Abstract
Mycoplasma genitalium was first isolated from the urethral swabs of two symptomatic men with urethritis in 1980. It is a sexually transmitted bacterium associated with a number of urogenital conditions in women like cervicitis, endometritis, pelvic inflammatory disease, infertility, and susceptibility to human immunodeficiency virus (HIV). However, M. genitalium may also act like a stealth pathogen at female reproductive tract, giving no symptoms. Its prevalence varies between different groups, with the average being 0.5–10% in the general population and 20–40% in women with sexually transmitted infections. The recommended treatment of this infection is azithromycin as a single 1-g dose. However, in recent years, macrolide resistance has increased which is significantly lowering the cure rate, being less than 50% in some studies. New treatment regimens need to be investigated due to increasing drug resistance. The discussion and suggestion of an algorithm for management of this infection is the highlight of this paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Mycoplasma and Ureaplasma species, members of the Mycoplasmataceae family, belong to the class Mollicutes. They are distinguished phenotypically from other bacteria by their minute size and total lack of a peptidoglycan-containing cell wall, providing them a unique phenotype and resistance to β-lactam antibiotics [1]. They are the smallest known free-living and self-replicating organisms and readily pass through filters that retain all other bacteria [2]. Their intracellular location protects mycoplasmas from the host’s immune system and antibiotics and promotes the establishment of latent or chronic infections [1]. Mycoplasma species are found in the mouth, the respiratory and genitourinary tract. Their role in pathogenesis, as opposed to being harmless components of the endogenous microbiota, remains ambiguous [3].
The first human mycoplasma species, Mycoplasma hominis, was isolated in 1937 by Dienes and Edsall. In 1944, Eaton described the isolation of Mycoplasma pneumoniae from sputum of a patient with primary pneumonia. In 1954, Shepard discovered from the urogenital tract of men with recurrent nongonococcal urethritis, a Mycoplasma with different morphological characteristics from those previously isolated, which gave rise to a new genus, Ureaplasma. The bacterium was named Ureaplasma urealyticum due to the fact that it used urea as an energy source.
First identified in 1980 from two men with acute nongonococcal urethritis [4], Mycoplasma genitalium (MG) specifically colonizes the male and female reproductive tract. It was one of the first microorganisms to be fully sequenced [5] and its genome was the first to be chemically synthesized [6]. MG is considered a sexually transmitted pathogen and has been associated with disorders that may affect multiple female reproductive organs such as the urethra, cervix and fallopian tubes. The focus of this review is to summarize studies on the role of MG in female reproductive tract infections, delineate current treatment options, and highlight the emergence of antibiotic resistance.
MG in the female reproductive tract
MG has been detected in the human urogenital, respiratory [7], and intestinal tract [8], with the urogenital tract being the most common site of colonization. Its prevalence varies between different groups, with the average being 0.5–10% in the general population [9] and 20–40% in women with sexually transmitted infections [10]. A study conducted in England [11] analyzed urine for the presence of MG in 4507 sexually active participants, aged 14 to 44 years. The prevalence in women was 1.3%, with the highest percentage (2.4%) seen in those 16–19 years old. In a study conducted in the USA, the prevalence of MG was 1.0% compared with 0.4%, 4.2%, and 2.3% for gonococcal, chlamydial, and trichomonal infections, respectively [9]. In Japan, a 2.8% prevalence of MG has been reported for female students [12]. In a cohort of high-risk Kenyan women, the prevalences of M. genitalium were 16.1% [13].
In Brazil, MG was identified by gene amplification in 28.1% of vaginal swab specimens, including those from healthy women [14]. Another Brazilian study [15] showed a prevalence of only 0.9% for MG in cervical samples. In samples from the general population, the summary prevalence estimate is 1.3% in countries with higher development and 3.9% in countries with lower development [16].
The population of women in the USA who are most infected by MG are those under 25 years of age, with a higher number of sexual partners, who are Black, had a prior pregnancy termination and who smoke [11, 17]. Other studies conducted in England [18] also demonstrated a similar prevalence profile and, in addition, demonstrated that bacterial vaginosis was an independent risk factor for MG acquisition.
