Abstract
Several epidemiological studies have demonstrated the existence of a correlation between high serum uric acid (SUA) levels, hypertension, and chronic kidney disease (CKD). Xantine oxidase inhibitors (XOI) are the most powerful uric acid lowering drugs, with presumed beneficial effects on cardiovascular and renal system. The multifactorial mechanism linking hyperuricemia with cardiovascular and renal diseases involves both the SUA level and the xanthine oxidase (XO) activity. In this context, the clinical research has been recently focused at assessing the efficacy of urate-lowering drugs active on XO in patients with abnormal blood pressure values and renal dysfunction. The mechanism of action responsible for the beneficial effect of XOI has not completely elucidated, and long-term studies involving large population samples are needed. In particular, XOI could play an important role in the management of hypertension and CKD, especially in patients not entirely controlled by conventional therapies. In the present review, we summarize the results of recent clinical trials that largely support a positive effect of allopurinol and febuxostat on blood pressure, glomerular filtration rate (GFR), and serum creatinine in different populations of patients. Will these drugs be considered a reliable choice or alternative to currently used drugs for the hypertension and kidney failure treatment? The debate is open, but much evidence is accumulating and supporting this role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperuricemia has recently raised a revitalized interest based on the evidence of a close correlation between serum uric acid (SUA) levels, hypertension, and chronic renal failure [1,2,3]. Several different papers have reported a possible pathogenetic role for increased SUA levels in subjects with metabolic syndrome, glucose intolerance, insulin resistance, dyslipidemia, abdominal obesity, and, in particular, hypertension [4, 5]. The mechanism linking hyperuricemia with cardiovascular and renal diseases is probably multifactorial and involves both the direct role of SUA level and the effects of the activation of xanthine oxidase (XO) [6]. Despite the evidence supporting a correlation between hyperuricemia, cardiovascular risk (CVR), and progression of chronic kidney disease (CKD), urate-lowering therapy (ULT) is recommended in daily clinical practice only in the presence of gout or urolithiasis [7]. In our review, we will summarize the evidence supporting the use of XOI in patients with hypertension and renal disease by emphasizing the importance of the plasma levels of uricaemia (SUA > 6 mg/dL) [8] and the different beneficial effects of a timely and appropriate treatment with XO inhibitors (XOI) on the cardiovascular and renal systems.
The Patho-Physiological Role of Uric Acid and XO
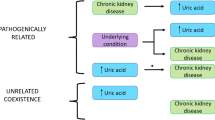
Uric acid is the end-product of the purine metabolism in liver, and for at least 95% is secreted by the kidney proximal tubule through different and specific carrier proteins. Uric acid is mainly excreted at renal level with only a minor portion excreted through the bowel [3]. Uric acid has a dual and opposite action depending on the environmental features and concentration, with some antioxidant properties in extracellular ambient and at a low concentration and a pro-oxidant activity at higher concentrations or intracellularly [3]. In the presence of ischemic damage, the pro-oxidant action of uric acid favors lipid oxidation with an evident inhibition of the reverse cholesterol transport and important pro-inflammatory effects at the tissue level [9]. Several longitudinal studies have confirmed that high SUA levels may be associated with an increased incidence of hypertension [10, 11•] and CKD progression [12] that can be the consequence of the pro-oxidant effect of uric acid. The pathophysiological mechanism underlying the correlation between the pro-oxidant activity of UA and the increased risk of cardio-renal disease involves the crucial role of XO, the main enzyme involved in the last few steps of UA production. XO catalyzes the conversion of hypoxanthine to xanthine and xanthine to uric acid, leading to the production of hydrogen peroxide (H2O2) and reactive oxygen species (ROS) [13]. The cytotoxic action of ROS, especially the increased synthesis of ONOO−, combined with the pro-oxidative action of UA determines a decrease of NO, resulting in endothelial dysfunction and in a more rapid progression of atherosclerotic disease [14] (Fig. 1). The endothelial dysfunction associated with hyperuricemia has several pathophysiological consequences by promoting platelet aggregation, vasoconstriction, proliferation of smooth muscle cells, fibrotic remodeling, and vascular inflammation that contribute to the development of CV diseases. In this broad context, the use of XO inhibitors could play a favorable role and could be taken into consideration for the prevention and treatment of CVD (Fig. 1).
