Abstract
Mitochondria are essential for the maintenance of normal physiological function of tissue cells. Mitochondria are subject to dynamic processes in order to establish a control system related to survival or cell death and adaptation to changes in the metabolic environment of cells. Mitochondrial dynamics includes fusion and fission processes, biogenesis, and mitophagy. Modifications of mitochondrial dynamics in organs involved in energy metabolism such as the pancreas, liver, skeletal muscle, and white adipose tissue could be of relevance for the development of insulin resistance, obesity, and type 2 diabetes. Mitochondrial dynamics and the factors involved in its regulation are also critical for neuronal development, survival, and function. Modifications in mitochondrial dynamics in either agouti-related peptide (AgRP) or pro-opiomelanocortin (POMC), circuits which regulates feeding behavior, are related to changes of food intake, energy balance, and obesity development. Activation of the sympathetic nervous system has been considered as a crucial point in the pathogenesis of hypertension among obese individuals and it also plays a key role in cardiac remodeling. Hypertension-related cardiac hypertrophy is associated with changes in metabolic substrate utilization, dysfunction of the electron transport chain, and ATP synthesis. Alterations in both mitochondrial dynamics and ROS production have been associated with endothelial dysfunction, development of hypertension, and cardiac hypertrophy. Finally, it might be postulated that alterations of mitochondrial dynamics in white adipose tissue could contribute to the development and maintenance of hypertension in obesity situations through leptin overproduction. Leptin, together with insulin, will induce activation of sympathetic nervous system with consequences at renal, vascular, and cardiac levels, driving to sodium retention, hypertension, and left ventricular hypertrophy. Moreover, both leptin and insulin will induce mitochondrial alterations into arcuate nucleus leading to signals driving to increased food intake and reduced energy expenditure. This, in turn would perpetuate white adipose tissue excess and its well-known metabolic and cardiovascular consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mitochondrion: Physiological and Pathophysiological Actions
Mitochondria are the cytoplasmic organelles founded in almost all human and animals cells except mature erythrocytes. Mitochondria are highly dynamic comprising at least six compartments: the outer membrane, inner boundary membrane of significantly larger surface area, intermembrane space, cristal membranes, intracristal space, and protein-rich matrix. They contain their own small mitochondrial DNA (mtDNA) and some RNA components, but the vast majority of the mitochondrial proteins are encoded by nuclear DNA, synthesized in the cytosol and then imported into the mitochondria post-transcriptionally [1, 2]. The mitochondrion plays a central role in cellular metabolism including pyruvate decarboxylation, tricarboxylic acid cycle, decarboxylation of fatty acids, or β-oxidation and degradation of branched amino acids. Furthermore, mitochondria contribute to calcium handling, regulation of programmed cell death, generation and control of reactive oxygen species (ROS), and biosynthetic processes taking place in the cytosol by providing intermediates like urea cycle, fatty acids, and heme synthesis [2, 3].
The principal role of mitochondria is to synthesize more than 95% of adenosine triphosphate (ATP) for cellular utilization. Production of ATP requires two major steps, oxidation of highly reducing metabolites and coenzymes such as nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) and phosphorylation of adenosine diphosphate to generate ATP to support various cellular functions (OXPHOS, oxidative phosphorylation) [4]. The mitochondrial respiratory system consists of four enzymatic multiheteromeric complexes (I–IV) embedded in the inner membrane of mitochondria and two individual mobile molecules, coenzyme Q (CoQ) and cytochrome c, along which the electrons liberated by the oxidation of NADH and FADH2 are passed and ultimately transferred to molecular oxygen. This respiratory process creates the electrochemical gradient of protons and membrane potential about 180 mV across the inner membrane that has the potential to do work. In this process, participates the complex V (ATP synthase) to phosphorylate matrix ADP by inorganic phosphate and to generate ATP [4, 5].
On the other hand, in the inner mitochondrial membrane, there are also mitochondrial uncoupling proteins (UCPs), which belong to the superfamily of mitochondrial transport proteins. There are five UCPs (named UCP1 to UCP5) found in mammals and they have similarities in their structures, but different tissue distributions. These proteins have the ability to dissipate the proton gradient generated by the respiratory chain, uncoupling the substrate oxidation from the production of ATP and generating heat instead of energy. Furthermore, UCPs accelerate mitochondrial respiration and consequently reduce the production of ROS being, therefore, an additional element of defense against oxidative stress [6].