Serological and epidemiologic studies strongly indicate that MG is sexually transmitted [19]. There is a high concordance of MG between partners [8, 20] and this agreement is inclusive of genotypes [21]. The prevalence of MG detection increases 10% with each additional sexual partner [9] reaching a reported prevalence of 42% in women with high-risk sexual behavior [20]. Frequently, MG is present in association with other STIs. The reported prevalence of MG co-infection with Neisseria gonorrhoeae, C. trachomatis, and Trichomonas vaginalis was 37.9%, 10.6%, and 7.6% respectively [22].
Transmission by penis-anus contact has been established [23] and MG has been detected by nucleic acid amplification testing in anorectal specimens [24]. Mother-to-child transmission during childbirth has been scarcely studied, but MG has been detected in the respiratory tract of a newborn [25].
Studies in North American women demonstrated that MG may be rapidly increasing in incidence [17]. A study analyzing cervical specimens from 1984 to 1986 in a STD clinic in Seattle, USA, noted MG in 50 (7%) of 719 women [17]. A subsequent study showed an MG prevalence of 19.3% [26].
As occurs following a C. trachomatis infection, MG infections in women are often asymptomatic [8]. In STD clinics, approximately 40–75% of female carriers lacked distinguishing symptoms [27]. In these women, a diagnosis of MG was characterized by an increased number of cervical leukocytes [3]. On the other hand, this organism has been associated with many adverse disease outcomes, such as urethritis or nongonococcal urethritis in men and many adverse reproductive sequelae in women, including cervicitis, endometritis, and pelvic inflammatory disease (PID).
Routine screening for Mycoplasma genitalium infection has been proposed, but prevalence rates are not well established [16]. The low prevalence estimates in the general population, pregnant women, and asymptomatic clinic-based patients do not support universal screening for M. genitalium [16].
The association between MG and bacterial vaginosis is controversial.
Data are limited and inconsistent, with some studies demonstrating increased risk of MG infection among women with BV [13, 18, 28], one demonstrating decreased risk [17], and two demonstrating no relationship [29, 30].
The first evidence of cervicitis due to MG was reported in 1997 [31]; MG was detected in 5 (9%) of 57 women with cervicitis compared with none of 79 women without this condition. Subsequent studies [32, 33] verified this relationship. A more recent meta-analysis [34], found a significant association between MG and cervicitis (pooled odds ratio (OR) 1.66).
About pelvic inflammatory disease, a high proportion of the causative organism remains unknown. It is difficult to make a microbiological diagnosis of PID [19]. Several studies attempted to demonstrate an association between MG and PID [8, 33, 34]. MG has been detected in the endometrium and fallopian tubes in women with acute pelvic infection [35]. Haggerty et al. [36] found MG in 88 (15%) of 586 women with PID. A prospective study [18] showed that women with MG had twice the risk of developing PID within a 12-month time period, compared with uninfected women. A meta-analysis [34] showed a significant association between MG and PID (pooled OR 2.14).
Additional evidence consistent with MG being a cause of PID is the ability of this organism to adhere to the lining of the fallopian tube and to initiate damage to the cilia [8]. MG has been shown to experimentally induce endometritis and salpingitis in nonhuman primates [8, 37] and hydrosalpingitis in rats [37]. Prior infection with MG has also been associated with uterine factor infertility [8].
Pregnancy and MG
Studies of preterm birth and MG have included a mix of case–control and cohort studies. However, many had low prevalence of MG, limiting statistical power. Six studies [38,39,40,41,42,43] were consistent with a role for MG in premature birth, while two others [44, 45] suggested that MG was an independent risk factor for preterm delivery. Averbach et al. reported an increased risk of preterm delivery, ranging from a nonsignificant 30% increase among low-risk women attending a community health center [42]. In a meta-analysis [34], MG was associated with preterm birth (pooled OR 1.89) and spontaneous abortion (pooled OR 1.82).
MG and infertility
It is well known that PID can lead to serious reproductive problems including infertility, ectopic pregnancy, and recurrent infections. Seroepidemiological studies [8, 46] have shown an association with tubal factor infertility, with 17 to 22% of women with this condition having MG antibodies, compared with 4 to 6% of women with patent tubes. Serological surveys based on detection of MG using gene amplification also indicated an association between MG detection and an increased risk for tubal factor infertility (pooled OR 2.43) [34].
