Abstract
Genital mycoplasmas are commonly found in the female genital tract. Despite ongoing debate, the evidence that they cause lower genital tract disease in women remains sparse. The data that Mycoplasma genitalium is primarily transmitted sexually are accumulating, but its role as a cause of symptomatic urethritis or cervicitis is open to debate. Although Mycoplasma hominis may be a co-factor in bacterial vaginosis, it has otherwise not been implicated as a cause of lower tract disease. Now that Ureaplasma urealyticum has been divided into U. urealyticum and Ureaplasma parvum, their role in causing urethritis and cervicitis remains even more unclear. To date, no convincing evidence exists that antimicrobial therapy should be directed solely at these organisms when treating women with urethritis, bacterial vaginosis, trichomoniasis, or cervicitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genital mycoplasmas refer to organisms from the genera Mycoplasma and Ureaplasma. Most health care providers are familiar with Mycoplasma pneumoniae, a cause of respiratory disease, as well as Mycoplasma hominis and Ureaplasma urealyticum, two organisms commonly found in the female genital tract. Over the decades since their discovery, the role of these latter two organisms in causing disease in the upper and lower female genital tract, as well as in pregnancy, has been much debated. Recently, the development of polymerase chain reaction (PCR) tests for Mycoplasma genitalium led to its identification as a possible cause of pelvic inflammatory disease (PID) [1]. Furthermore, the separation of certain Ureaplasma organisms into a distinct species, Ureaplasma parvum, has spurred new questions about the role of ureaplasmas in causing female genital tract disease. The purpose of this paper is to review the classification system and detection methods for genital mycoplasmas, evaluate the evidence for and against their role in causing lower genital tract infection in women, and discuss possible treatment modalities.
Detection of Genital Mycoplasmas
In terms of taxonomy, the genital mycoplasmas belong to the Mycoplasmataceae family and Molliculutes class. In the genus Mycoplasma, seven mycoplasmal strains have been identified in the genital tract: M. hominis, M. genitalium, M. fermentans, M. penetrans, M. pneumoniae, M. primatum and M. spermatophilum. Of these, current consensus is that M. fermentans, M. penetrans, M. pneumoniae, M. primatum, and M. spermatophilum do not cause genital tract disease [2, 3]. Most genital mycoplasmas are facultative anaerobes. They are flask-shaped cells that replicate in a parasitic manner. For vaginal infections such as bacterial vaginosis, vaginal flora are frequently visualized and quantified via Gram stain. Because genital mycoplasmas lack a cell wall and are thus resistant to Gram stain, the presence of genital mycoplasmas in studies of vaginal infections that rely on Gram stain criteria can easily be missed.
Historically, the gold standard for detection of M. hominis and U. urealyticum has been culture. For M. genitalium, culture has always been considered difficult. Over recent years, PCR has gained in popularity as a method for finding all genital mycoplasmas in research settings and seems to be more reliable. For example, Petrikkos and colleagues [4] examined vaginal specimens collected from 203 asymptomatic women in Greece. When culture was used as the reference standard, they found that the sensitivity and specificity of PCR for the detection of U. urealyticum were 94.2% and 92% and for M. hominis were 95.6% and 86.9%. However, when PCR instead of culture was the reference standard, the sensitivity and specificity of culture was 92.4% and 93.8% for U. urealyticum and 63% and 98.8% for M. hominis. In addition to better performance of the test results, PCR had an added advantage of giving more immediate results. More recently, McIver and colleagues [5•] used a four-multiplex PCR for the detection of 19 microorganisms in cervical swabs from women attending an Australian sexual health clinic. This approach included a single round PCR to detect M. genitalium, M. hominis, U. urealyticum, and U. parvum. Although there was some difficulty in data analysis because of cross reaction between the ureaplasmas and weak reactions for U. parvum caused by wild strains of M. hominis, the authors concluded that this technique could at some point be adapted for testing in clinical situations. Currently, a variety of PCR techniques for genital mycoplasmas have been used in published studies. There are little data to compare one PCR method to another. From a practical perspective for clinicians, it should be emphasized that the performance of commercially available PCR tests for genital mycoplasmas remains unstudied and unvalidated.
