Abstract
Leukotriene modifiers represent a potentially useful agent in the treatment of chronic rhinosinusitis. Currently, leukotriene-modifying agents are FDA approved for treatment of asthma and allergic rhinitis. Leukotrienes are inflammatory agents released by mast cells and eosinophils. They enact inflammatory changes by increasing edema, recruiting inflammatory cells, increasing cytokine and collagen production, and increasing vascular permeability. Leukotriene-modifying agents include leukotriene receptor antagonists and 5-lipoxygenase inhibitors, both of which act to decrease downstream leukotriene effects. Currently, there are no specific guidelines for the use of antileukotriene agents in chronic rhinosinusitis. They are often used as adjunct therapy for the medical management of Samter’s (aspirin) triad patients and patients with chronic rhinosinusitis with nasal polyposis. The literature studying the benefits of antileukotrienes in chronic rhinosinusitis is limited, but existing data does show some benefit of using these agents in specific cases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic rhinosinusitis
- Leukotriene
- Montelukast
- Allergic rhinitis
- Aspirin triad
- Nasal polyposis
- Montelukast
-
Leukotriene-modifying agents have been shown to be beneficial in asthma and allergic rhinitis.
-
Leukotriene-modifying agent may have benefit in chronic rhinosinusitis with nasal polyposis (aspirin triad) as adjuvant therapy in select patients.
-
Further randomized controlled trials are needed to more fully assess benefits of leukotriene-modifying agents.
Introduction
Leukotriene modifiers have been key agents for treatment of asthma and lower airway disease. Under a united airway model, it has been postulated that leukotriene modifiers may be of benefit in reducing inflammation in the upper airway and specifically in the nasal mucosa. In allergic rhinitis, leukotriene modifiers have been shown to be of benefit in symptomatic relief [1, 2]. However, evidence to date in regard to leukotriene-modifying agents in chronic rhinosinusitis (CRS) is limited.
There is considerable overlap between allergic rhinitis and CRS [3]. Chronic allergen exposure in patients leads to increased inflammatory cell recruitment and inflammatory cascades in nasal mucosal tissue. This leads to increased inflammation (both acute and chronic), nasal edema, fibrosis, goblet cell hyperplasia, and mucous production [4–6]. The allergic milieu subsequently leads to nasal polyp formation and CRS. The exact mechanisms associated with the development of CRS, however, have not yet been elucidated.
Chronic rhinosinusitis is stratified into two groups: chronic rhinosinusitis with nasal polyposis (CRSwNP) and chronic rhinosinusitis without nasal polyposis. The two groups encompass very different inflammatory environments. Chronic rhinosinusitis with nasal polyposis usually exhibits lower regulatory T cell activity, with increased eosinophilia, IgE production, and Th2 activation, with IL-3 and IL-5 production. On the other hand, CRS without polyposis usually entails an environment with increased fibrosis, neutrophilic inflammation, and Th1 activation, with IFN-γ, IL-2, and TNF-β production [7].
Current guidelines for medical management of CRS most frequently recommend treatment with daily intranasal corticosteroids with short courses of oral corticosteroids as needed [8–10]. Additional therapeutic options including antibiotics, nasal rinses, and leukotriene-modifying agents are often used as adjunct therapy. The current guidelines from the European position paper on rhinosinusitis and nasal polyps do not recommend the use of leukotriene-modifying agents, citing a lack of current supportive evidence [8]. The clinical practice guidelines from Otolaryngology – Head and Neck Surgery journal acknowledge the role of allergy in CRS, but do not comment on the use of leukotriene modifiers specifically.
Mechanisms of Action
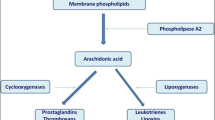
Leukotriene-modifying agents act in one of two ways: decreasing the production of leukotrienes by inhibiting 5-lipooxygenase function or acting as competitive leukotriene receptor antagonists. See Fig. 18.1 for a depiction of the leukotriene pathway. The enzyme phospholipase A2 is initially activated, which then cleaves arachidonic acid from cell membrane phospholipids via hydrolysis. The enzyme 5-lipoxygenase then converts arachidonic acid to LTA4. This enzyme is the target of the inhibitor zileuton. Activity of 5-lipoxygenase is enhanced by the protein 5-lipoxygenase-activating protein. LTC4 synthase adds glutathione to LTA4 to produce LTC4. LTA4 hydrolase converts LTA4 into LTB4. Specific transporters target LTB4 and LTC4 to export these leukotrienes extracellularly. γ-Glutamyl leukotrienase converts LTC4 to LTD4 extracellularly. LTE4 is created extracellularly as well by a dipeptidase. These cysteinyl leukotrienes (LTC4, LTD4, and LTE4) act as the primary proinflammatory molecules of this pathway [11, 12]. These leukotrienes bind primarily to two G protein-coupled receptors, CysLT1 and CysLT2, in target cells. Montelukast, zafirlukast, and pranlukast act as antagonists of CysLT1 specifically. When leukotriene receptors are activated in target cells, there is an increase in intracellular calcium and decrease in cAMP, with subsequent activation of protein kinases and proinflammatory downstream effects. Notably, the pathway is active in nasal mucosa as well as the lower airway. As is seen in patients with aspirin triad, patients may have effects in the nasal mucosa (polyposis) and the lower respiratory tract (asthma).