MG and HIV infection
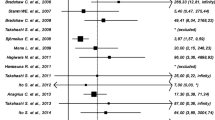
MG can also impact women’s health through its relationship with HIV. In HIV-positive women, cervicitis caused by MG was more frequent than in HIV-negative women [21]. In addition, MG was found more often in endometrial biopsies in HIV-positive women [34]. This association between MG and HIV infection was strongly supported by a meta-analysis encompassing 19 eligible studies [47]. Among them, 17 studies revealed that women infected with MG had a higher likelihood to be infected with HIV than those who were negative for MG. This association was statistically significant (P < 0.05) in 12 of the studies. The OR for the 17 studies was 1.40 (95% CI 1.13–1.72) to 5.96 (95% CI 0.73–48.62). Studies in sub-Saharan Africa [48, 49] concluded that MG facilitated HIV transmission. If MG eradication failed, there was an increased risk of HIV transmission.
MG diagnosis
Given the difficulty with culturing the organism and the lack of standardized serological tests for MG, investigations of the relationship between MG and disease lagged. However, the later utilization of NAATs in the form of polymerase chain reaction (PCR) to detect MG resulted in an influx of studies on the prevalence and clinical manifestations of MG infection [4].
Today, PCR is the method of choice for diagnosis. The sensitivity and specificity of this technique for MG detection have been reported to be 98.5% and 100%, respectively [50]. PCR also allows for the detection of macrolide resistance due to mutation in the MG gene coding for 23S rRNA [51], with 100% sensitivity and a specificity of 96.2% [52]. New gene amplification technologies can simultaneously detect MG and its most common mutations [52]. The increased resistance of MG to macrolides influenced the European Society of Dermatology and Venereal Diseases to recommend new guidelines in 2016, recommending that all positive tests for MG are followed by tests of detection for macrolide resistance [25].
Treatment and antibiotic resistance
All Mycoplasmas, including MG, have no cell wall and, consequently, β-lactam antibiotics and other antibiotics that react with this structure are ineffective. Only a select number of antibiotics such as tetracyclines, macrolides, and fluoroquinolones are effective against Mycoplasmas. In MG, the antibiotic most often used initially for treatment is the macrolide azithromycin [53]. Initial in vitro studies [54] showed that MG had high susceptibility to tetracyclines and macrolides, especially azithromycin; they had a reduced susceptibility to the older quinolones ofloxacin and ciprofloxacin [54].
Azithromycin 1 g is recommended as first-line therapy in the majority of international treatment guidelines [25, 53] but there is a growing concern that the resistance of MG to macrolides is significantly lowering the cure rate, being less than 50% in some studies [55, 56]. This macrolide resistance is strongly associated with the presence of a mutation in the MG gene coding for 23S ribosomal RNA [51]. Resistance to macrolides in MG is rapidly increasing and the prevalence of this mutation varies geographically, being found in about 30–40% of the MG isolates [57,58,59].
While still effective for the treatment of C. trachomatis, the efficacy of 1 g of azithromycin for M. genitalium has decreased from 85.3% prior to 2009 to 67.0% after 2009, and is now as low as 60.0% [60]. Although an extended treatment of azithromycin has been proposed (500 mg on the first day, followed by 250 mg for 4 days), it has not always been shown to be effective [61]. Existing data are insufficient to conclude that one azithromycin regimen is superior to another. However, the 1.5-g regimen given over the course of 5 days may be preferable to a single 1-g dose because of the possibly diminished risk of resistance associated with a longer course of treatment [62].
Comparing azithromycin with doxycycline, the former had better efficacy in the treatment of MG [62]. Doxycycline has low efficacy [55, 56, 63], with cure rates of only 30–40%, while 1 g azithromycin in a single dose had approximately an 85% cure rate in macrolide-susceptible infections [63]. However, a more recent study conducted in Seattle, in the context of higher levels of circulating macrolide resistance, showed no difference between azithromycin and doxycycline, with cure rates of 40% vs 30%, respectively [64].