Finally, it is also apparent that, in addition to the technique for detecting genital mycoplasmas, the site of sampling is also important. For example, in a study that included 304 Norwegian women testing positive for M. genitalium, 62% were positive in the first voided urine, 86% on cervical swab, and only 47% at both sites [6•]. On the other hand, in 102 French women where multiple sites were swabbed simultaneously, M. genitalium was found more frequently on urethral and vaginal swabs (39%) than cervical swab (29%) [7].
Prevalence of Genital Mycoplasmas
With the above limitations in mind, one can find multiple studies, summarized in Table 1, that evaluate the prevalence of genital mycoplasmas in various populations. For most of these studies, the target population was women thought to be at high risk for STI, and exclusion criteria included recent antibiotic treatment. Ureaplasmas as a whole are more prevalent than other mycoplasmas in the female urogenital tract, and U. parvum was found more often than U. urealyticum. When comparing the prevalence of M. genitalium to chlamydia and gonorrhea on cervical swabs (Table 2), M. genitalium was detected about as often as Chlamydia trachomatis and more frequently than Neisseria gonorrhoeae. Furthermore, it is worth noting that co-infection of M. genitalium and C. trachomatis was not uncommon, with about 10% to 40% of women with chlamydia also having M. genitalium.
Mycoplasma genitalium
First characterized in 1981 by Tully and colleagues from 2 of 13 men with nongonococcal urethritis (NGU), M. genitalium has the smallest free-living bacterial genome characterized from nature [8]. Although most genital mycoplasmas can be grown on specific culture media, M. genitalium in particular is quite fastidious. With the development of specific PCR assays, detection of this organism has been facilitated and has led to more research on its transmission and pathogenicity.
As will be discussed, the disease process caused by M. genitalium is debated. However, based on results of several studies, it seems that M. genitalium can for the most part be considered a sexually transmitted organism. For example, a study in a Swedish sexually transmitted disease (STD) clinic population found that 56% of partners of women positive for M. genitalium also tested positive, compared to only 5% of those where the women did not have this organism (P < 0.001) [9]. Furthermore, in a longitudinal study of 383 adolescent women, Tosh and colleagues [10] found M. genitalium in 78 of 3110 (2.5%) vaginal swabs, but only one was from a woman who denied ever having vaginal intercourse. As further evidence of the sexual transmission of the organism, they noted that women testing positive were more likely to have had more partners in the previous 3 months and to have a male partner also testing positive.
When investigating M. genitalium and its contribution to lower genital tract disease, many of the studies focused initially on male urethritis, then later on its role in causing mucopurulent cervicitis (MPC) in women. In the upper genital tract, some researchers feel that M. genitalium can cause PID independently of other known causes [1]. PID studies suggest that M. genitalium cases may be similar to chlamydial ones in terms of severity of symptoms and signs [11]. However, its role in causing lower genital tract disease remains even more debatable.
In the case of MPC, M. genitalium is thought by some to be a clear cause of this syndrome. In a 2003 study of 719 women attending an STD clinic in Seattle from 1984 to 1986, MPC was found in 215 women, with 11% testing positive for M. genitalium [12]. Furthermore, compared to women who tested negative for M. genitalium, women who tested positive complained more frequently of abnormal vaginal discharge (P = 0.04) or of a brown/bloody vaginal discharge (P < 0.001). Furthermore, they had more findings on examination (cervical mucopus, easily induced bleeding) and on microscopy (higher counts of vaginal and cervical polymorphonuclear leukocytes). Similarly, a 2006 Texas study evaluating the presence of M. genitalium and genitourinary symptoms and signs found more frequent complaints of vaginal discharge in women with the organism (23%) than those without (6%) [13]. In this latter study, both culture and PCR were used to detect M. genitalium. Of note, the authors found that, although PCR detected 10 times more positive women (256) than culture (26), the rate of symptomatic discharge was the same in both groups of positive women. Although cervical findings on targeted physical examination were similar between positive and negative women, no information was available on the number of polymorphonuclear leukocytes on microscopy.