Leukotrienes are produced and secreted by mast cells, eosinophils, and basophils. Normal nasal mucosa has been shown to express leukotriene receptors [13], and nasal polyps, in particular, demonstrate increased upregulation of leukotriene receptors [14]. In the nasal and paranasal sinus mucosa, leukotrienes work by increasing nasal mucosa blood flow, increasing nasal airway edema and resistance, and recruiting and maturing of inflammatory cells. Additionally, they play roles in increasing cytokine and collagen production and increasing vascular permeability with plasma protein exudation [4–6].
Indications
There are no established indications for the use of leukotriene-modifying agents in CRS. These agents are FDA approved for use in patients with allergic rhinitis and asthma and have been shown to be of benefit in these patients [1]. They have been used frequently in the treatment of aspirin triad and CRSwNP. As discussed below, limited studies in regard to use in CRS suggest a benefit of leukotriene-modifying agents in specific cases.
Specific Therapy
The most frequently used leukotriene-modifying agent is montelukast. It is a leukotriene receptor antagonist, specifically against CysLT1. It is usually prescribed as one 10 mg tablet daily. The other commonly used leukotriene receptor antagonist is zafirlukast. Zileuton is the only 5-lipoxygenase inhibitor currently on the market.
In general, these drugs are fairly well tolerated. The most common side effect of zileuton, montelukast, and zafirlukast is headache (25 %, 18 %, and 13 % of patients, respectively). Montelukast and zafirlukast are class B pregnancy category drugs. These agents have been linked to neuropsychiatric changes, with increased agitation, disorientation, irritability, vivid dreams, depression, and suicidal ideation. Additionally, cases have been described of increased eosinophilia and vasculitis in montelukast. Unlike, zafirlukast and zileuton, liver function tests are not necessary for patients taking montelukast. Zafirlukast has been associated with Churg-Strauss syndrome and hepatitis. Careful surveillance should be undertaken when starting patients on this medication, and baseline liver function tests should be performed. It is contraindicated in patients with existing Churg-Strauss syndrome and patients with cirrhosis or liver disease. Zileuton is a class C pregnancy category drug. This drug, as well, can exacerbate underlying liver disease and is contraindicated in patients with a history of liver disease or heavy alcohol use. Zileuton also can cause similar neuropsychiatric effects. It additionally has a higher incidence of gastrointestinal symptoms.
Clinical Efficacy Data (Including Expert Opinion)
A few randomized clinical trials exist studying the effects of antileukotrienes in CRS. There are additionally multiple case series documenting the effects of antileukotrienes on patients with CRS, with the majority of these studying aspirin triad patients.
Leukotriene-modifying agents in comparison to placebo have been studied in CRSwNP. Pauli et al. performed a randomized clinical trial comparing montelukast against placebo in CRSwNP patients [15]. The montelukast group had significant improvements in health-related quality of life symptoms, including headaches, sleep, and emotional problems, as well as objective measurement of polyp burden. Schaper et al. [16] performed a crossover study comparing montelukast to placebo in patients with CRSwNP who also had mild to moderate asthma. Overall, patients demonstrated statistically significant improvement in nasal symptoms (nasal obstruction, rhinorrhea, itching), reduction in nasal edema, increased nasal airflow, and decreased local and systemic eosinophil counts while on montelukast in comparison to placebo. A case series by Kutting et al. [17] provided montelukast to patients who had undergone multiple previous endoscopic surgeries for sinonasal polyposis. Seven out of nine patients noted significant improvement in symptoms up to 1 year out. Notably, when two patients discontinued montelukast, they suffered recurrence of their symptoms. These studies do suggest that antileukotriene medications have an overall beneficial effect when compared to placebos.