Moxifloxacin is the second-line antibiotic most used for persistent MG infection. Initial results indicated a 100% cure rate [59]. However, resistance has increased and this treatment is now ineffective in up to 30% of cases, mainly in the Asia-Pacific region [58]. In Europe, routine testing for resistance to moxifloxacin is not indicated due to its low prevalence (< 5%) [25]. However, an FDA safety review has shown that fluoroquinolones when used systemically are associated with disabling and potentially permanent serious side effects that can occur together. These side effects can involve the tendons, muscles, joints, nerves, and central nervous system [65].
A proportion of MG isolates exhibit resistance to multiple antibiotics, resulting in only a few remaining treatment options [66]. Multidrug-resistant MG strains are frequently reported in the Pacific: in Australia were identified in 9.8% and in Japan in up to 30.8% of the patients screening for STIs [60].
Pristinamycin is the only antibiotic with documented anti-MG activity after failure with azithromycin and moxifloxacin [56]. However, pristinamycin is expensive and has not been well evaluated [67, 68].
A test of cure (TOC) should be performed routinely on all infected women due to this high prevalence of resistance to macrolides. There is clinical evidence that many women enter into an asymptomatic or only mildly symptomatic stage after treatment but with MG persistence and the subsequent continued risk of spreading the infection to others [25]. Therefore, the TOC should be performed no earlier than three weeks after the start of treatment. If MG is detected, treatment with moxifloxacin should be initiated [56]. This is according to the European guidelines [25], which differ from the US Center for Disease Control guidelines [53] where TOC is not recommended for asymptomatic women.
Increased resistance to MG will probably become more prevalent in the near future and make effective treatment even more challenging. Figure 1 details a suggestion of management including the European guidelines [25].
Future perspectives
Much has been discovered about MG since its identification in 1997, but many doubts persist about these small pathogens. Despite the increase in MG-related research, they are not evaluated and remain unidentified in many clinical situations. Several factors contribute to this: the majority of women who are infected with MG are asymptomatic, many clinics and physicians are not aware of their identity, and diagnostic examinations by gene amplification are expensive and not always available, especially in many public health systems. However, like many other STIs, the prevalence of MG is increasing, especially among teenagers and young adults. The rapid emergence of antibiotic resistance in MG reinforces the need for detection and prompt treatment of these infections.
References
McGowin CL, Totten PA (2017) The unique microbiology and molecular pathogenesis of Mycoplasma genitalium. J Infect Dis 216(suppl_2):S382–S388
Suthers PF, Dasika MS, Kumar VS, Denisov G, Glass JI, Maranas CD (2009) Genome-scale metabolic reconstruction of mycoplasma genitalium, iPS189. PLoS Comput Biol 5(2):e1000285
Dehon PM, McGowin CL Mycoplasma genitalium infection is associated with microscopic signs of cervical inflammation in liquid cytology specimens. J Clin Microbiol 52(7):2398–2405 [s.l.], 23 abr. 2014. American Society for Microbiology
Gaydos CA (2017) Mycoplasma genitalium: accurate diagnosis is necessary for adequate treatment. J Infect Dis 216(suppl_2):S406–S411
Fookes MC, Hadfield J, Harris S, Parmar S, Unemo M, Jensen JS, Thomson NR (2017) Mycoplasma genitalium: whole genome sequence analysis, recombination and population structure. BMC Genomics 18:1. https://doi.org/10.1186/s12864-017-4399-6
Gibson DG, Benders GA, Andrews-Pfannkoch C et al (2008) Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 319:1215–1220
Patel KK, Salva PS, Webley WC (2011) Colonization of paediatric lower respiratory tract with genital Mycoplasma species. Respirology 16(7):1081–1087 [s.l.], 26 set. Wiley
Taylor-Robinson D, Jensen JS (2011) Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev, p. 498–514
Manhart L, Holmes K, Hughes JP, Houston L, Totten PA (2007) Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health 97:1118–1125