Although multiple studies [9, 12, 13, 14••] suggest that M. genitalium causes cervicitis, not all investigators would agree. In a French STD clinic population, researchers found a high prevalence of M. genitalium (38%) without an association with cervicitis or symptoms [7]. Although they concluded that M. genitalium is a frequently encountered bacterium in the female genital tract with no associated pathology, the lack of asymptomatic controls may diminish the validity of their findings. In a 2008 study of women recruited from an urban teen health center or emergency department, Huppert and colleagues [15] found no association with vaginal symptoms or cervicitis but interestingly found correlations with C. trachomatis infection (OR 2.5, 95% CI 1.4–4.4) and sexual contact (OR 2.0, 95% CI 1.1–3.2). These authors concluded that M. genitalium is a sexually transmitted organism, but that its role as a pathogen needs to be explored further. Similarly, in their study of adolescent women, Tosh and colleagues [10] found no statistically significant association between symptoms (self-report of itching, burning, or dyspareunia, but not abnormal discharge) and signs when women with this M. genitalium were compared to controls.
Similarly to men, M. genitalium may play a role in causing urethritis in women. In a Norwegian study, Moi and colleagues [6•] found that urinary complaints were similar between women with and without M. genitalium, but that those who had the organism had more polymorphonuclear leukocytes in their first voided urine than negative women. Likewise, Anagrius and colleagues [16] found that M. genitalium was detected three times more often in women with urethritis than in those without (9.2% compared to 2.6%). However, the diagnosis of urethritis was based on microscopy of first voided urine specimens, and the rates of dysuria and urinary urgency were similar between infected and uninfected women.
To date, most studies of M. genitalium have focused on prevalence, or, with lower tract disease, its role in causing symptomatic cervicitis or urethritis. Because other vaginal infections, such as bacterial vaginosis (BV), were either excluded in the entry criteria for studies or considered a confounding factor, little is known about the role of M. genitalium in causing such infections. However, in one study that included 15 women with BV [17], none had the organism. Furthermore, the finding that it may be inversely associated with the presence of BV suggests that it plays little role with this condition [12].
Mycoplasma hominis
M. hominis seems to occur more commonly than M. genitalium (Table 1). Consistently, it seems to be found more often in women with bacterial vaginosis. For example, a 2000 study by Keane and colleagues [17] investigated M. hominis, M. genitalium, and ureaplasmas as potential causes for BV. In this relatively small study of 38 women, M. hominis was the only genital mycoplasma detected significantly more often in women with BV than in those without (53% vs 0%, P = 0.0001). Taylor-Robinson and Rosenstein [18] noted that M. hominis is present in low numbers in the healthy vagina and is thought of as a commensal organism, but its number is increased “perhaps by 10,000-fold or more” in women with BV. Studies using quantitative PCR have also shown that women with BV have higher quantities of M. hominis and that levels of this organism correlate with Gram stain criteria for BV [19]. However, as noted in a thorough review of vaginal microflora and BV [20], the vaginal microflora of women with BV is highly complex and variable, and the mere presence of M. hominis in women with BV by no means answers the difficult question of what role, if any, it plays in this disease. Although such a review is beyond the scope of this article, it is worth noting that treatment for BV with either topical metronidazole gel or clindamycin ovules successfully decreases M. hominis colonization rates [21]. On a separate note, some authors have noted that M. hominis can be found within trichomonads, and have developed in vitro models suggesting that M. hominis may play a role in increasing the cytopathic effects of trichomoniasis on vaginal epithelial cells [22•]. However, similar to the findings with BV, the clinical implications remain unclear.
Although M. hominis may be a co-factor in BV and trichomoniasis, the question remains whether it can, on its own, cause lower genital tract symptoms. In a study of 996 Swedish women, 12% of women had M. hominis isolated on culture [23]. Not surprisingly, 41% had BV diagnosed by Amsel criteria. They found, however, that after adjusting for concomitant BV, women with M. hominis were more likely to complain of a fishy odor and to have a positive amine test, a vaginal pH greater than 4.7, and clue cells on microscopy. Other vaginal complaints and findings were similar between those with and without the organism. The authors concluded that M. hominis may have clinical characteristics of women with BV even though the criteria for BV were not met. More recently, in a British study of 1200 women [24] where BV was diagnosed clinically but also confirmed by Nugent score, M. hominis did not seem to be associated with any vaginal symptoms when it was found as a single organism, not associated with other infections. Similar to other studies, the authors did find M. hominis more frequently in women with BV.