Other studies have compared leukotriene modifiers to standard nasal corticosteroids. Mostafa et al. performed a randomized controlled study treating CRS patients with sinonasal polyps postoperatively with either montelukast or beclomethasone [18]. Both groups demonstrated symptomatic improvement postoperatively, with the montelukast group showing greater improvement in postnasal drip, headache, and itching and the beclomethasone group having greater improvement in dysosmia and nasal obstruction. There was no difference in nasal polyp recurrence rates. There was no combination group or negative control group postoperatively in this study, however. Another study by Vuralkan et al. compared treatment with montelukast versus mometasone postoperatively in patients with CRSwNP, with both groups showing improvement on SNOT-22 scores and decreased sinonasal disease on CT imaging [19]. Mometasone performed slightly better than montelukast in preventing polyp recurrence. Overall, studies do not support replacement of standard nasal corticosteroid therapy with leukotriene-modifying agents, but do not symptomatic benefits and objective decrease in nasal polyposis with antileukotriene therapy.
Additional studies have focused on adding leukotriene inhibitors to patients already taking nasal corticosteroid regimens. Parnes and Chuma studied the effect of adding zileuton or zafirlukast to standard therapy in patients with CRS with sinonasal polyposis [20]. Seventy-two percent of patients reported symptomatic improvement with added antileukotriene therapy, although there were no control or comparison groups in this study. Nonaka et al. added montelukast to the treatment regimen of 20 CRSwNP patients who were already taking inhaled corticosteroids for over 1 year [21]. After 1 year of combined treatment, significant reductions in nasal polyp size, sinus disease burden, and peripheral eosinophil counts were noted. Similarly, Kieff and Busaba added montelukast to patients with nasal polyposis who had been taking intranasal corticosteroids for greater than 6 months [22]. After addition of montelukast, 71 % of patients noted symptomatic improvement, and biopsies of nasal polyps demonstrated a significant reduction in eosinophil burden. The greatest effect of montelukast, notably, was found in patients with documented allergic rhinitis, indicating that leukotriene-modifying agents may be ideally suited in CRS patients with an allergic component. Stewart et al. [23] tested for additional benefits of adding montelukast to an oral steroid course and nasal steroids for 8 weeks. They identified statistically significant improvement in headache, facial pain, and sneezing in the montelukast group, although these benefits did not last after discontinuing therapy. Ragab et al. [24] also studied the addition of montelukast to patients with CRSwNP and asthma refractory to treatment with intranasal steroids. The researchers subdivided their subjects into those with aspirin sensitivity and those without. Subjective improvement was noted in patients who were aspirin tolerant. However, other measures such as rhinometry and nasal inspiratory peak flow did not improve. Aspirin sensitivity was not associated with improvement on montelukast in this study. Overall, these studies suggest symptomatic improvement with the addition of leukotriene-modifying therapy to standard steroid therapy, although many of the studies reviewed did not have control groups.
Ulualp et al. [25] surveyed patients with aspirin triad who had functional endoscopic sinus surgery and who underwent trials of postoperative zafirlukast (16) or zileuton (2). Patients reported improvement in CRS symptoms, ranging from slight (seven) to moderate (two) to very good (three).
Notably, in all these studies, leukotriene-modifying agents appeared to be well tolerated. Side effects were minimal in studies that commented on tolerance to medications.
Conclusion
Current evidence does not support the use of leukotriene-modifying drugs in all cases of CRS. The current European Position Paper on Rhinosinusitis and Nasal Polyps 2012 does not recommend the use of leukotriene-modifying agents for the treatment of CRS [8] as there is insufficient data in the literature to support their use. The current clinical practice guideline for adult sinusitis in Otolaryngology – Head and Neck Surgery journal does not comment on the role of leukotriene-modifying agents in CRS [10]. A meta-analysis and systematic review by Wentzel et al. in 2013 [26] did find some mild benefit in patients with CRSwNP in symptom management. Overall, the use of leukotriene-modifying agents may be warranted in specific cases of CRS, such as patients with aspirin triad. In patients with a clear component or history of allergic rhinitis or asthma in conjunction with CRSwNP, a trial of leukotriene modifiers may be beneficial. Additionally, patients who do not tolerate, or fail, intranasal corticosteroids therapy may benefit from a trial of leukotriene-modifying agents. The effects of these medications, however, may not last after cessation of treatment. Overall, the use of these medications in carefully chosen patients may provide additional benefit to standard therapy for CRS and in particular CRSwNP. Leukotriene-modifying agents are generally well tolerated, with few side effects, the most notable being headaches, neuropsychiatric changes, and liver injury. Current reports in the literature have small population sizes, with at most a few dozen patients receiving treatments. Given the lack of large, randomized, blinded clinical studies involving leukotriene-modifying agents, further research into the application of these agents in chronic rhinosinusitis is warranted.