McGowin CL, Anderson-Smits C (2011) Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog 7(5):e1001324
Sonnenberg P, Ison CA, Clifton S et al (2015) Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol 44(6):1982–1994
Hamasuna R, Imai H (2008) Prevalence of mycoplasma genitalium among female students in vocational schools in Japan. Sex Transm Infect 84(4):303–305
Lokken EM, Balkus JE, Kiarie J et al (2017) Association of recent bacterial vaginosis with acquisition of Mycoplasma genitalium. Am J Epidemiol 186(2):194–201
Campos et al (2015) Prevalence of Mycoplasma genitalium and Mycoplasma hominis in urogenital tract of Brazilian women. BMC Infect Dis 15:60
Rodrigues MM, Fernandes PÁ, Haddad JP (2011) Frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma species in cervical samples. J Obstet Gynaecol 31(3):237–2241
Baumann L, Cina M, Egli-Gany D et al (2018) Prevalence of Mycoplasma genitalium in different population groups: systematic review and meta-analysis. Sex Transm Infect 94(4):255–262
Manhart LE, Critchlow CW, Holmes KK, Dutro SM et al (2003) Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 187(4):650–657
Oakeshott P, Aghaizu A, Hay P et al (2010) Is Mycoplasma genitalium in women the new chlamydia? A community-based prospective cohort study. Clin Infect Dis 51(10):1160–1166
Daley G et al (2014) Mycoplasma genitalium: a review. Int J STD AIDS 25(7):475–487 [s.l.], 11 fev. SAGE Publications
Thurman AR et al (2010) Mycoplasma genitalium symptoms, concordance and treatment in high-risk sexual dyads. Int J STD AIDS 21:177–183
Ma L et al (2008) Short tandem repeat sequences in the Mycoplasma genitalium genome and their use in a multilocus genotyping system. BMC Microbiol 8:130
Sethi S, Singh G, Samanta P, Sharma M (2012) Mycoplasma genitalium: an emerging sexually transmitted pathogen. Indian J Med Res 136(6):942–955
Edlund M, Blaxhult A, Bratt G (2012) The spread of Mycoplasma genitalium among men who have sex with men. Int J STD AIDS 23:455–456
Lillis RA, Nsuami MJ, Myers L, Martin DH (2011) Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in women. J Clin Microbiol 49:1990–1992
Jensen JS, Cusini M, Gomberg M, Moi H (2016) 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 30:1650–1656. https://doi.org/10.1111/jdv.13849
Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC (2009) Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis 36:598–606
Falk L, Fredlund H, Jensen J (2005) Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect 81(1):73–78
Mavedzenge SN, Müller EE, Lewis DA et al (2015) Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis 211(9):1388–1398
Lawton B, Rose SB, Bromhead C et al (2008) High prevalence of Mycoplasma genitalium in women presenting for termination of pregnancy. Contraception. 77(4):294–298
Cohen CR, Nosek M, Meier A et al (2007) Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis 34(5):274–279
Uno M et al (1997) Mycoplasma genitalium in the cervices of Japanese women. Sex Transm Dis 24:284–286
Bjartling C, Osser S, Persson K (2010) The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 117:361–364
Moi H, Reinton N, Moghaddam A (2009) Mycoplasma genitalium in women with lower genital tract inflammation. Sex Transm Infect 85:10–14
Lis R, Rowhani Rahbar A, Manhart LE (2015) Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 6(3):418–426
Cohen CR, Mugo NR, Astete SG et al (2005) Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect 81:463–466
Haggerty CL, Totten PA, Astete SG et al (2008) Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect 84:338–342
McGowin CL, Spagnuolo RA, Pyles RB (2010) Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect Immun 78:726–736
Garcia P et al (2006) Risk of preterm birth associated with Mycoplasma genitalium infection. Am J Obstet Gynecol 195:S53
Kataoka S et al (2006) Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol 44:51–55
Labbe AC et al (2002) Mycoplasma genitalium is not associated with adverse outcomes of pregnancy in Guinea-Bissau. Sex Transm Infect 78:289–291
Lu GC et al (2001) Midtrimester vaginal Mycoplasma genitalium in women with subsequent spontaneous preterm birth. Am J Obstet Gynecol 185:163–165
Averbach SH, Hacker MR, Yiu T, Modest AM, Dimitrakoff J, Ricciotti HA (2013) Mycoplasma genitalium and preterm delivery at an urban community health center. Int J Gynaecol Obstet 123:54–57