Ureaplasma urealyticum and Ureaplasma parvum
In 1999 research by Kong and colleagues [25] led to the establishment of a new species in the genus Ureaplasma; the organism formerly classified as one species (U. urealyticum) was separated into two individual species, U. urealyticum (previously U. urealyticum biovar 2) and U. parvum (previously U. urealyticum biovar 1). It is clear that ureaplasmas are common in the female genital tract (Table 1), but our earlier review [26] found no clear evidence implicating them as a cause of urethritis or cervicitis. However, because all of the reviewed studies were done prior to the new classification system, several questions must be raised. Were these studies of U. urealyticum, U. parvum, or both? If older studies could not find an association between ureaplasmas and a specific disease, is it because no association exists or because the association was lost within the old classification system? Because most ureaplasmas in the lower genital tract are actually U. parvum [5•], are older studies of U. urealyticum actually studies of primarily U. parvum? These questions remain unanswered and lead to many limitations in evaluating current literature.
Similar to the data with M. genitalium and M. hominis, some studies suggest that genital ureaplasmas may be associated with vaginal symptoms. For example, in a study of women with symptoms suggestive of urethritis or cervicitis, Schlicht and colleagues [27] found ureaplasmas in 54% of symptomatic and 16% of asymptomatic women. On the other hand, deFrancesco and colleagues [28•] found genital ureaplasmas in 82% of symptomatic and 98% of asymptomatic women, where symptoms consisted of vaginal or cervical discharges, burning sensation, or dysuria. However, to further underscore the complexity of this issue, the authors found both positive and negative associations between specific serovars and biovars of ureaplasmas and symptoms in women. Finally, ureaplasmas do not seem to be associated with BV. Unlike M. hominis, it is not even thought of as a potential co-factor in the disease. Ureaplasmas can be found in large numbers of women both with (65%) and without BV (48%) [17]. When evaluating specifically for U. urealyticum and U. parvum in vaginal samples obtained from women with documented endometritis, no association was found between BV and either organism [29].
Treatment of Genital Mycoplasmas
If the genital mycoplasmas are eventually found to cause specific lower genital tract diseases, then choosing a therapy aimed at these organisms may at some point become important. For now, in our view, no clear data exist to support changing treatment recommendations for cervicitis, urethritis, or BV to address the genital mycoplasmas specifically. Nevertheless, some authors have investigated the efficacy of various antibiotic regimens to effect a microbiological cure specifically of mycoplasmas.
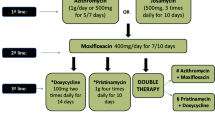
Because genital mycoplasmas lack a cell wall, they are inherently resistant to all cell wall synthesis inhibitors (eg, β-lactam antibiotics). Treatment is therefore restricted to agents such as tetracyclines, macrolides, and fluoroquinolones. In a study comparing various quinolones, doxycycline, and erythromycin in vitro, activity was highest with moxifloxacin [30]. In a clinical study that focused on microbiological results, with repeat tests obtained 10 days after treatment, a single 1-g dose of azithromycin had a similar (> 90%) microbiological eradication of ureaplasmas as 7 days of doxycycline, 100 mg daily [31]. Separately, Bjornelius and colleagues [32•] investigated the differential response to doxycycline and azithromycin in patients recruited from multiple sites in Norway and Sweden. Of the 27 women with cervicitis and a positive test for M. genitalium who received a course of doxycycline, only 37% had a negative test. On the other hand, of 17 women initially treated with azithromycin, the eradication rate was 88%. Of the six who failed doxycycline and then received a 5-day course of azithromycin (500 mg on day 1, 250 mg daily on days 2–5), all had a negative follow-up culture. Interestingly, the resolution of symptoms and signs seemed similar between those with and without microbiological cure. Finally, in a study of 319 men and women where microbiological cure alone was evaluated and follow-up tests were available [33], azithromycin—given as one dose (1 g), two doses (1 g repeated in 5–7 days), or five doses (500 mg day 1, then 250 mg daily on days 2–5)—had similar eradication rates of 74% to 79%. Ofloxacin (200 mg twice daily for 10 days) eradicated the organism in 4 of 9 (44%) and moxifloxacin (400 mg daily for 7 days) in 3 of 3 (100%) patients. Finally, with M. genitalium often found concomitantly with Chlamydia, it is important to note that one study by Thurman and colleagues [34] found no difference between standard therapies for uncomplicated C. trachomatis infection and antimicrobial therapy in eliminating M. genitalium. As noted earlier, Austin and colleagues [21]found that treatment with either topical metronidazole or clindamycin was effective at decreasing colonization of M. hominis in women who were being treated for BV.