Hyperuricemia and Hypertension
Several studies have demonstrated a close correlation between hyperuricemia and cardio-metabolic disorders, in particular with hypertension [15, 16]. In a large meta-analysis involving 18 prospective cohort studies, Grayson et al. have demonstrated an increase of 13% of the incidence of hypertension for every increase of 1% of SUA levels [17••]. More recently, in a study carried out in an overall healthy population, we have reported an increase of systolic blood pressure (SBP) in subjects with a progressive worsening of SUA levels [18]. Moreover, after 4 years of follow-up, SBP values were higher in subjects with untreated hyperuricemia than in those undergoing allopurinol treatment and showing lower SUA levels. This association between hypertension and high SUA values was detected in different racial groups [19,20,21], and according to the many prospective studies, hyperuricemia may be considered as an independent risk factor for hypertension [1, 22]. It has been demonstrated that elevated SUA levels may trigger the renin-angiotensin system, either directly inhibiting the NO synthesis in the juxtaglomerular apparatus, as in an indirect way, stimulating the proliferation of smooth muscle cells of the afferent arteriola wall with a consequent reduction of renal perfusion [23].
Hyperuricemia and CKD
The results of several epidemiological studies allow us to consider hyperuricemia as an independent risk factor for development and progression of CKD [7, 12, 24,25,26]. Elevated SUA levels lead to the formation of urate crystals in the nephron collecting ducts with tubular obstruction; moreover, hyperuricemia causes progressive damage to kidney structure with an independent crystal mechanism, through endothelial dysfunction, oxidative stress, and the renin-angiotensin-aldosterone system activation [23, 27] (Fig. 2). These mechanisms inevitably lead to the development or progression of kidney disease, in fact in a wide epidemiological investigation. Weiner et al. reported an increased risk for incident kidney disease (7%, defined as a glomerular filtration rate (GFR) < 60 mL/min) for every increase of 1 mg/dL of baseline SUA levels after adjusting for multiple metabolic and cardio-renal parameters [28]. In our previously mentioned research, we found that a worsened or untreated hyperuricemia is related to high levels of FPG, a key parameter which can lead to hyperinsulinemia and a diagnosis of metabolic syndrome [18]. It is well-known that high insulin values cause an increase of sodium reabsorption in the renal tubule and a reduced excretion of uric acid with negative consequences for kidney function [29,30,31]. Moreover, in subjects with renal impairment, SUA levels > 6 mg/dL cause an increased risk of end-stage renal disease (ESRD), especially in women [32].
The Urate Lowering Therapy
The ULT, in current clinical practice, is based on the use of XOI, which are considered high-grade drugs with uricostatic effect. Allopurinol is the most well-known among this class of drugs, together with its main active metabolite, the Oxypurinol, and has a good safety, efficacy, and tolerability profile [33]. Febuxostat is a new and emerging selective XOI, found in different studies more effective than allopurinol to lower SUA levels and counteract inflammatory processes on endothelial cells [34•, 35, 36]. The efficacy and safety of febuxostat has been confirmed in several trials, including in subjects with CKD and treated hemodialysis [37]. For instance, Mitsuboshi et al. claimed, in a recent short report, that febuxostat at low dosage (10 mg/day) is a safer and more effective therapy than allopurinol (100 mg/day) also in hemodialysis subjects after 16 months of follow-up [38]. In the recent past, some authors have evaluated and often confirmed the power, efficacy, and low toxicity of a new inhibitor of XO, the 3,4-dihydroxy-5-nitrobenzaldehyde (DHNB), but especially its direct antioxidant effect [39].
The uricosuric drugs (probenecid, benzbromarone, and the most recent—lesinurad) are considered second choice for the hyperuricemia treatment and are mainly used in subjects intolerant to XOI; moreover, it is also possible to use some uric acid metabolism stimulants such as polyethylene glycolated uricases (pegloticase and pegadricase) for short periods and in patients refractory to conventional therapy (Table 1).
