA. IL-4 regulates MEK expression required for lysophosphatidic acid-mediated chemokine generation by human mast cells. J Immunol. 2005;175:5430–8.
Gevaert P, Lang-Loidolt D, Lackner A, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41.
•• Kim S, Marigowda G, Oren E, et al. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–1343. Anti-IL-5 treatment reduces eosinophil number and therefore leukotriene levels in sinus tissue. An additional benefit would be the reduction of steroid levels required in the treatment of chronic sinusitis and associated nasal polyposis.
Bain BJ. Relationship between idiopathic hypereosinophilic syndrome, eosinophilic leukemia, and systemic mastocytosis. Am J Hematol. 2004;77:82–5.
Koury MJ, Newman JH, Murray JJ. Reversal of hypereosinophilic syndrome and lymphomatoid papulosis with mepolizumab and imatinib. Am J Med. 2003;115:587–9.
Amrol D, Murray JJ. Alternative medical treatment strategies for chronic hyperplastic eosinophilic sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2005;13:55–9.
Wallwork B, Coman W, Mackay-Sim A, et al. Effect of clarithromycin on nuclear factor-kappa B and transforming growth factor-beta in chronic rhinosinusitis. Laryngoscope. 2004;114:286–90.
Katsuta S, Osafune H, Takita R, et al. Therapeutic effect of roxithromycin on chronic sinusitis with nasal – polyps clinical, computed tomography, and electron microscopy analysis. Nihon Jibiinkoka Gakkai Kaiho. 2002;105:1189–97.
Bradley JD, Dmitrienko AA, Kivitz AJ, et al. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J Rheumatol. 2005;32:417–23.
Miyake A, Yamamoto H, Takebayashi Y, et al. The novel natural product YM-26567-1 [(+)-trans-4-(3-dodecanoyl-2,4,6- trihydroxyphenyl)-7-hydroxy-2-(4-hydroxyphenyl)chroman]: a competitive inhibitor of group II phospholipase A2. J Pharmacol Exp Ther. 1992;263:1302–7.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinke, J.W., Kennedy, J.L. Leukotriene Inhibitors in Sinusitis. Curr Infect Dis Rep 14, 147–154 (2012). https://doi.org/10.1007/s11908-012-0245-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-012-0245-9