Conclusions
From a practical point of view, we feel that it is premature to recommend testing and treatment aimed specifically at the genital mycoplasmas. The best methods for testing remain unknown or unvalidated in broad patient populations. In research studies that may be biased by their study populations, M. genitalium may have a modest role in causing MPC and urethritis, but it should be emphasized that its role in causing symptomatic lower tract disease is still highly debatable. With M. hominis and the ureaplasmas, they are found commonly, and their role as stand-alone pathogens remain questionable at best. There are no current data to show that a positive test result for any of these organisms should alter standard recommendations for managing urethritis, vaginal infections, or cervicitis. However, given the complexity of the vaginal microflora and our relatively limited understanding of lower genital tract infections, further studies of genital mycoplasmas are important so that they may lead to a clearer understanding of the role these common organisms play in causing disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Haggerty CL, Totten PA, Astete SG, et al.: Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect 2008, 84:338–342.
Taylor-Robinson D: The role of mycoplasmas in pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 2007, 21:425–438.
Hartmann M: Genital mycoplasmas. J Dtsch Dermatol Ges 2009, 7:371–377.
Petrikkos GL, Hadjisoteriou M, Daikos GL: PCR versus culture in the detection of vaginal Ureaplasma urealyticum and Mycoplasma hominis. Int J Gynaecol Obstet 2007, 97:202–203.
• McIver CJ, Rismanto N, Smith C, et al.: Multiplex PCR testing detection of higher-than-expected Rates of cervical mycoplasma, ureaplasma, and trichomonas and viral agent infections in sexually active Australian women. J Clin Microbiol 2009, 47:1358–1363. This interesting article investigated the use of multiplex PCR to detect various STDs. One array in particular was used to detect the four bacteria discussed extensively in this article.
• Moi H, Reinton N, Moghaddam A: Mycoplasma genitalium in women with lower genital tract inflammation. Sex Transm Infect 2009, 85:10–14. This article investigates M. genitalium and its possible role in female urethritis with well-defined parameters.
Casin I, Vexiau-Robert D, De La Salmoniere P, et al.: High prevalence of Mycoplasma genitalium in the lower genitourinary tract of women attending a sexually transmitted disease clinic in Paris, France. Sex Transm Dis 2002, 29(6):353–359.
Tully JG, Taylor-Robinson D, Cole RM, Rose DL: A newly discovered Mycoplasma in the human urogenital tract. Lancet 1981, 1:1288–1291.
Falk L, Fredlund H, Jensen JS: Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect 2005, 81:73–78.
Tosh AK, Van Der Pol B, Fortenberry JD, et al.: Mycoplasma genitalium among adolescent women and their partners. J Adolesc Health 2007, 40:412–417.
Short VL, Totten PA, Ness RB, et al.: Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis 2009, 48:41–47.
Manhart LE, Critchlow CW, Holmes KK, et al: Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003, 187:650–657.
Korte JE, Baseman JB, Cagle MP, et al.: Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am J Reprod Immunol 2006, 55:265–275.
•• Gaydos C, Maldeis NE, Hardick A, et al.: Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis 2009, 36:598–606. A cross-sectional study investigating the prevalence of various STDs and their contribution to cervicitis. Data were well summarized and the authors noted a significant association between M. genitalium and cervicitis
Huppert JS, Mortensen JE, Reed JL, et al.: Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008, 35(3):250–254.