References
Nayak A, Langdon RB. Montelukast in the treatment of allergic rhinitis: an evidence-based review. Drugs. 2007;67(6):887–901.
Cingi C, Gunham K, Gage-White L, Unlu H. Efficacy of leukotriene antagonists as concomitant therapy in allergic rhinitis. Laryngoscope. 2010;120(9):1718–23.
Kennedy JL, Borish L. Chronic sinusitis pathophysiology: the role of allergy. Am J Rhinol Allergy. 2013;27(5):367–71.
Bisgaard H, Olsson P, Bende M. Effect of leukotriene D4 on nasal mucosal blood flow, nasal airway resistance and nasal secretion in humans. Clin Allergy. 1986;16:289–97.
Busse W, Kraft M. Cysteinyl leukotrienes in allergic inflammation: strategic target for therapy. Chest. 2005;127:1312–26.
Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55:7–16.
Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–9.
Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23(3):1–298.
Cain RB, Lal D. Update on the management of chronic rhinosinusitis. Infect Drug Resist. 2013;6:1–14.
Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31.
Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochim Biophys Acta. 2011;1810(11):1096–102.
Peters-Golden M, Henderson Jr WR. Leukotrienes. N Engl J Med. 2007;357(18):1841–54.
Shirasaki H, Kanaizumi E, Watanabe K, et al. Expression and localization of the cysteinyl leukotriene 1 receptor in human nasal mucosa. Clin Exp Allergy. 2002;32(7):1007–12.
Chao SS, Graham SM, Brown CL, et al. Cysteinyl leukotriene 1 receptor expression in nasal polyps. Ann Otol Rhinol Laryngol. 2006;115(5):394–7.
Pauli C, Fintelmann R, Klemens C, et al. Polyposis nasi – improvement in quality of life by the influence of leukotriene receptor antagonists. Laryngorhinootologie. 2007;86(4):282–6.
Schaper C, Noga O, Koch B, et al. Anti-inflammatory properties of montelukast, a leukotriene receptor antagonist in patients with asthma and nasal polyposis. J Investig Allergol Clin Immunol. 2011;21(1):51–8.
Kutting B, Nieschalk M, Brehler R. A new concept for treatment of sinonasal polyposis. Allergy. 2000;55(11):1091–2.
Mostafa BE, Abdel Hay H, Mohammed HE, Yamani M. Role of leukotriene inhibitors in the postoperative management of nasal polyps. ORL J Otorhinolaryngol Relat Spec. 2005;67(3):148–53.
Vuralkan E, Saka C, Akin I, Hucumenoglu S, et al. Comparison of montelukast and mometasone furoate in the prevention of recurrent nasal polyps. Ther Adv Respir Dis. 2012;6(1):5–10.
Parnes SM, Chuma AV. Acute effects of antileukotrienes on sinonasal polyposis and sinusitis. Ear Nose Throat J. 2000;79(1):18–20.
Nonaka M, Sakanushi A, Kusama K, et al. One-year evaluation of combined treatment with an intranasal corticosteroid and montelukast for chronic rhinosinusitis associated with asthma. J Nippon Med Sch. 2010;77(1):21–8.
Kieff DA, Busaba NY. Efficacy of montelukast in the treatment of nasal polyposis. Ann Otol Rhinol Laryngol. 2005;114(12):941–5.
Stewart RA, Ram B, Hamilton G, et al. Montelukast as an adjunct to oral and inhaled steroid therapy in chronic nasal polyposis. Otolaryngol Head Neck Surg. 2008;139(5):682–7.
Ragab S, Parikh A, Darby YC, Scadding GK. An open audit of montelukast, a leukotriene receptor antagonist, in nasal polyposis associated with asthma. Clin Exp Allergy. 2001;31(9):1385–91.
Ulualp SO, Sterman BM, Toohill RJ. Antileukotriene therapy for the relief of sinus symptoms in aspirin triad disease. Ear Nose Throat J. 1999;78(8):604–6.
Wentzel JL, Soler ZM, Deyoung K, et al. Leukotriene antagonists in nasal polyposis: a meta-analysis and systematic review. Am J Rhinol Allergy. 2013;27(6):482–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zacharek, M.A., Birkeland, A.C. (2015). Leukotriene Modifiers. In: Batra, P., Han, J. (eds) Practical Medical and Surgical Management of Chronic Rhinosinusitis. Springer, Cham. https://doi.org/10.1007/978-3-319-16724-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-16724-4_18
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16723-7
Online ISBN: 978-3-319-16724-4
eBook Packages: MedicineMedicine (R0)