Short VL et al (2010) Mycoplasma genitalium among young, urban pregnant women. Infect Dis Obstet Gynecol 2010:984760
Edwards RK et al (2006) Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J Matern Fetal Neonatal Med 19:357–363
Hitti J et al (2010) Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis 37:81–85
Svenstrup HF et al (2008) Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil Steril 90:513–520
Napierala Mavedzenge S, Weiss HA (2009) Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS (London, England) 23(5):611–620
Vandepitte J, Weiss HA, Bukenya J et al (2014) Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect 90:545–549
Manhart LE (2012) Another STI associated with HIV-1 acquisition: now what? AIDS 26:635–637
Tabrizi SN, Su J, Bradshaw CS (2017) Prospective evaluation of ResistancePlus MG, a new multiplex quantitative PCR assay for detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol 55(6):1915–1919
Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R (2008) Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis 47:1546–1553. https://doi.org/10.1086/593188
Tabrizi SN, Tan LY, Walker S, Twin J, Poljak M, Bradshaw CS, Fairley CK, Bissessor M, Mokany E, Todd AV, Garland SM (2016) Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology. PLoS One 11:e0156740. https://doi.org/10.1371/journal.pone.0156740
Workowski KA, Bolan GA, Centers for Disease Control and Prevention (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137
Jensen JS, Bradshaw C (2015) Management of Mycoplasma genitalium infections - can we hit a moving target? BMC Infect Dis 15:343. Published 2015 Aug 19. https://doi.org/10.1186/s12879-015-1041-6
Lau A, Bradshaw CS, Lewis D, Fairley CK, Chen MY, Kong FYS, Hocking JS (2015) The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis 61(9):1389–1399
Bissessor M, Tabrizi SN, Twin J, Abdo H, Fairley CK, Chen MY, Vodstrcil LA, Jensen JS, Hocking JS, Garland SM, Bradshaw CS (2015) Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis 60:1228–1236
Nijhuis RH, Severs TT, Van der Vegt DS, Van Zwet AA, Kusters JG (2015) High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother 70:2515–2518
Kikuchi M, Ito S, Yasuda M et al (2014) Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother 69:2376–2382
Salado-Rasmussen K, Jensen JS (2014) Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis 59:24–30
Braam JF, van Dommelen L, Henquet CJM, van de Bovenkamp JHB, Kusters JG (2017) Multidrug-resistant Mycoplasma genitalium infections in Europe. Eur J Clin Microbiol Infect Dis 36(9):1565–1567
Horner P, Blee K, Adams E (2014) Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1 g! Curr Opin Infect Dis 27:68–74
Manhart et al (2011) Mycoplasma genitalium: should we treat and how? Clin Infect Dis 53(S3):S129–S142
Mena LA, Mroczkowski TF, Nsuami M, Martin DH (2009) A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis 48:1649–1654
Manhart LE, Gillespie CW, Lowens MS et al (2013) Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis 56:934
FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together - FDA Drug Safety Communication issued July 26, 2016 - https://www.fda.gov/drugs/drugsafety/ucm500143.htm)
Gundevia Z, Foster R, Jamil MS, McNulty A (2015) Positivity at test of cure following first-line treatment for genital Mycoplasma genitalium: follow-up of a clinical cohort. Sex Transm Infect 91:11–13
Couldwell DL, Lewis DA (2015) Mycoplasma genitalium infection: current treatment options, therapeutic failure, and resistance-associated mutations. Infect Drug Resist 8:147–161
Bradshaw C, Twin J, Bissessor M et al (2015) 006.1 The efficacy of pristinamycin for mycoplasma genitalium – an increasing multidrug resistant pathogen. Sex Transm Infect 91:A37
Author information
Authors and Affiliations
Contributions
Newton Sergio de Carvalho: Manuscript writing, data collection.
Gabriele Palú: Manuscript writing.
Steven S. Witkin: Data management, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Carvalho, N.S., Palú, G. & Witkin, S.S. Mycoplasma genitalium, a stealth female reproductive tract. Eur J Clin Microbiol Infect Dis 39, 229–234 (2020). https://doi.org/10.1007/s10096-019-03707-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03707-8