Anagrius C, Lore B, Jensen JS: Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex Transm Infect 2005, 81:458–462.
Keane FEA, Thomas BJ, Gilroy CB, et al.: The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS 2000, 11:356–360.
Taylor-Robinson D, Rosenstein IJ: Is Mycoplasma hominis a vaginal pathogen? Sex Transm Infect 2001, 77:302.
Sha BE, Chen HY, Wang QJ, et al.: Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol 2005, 43:4607–4612.
Srinivasan S, Fredricks DN: The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008, 2008:750479.
Austin MN, Beigi RH, Meyn LA, Hillier SL: Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol 2005, 43:4492–4497.
• Vancini RG, Pereira-Neves A, Borojevic, et al.: Trichomonas vaginalis harboring Mycoplasma hominis increases cytopathogenicity in vitro. Eur J Clin Microbiol Infect Dis 2008, 27:259–267. This article investigates that connection between M. hominis and T. vaginalis at the cellular level.
Mårdh PA, Elshibly S, Kallings I, Hellberg D: Vaginal flora changes associated with Mycoplasma hominis. Am J Obstet Gynecol 1997, 176:173–178.
Arya OP, Tong CY, Hart CA, et al.: Is Mycoplasma hominis a vaginal pathogen? Sex Transm Infect 2001, 77:58–62.
Kong F, James G, Zhenfang M, et al.: Phylogenetic analysis of Ureaplasma urealyticum-support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacter 1999, 49:1879–1889.
Nyirjesy P: Nongonococcal and nonchlamydial cervicitis. Cur Infect Dis Reports 2001, 3:540–545.
Schlicht MJ, Lovrich SD, Sartin JS, et al.: High prevalence of genital mycoplasmas among sexually active young adults with urethritis or cervicitis symptoms in La Crosse, Wisconsin. J Clin Microbiol 2004, 42:4636–4640.
• DeFrancesco MA, Negrini R, Pinsi G, et al.: Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Clin Microbiol Infect Dis 2009, 28:641–646. The authors investigated the two ureaplasma biovars and suggest differentiating further by strains suspecting a difference in virulence.
Haggerty CL, Totten PA, Ferris M, et al.: Clinical characteristics of bacterial vaginosis among women testing positive for fastidious bacteria. Sex Transm Infect 2009, 85:242–248.
Bebear CM, de Barbeyrac B, Pereyre S, et al.: Activity of Moxifloxacin against the urogenital mycoplasmas, Ureaplasmas spp., Mycoplasmas hominis, Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect 2008, 14:801–805.
Guven MA, Gunyeli I, Dogan M, et al.: The demographic and behavioural profile of women with cervicitis infected with Chlamydia trachomatis, Mycoplasma hominis, and Ureaplasma urealyticum and the comparison of two medical regimens. Arch Gynecol Obstet 2005, 272:197–200.
• Bjornelius E, Anagrius C, Bojs G, et al.: Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex Transm Infect 2008, 84:72–76. This article describes a study of the microbiological eradication of M. genitalium.
Jernberg E, Moghaddam A, Moi H: Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int J STD AIDS 2008, 19:676–679.
Thurman AR, Musatovova O, Perdue S, et al.: Mycoplasma genitalium symptoms, concordance and treatment in high-risk sexual dyads. Int J STD AIDS 2010, 21:177–183.
Lanzafame M, Delama A, Lattuada E, et al.: Prevalence and clinical significance of Ureaplasma urealyticum and Mycoplasma hominis in the lower genital tract of HIV-1-infected women. Infez Med 2006, 14:213–215.
Ross JDC, Brown L, Saunders P, et al.: Mycoplasma genitalium in asymptomatic patients: implications for screening. Sex Transm Infect 2009, 85:436–437.
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, M.A., Nyirjesy, P. Role of Mycoplasma and Ureaplasma Species in Female Lower Genital Tract Infections. Curr Infect Dis Rep 12, 417–422 (2010). https://doi.org/10.1007/s11908-010-0136-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-010-0136-x