The Role of XO Inhibitors in Cardio-Renal Diseases
XO Inhibitors and Hypertension
Considering the just mentioned close correlation between hyperuricemia and hypertension, as well as the crucial pathophysiologic role of XO in purine metabolism and in inducing endothelial dysfunction besides its negative consequences (Fig. 1), it could be easy to infer the cardiovascular benefits of XOI. To date, there are still few observational clinical studies conducted on patients with regard to the antihypertensive efficacy of XOI; in fact, the mechanism behind this effect has not yet fully elucidate. The administration of allopurinol (200 mg twice daily) in a randomized, double-blind, placebo-controlled trial of 30 adolescents, with a recent hypertension diagnosis and with levels of SUA > 6 mg/dL, has led to a significant reduction of BP values in 20 of the subjects enrolled [41]. In a more recent study carried out on 60 obese adolescents with hypertension, both treatment with allopurinol and probenecid induced a significant reduction of BP [42]. Kanbay et al. reported in a study on 48 subjects with high SUA values a significant reduction of BP values after 3 months of treatment with allopurinol (300 mg/day) [43•]. On the other hand, in another study, 8-week treatment with allopurinol (150 mg/day) did not have antihypertensive effects in subjects previously randomized to either perindopril or hydrochlorothiazide [44]. MacIsaac et al. claimed important cardiovascular benefits on a large population of elderly adults (> 65 aged) with hypertension after regular and long-term intake of high dosage allopurinol (≥ 300 mg daily) [45].
Can the antihypertensive effect of allopurinol be dose-dependent or manifest in the long-term? There are still doubts as to the improvement of BP values, tested in several studies on rats [46] and still few on people [45, 47], may be due solely to the reduction of SUA levels or to the antioxidant effects derived from the XO inhibition; it remains to be clarified which one of them is the most decisive action on hypertension.
XO Inhibitors and CKD
A few number of randomized clinical trials analyzed the therapeutic role of XOI in CKD, the pharmacokinetic and pharmacodynamic characteristics of these drugs, the duration of treatment, and the onset of severe adverse events in relation to GFR or the stage of renal failure. In the past, some studies have shown that allopurinol treatment is associated with a slowing down of renal chronic disease, a decrease in kidney function impairment with significant increase of GFR values, and a drop in dialysis treatment [48,49,50]. In more recent past, Satirapoj et al. reported in a study on 44 patients with CKD stages II–III, after 12 weeks of allopurinol treatment (50 mg once daily), a significant improvement of GFR values (43.22 ± 14.44 vs 45.34 ± 16.09 mL/min/1.73 m2, p = 0.029) [51]. Krishnamurthy et al. in a retrospective cohort study found in 50 subjects with hyperuricemia an increase in GFR of 11.9 mL/min/1.73m2 compared to the control group (95% confidence interval, 4.8–11.9 mg/d dose; P = 0.01), besides a reduction of 0.10 mg/dL of serum creatinine levels (95% confidence interval, 0.003–0.20 mg/dL; P = 0.04) [52]. It remains to be clarified whether the improvement of renal function is only linked to the reduction of SUA levels or there is another mechanism, based on the antioxidant properties of these drugs, that we can consider to be the main responsible of this positive effect.
Conclusion
Can the XOI actually fall into the category of drugs for the prevention of cardiovascular and chronic kidney diseases? The debate is open, as it will be necessary to conduct several randomized clinical trials to confirm the antihypertensive efficacy of high dosage allopurinol administration or long-term febuxostat therapy, and verify the subsequent cardiovascular and renal outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Borghi C, Cicero AF. Serum uric acid and cardiometabolic disease: another brick in the wall? Hypertension. 2017;69(6):1011–3.
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729–41.
Gustafsson D, Unwin R. The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013;14:164.
Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120(5):442–7.
Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN heumatology. 2014;2014:852954.
Galassi FM, Borghi C. A brief history of uric acid: from gout to cardiovascular risk factor. Eur J Intern Med. 2015;26(5):373.
Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
McGillicuddy FC, de la Llera MM, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63:102–10.
• Loeffler LF, Navas-Acien A, Brady TM, Miller ER 3rd, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey. Hypertension. 2012;59:811–7. Impressive epidemiological data on relationship between uric acid level and hypertension in young people.
Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803.
Ce B, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606.
Sabán-Ruiz J, Alonso-Pacho A, Fabregate-Fuente M, et al. Xanthine oxidase inhibitor febuxostat as a novel agent postulated to act against vascular inflammation. Antiinflamm Antiallergy Agents Med Chem. 2013;12(1):94–9.
Krishnan E, Kwoh CK, Schumacher HR, Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49:298–303.
Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–30.
•• Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63(1):102–10. A definitive demonstration of the association between hyperuricemia and incident hypertension.
Cicero AF, Rosticci M, Bove M, Fogacci F, Giovannin M, Urso R, et al. Serum uric acid change and modification of blood pressure and fasting plasma glucose in an overall healthy population sample: data from the Brisighella heart study. Ann Med. 2017;49(4):275–82.
Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21.
Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa. Jpn Hypertens Res. 2004;27:835–41.
Emokpae AM, Abdu A. Serum uric acid levels among Nigerians with essential hypertension. Niger J Physiol Sci. 2013;28:41–4.
Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. 2007;18:287–92.
Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–75.
Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50:239–47.
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–13.
Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–11.
Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–62.
Chien KL, Lin HJ, Lee BC, Hsu HC, Lee YT, Chen MF. A prediction model for the risk of incident chronic kidney disease. Am J Med. 2010;123(9):836–846.e2.
Galvan AQ, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Phys. 1995;268:1–5.
Muscelli E, Natali A, Bianchi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9:746–52.
Artunc F, Schleicher E, Weigert C, Fritsche A, Stefan N, Häring HU. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol. 2016;12(12):721–37.
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–50.
Gliozzi M, Malara N, Muscoli S, Mollace V. The treatment of hyperuricemia. Int J Cardiol. 2016;213:23–7.
• Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. Direct demonstration of superiority of febuxostat vs. allopurinol as uric acid lowering drug.
Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–8.
Li S, Yang H, Guo Y, Wei F, Yang X, Li D, et al. Comparative efficacy and safety of urate lowering therapy for the treatment of hyperuricemia: a systematic review and network meta-analysis. Sci Rep. 2016;6:33082.
Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ. Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther. 2005;12:22–34.
Mitsuboshi S, Yamada H, Nagai K, Okajima H. Switching from allopurinol to febuxostat: efficacy and tolerability in hemodialysis patients. J Pharm Health Care Sci. 2015;1:28.
Jian-Ming L, Yao Q, Chen C. 3,4-Dihydroxy-5-nitrobenzaldehyde (DHNB) is a potent inhibitor of xanthine oxidase: a potential therapeutic agent for treatment of hyperuricemia and gout. Biochem Pharmacol. 2013;86:1328–37.
Bove M, Cicero AF, Veronesi M, Borghi C. An evidence-based review on urate-lowering treatments: implications for optimal treatment of chronic hyperuricemia. Vasc Health Risk Manag. 2017;13:23–8.
Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32.
Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60:1148–56.
• Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–33. First suggestion of the blood pressure lowering effect of a serum uric acid lowering agent.
Kostka-Jeziorny K, Uruski P, Tykarski A. Effect of allopurinol on blood pressure and aortic compliance in hypertensive patients. Blood Press. 2011;20:104–10.
MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension. 2016;67(3):535–40.
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6.
Beattie CJ, Fulton RL, Higgins P, Padmanabhan S, McCallum L, Walters MR, et al. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension. 2014;64:1102–7.
Fairbanks LD, Cameron JS, Venkat-Raman G, Rigden SP, Rees L, Van'T Hoff W, et al. Early treatment with allopurinol in familial juvenile hyperuricaemic nephropathy (FJHN) ameliorates the long-term progression of renal disease. QJM. 2002;95:597–607.
Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–9.
Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–93.
Satirapoj B, Wirajit O, Burata A, Supasyndh O, Ruangkanchanasetr P. Benefits of allopurinol treatment on blood pressure and renal function in patients with early stage of chronic kidney disease. J Med Assoc Thail. 2015;98(12):1155–61.
Krishnamurthy AL, Lazaro D, Stefanov DG, Blumenthal D, Gerber D, Patel S. The effect of allopurinol on renal function. J Clin Rheumatol. 2017;23(1):1–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Prof. Borghi is a scientific consultant for Menarini International SpA. Dr. Bove and Dr. Cicero declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Antihypertensive Agents: Mechanisms of Drug Action
Rights and permissions
About this article
Cite this article
Bove, M., Cicero, A.F.G. & Borghi, C. The Effect of Xanthine Oxidase Inhibitors on Blood Pressure and Renal Function. Curr Hypertens Rep 19, 95 (2017). https://doi.org/10.1007/s11906-017-0793-3
Published:
DOI: https://doi.org/10.1007/s11906-017-0793-3