Mitochondrial Dynamics, Processes, and Factors Involved
Mitochondria are subject to dynamic processes in order to establish a control system related to survival or cell death and adaptation to changes in the metabolic environment of cells. Mitochondrial dynamics includes several processes: (a) The fusion or union of two mitochondria in a single; (b) the fission or division of a mitochondria into smaller ones; (c) the biogenesis required for cell growth and adaptation to increased oxidative stress and nutritional deprivation, and (d) mitophagy, a specialized form of autophagy that is intended to degrade the mitochondria and is closely related to the process of cell apoptosis.
The fusion and fission processes, termed mitochondrial remodeling, control mitochondrial morphology and result in more efficient organelles [7•]. Mitochondrial fusion is an evolutionarily conserved process in mammals mediated by three large GTPases of the dynamin superfamily. Mitofusin 1 (MFN1) and MFN2 are integral outer membrane proteins that mediate outer membrane fusion, and optic atrophy-1 (OPA1) has multiple isoforms associated with the inner membrane and mediates inner membrane fusion [8]. Genetic deletion of the fusion genes results in severe fragmentation of the mitochondrial network and abolished content exchange between mitochondria [9••]. The functions of OPA1 are controlled by alternative splicing and proteolysis of different isoforms, long and short isoforms (L-OPA1 and S-OPA1), and up to five isoforms of OPA1 have been described in mammals, varying among tissues, species, and reports. OPA1 can be cleaved by two distinct metalloproteases, Yme1L (ATP-dependent protease) and OMA1 (membrane potential-dependent protease), and the presence of both long and sort forms correlates with fusion-competent mitochondria [10, 11•]. Mitochondrial fission is also critical for cellular physiology and is mediated by dynamin-related protein 1 (DRP1), a large cytosolic GTPase that is recruited by receptor proteins (Fis1, Mff, MiD49, and MiD50) on the outer membrane, where it produces constriction required for mitochondrial fission and therefore control mitochondrial morphology. One of the best-known regulatory mechanisms for mitochondrial fission involves phosphorylation of DRP1, activating or inhibiting DRP1, depending on the site involved. Other processes as acetylation and S-nitrosylation are related with DRP1 regulation. Otherwise, fission process has been implicated in others functions, including the facilitation of mitochondrial transport, mitophagy, and apoptosis [12•, 13]. Mitochondria go through continuous cycles of selective fusion and fission, referred to as the “mitochondrial life cycle,” to maintain the quality of its function. Deregulation of fusion/fission events appears to be involved in several diseases.
Mitochondrial biogenesis is critical for the normal function of cells and two closely processes are related: proliferation, consisting in increasing the number of mitochondria per cell, and differentiation, whereby the organelle acquires suitable specific structural and functional characteristics for the development of specific functions of different cells in the organism. Mitochondrial biogenesis is not only produced in association with cell division but also it can be produced in response to an oxidative stimulus, to an increase in the energy requirements of the cells, to exercise training, to electrical stimulation, to hormones, during development, in certain mitochondrial diseases, etc. It is well known that mitochondria are produced and eventually after normal functioning they are degraded. Thus, the actual level of mitochondria in cells is dependent both on the synthesis and the degradation. Mitochondrial synthesis is stimulated by the PGC-1α–NRF1–TFAM pathway. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) is the major stimulator of mitochondrial biogenesis. PGC-1α expression is very high in tissues with highly developed mitochondrial systems and its expression is very important in physiological situations characterized by an increase in demand for energy as ATP or heat. PGC-1α modulates the expression of some proteins implicated in mitochondrial biogenesis through its interaction with transcription factors involved as nuclear respiratory factor 1 (NRF1). NRF1 stimulates the expression of mitochondrial transcription factor A (TFAM) which is a final effector activating the duplication of mitochondrial DNA molecules [14•].
Mitochondrial mass in a cell is regulated by a balance between biogenesis and degradation. When mitochondria are defective, aged, or there is an excess of them, they are removed through autophagy, a process termed mitophagy, a critical process for maintaining proper cellular functions. Mitophagy involves the encapsulation of mitochondria in autophagosome which fuse with lysosomes in order to degrade the mitochondria [7•]. The mechanisms associated mitophagy-dependent cellular stress, including the availability of nutrients, metabolic state of the cell, and the redox state. The mitophagy is mediated either by the PINK1-PARKIN signaling pathway or the mitophagic receptors NIX and BNIP3 and their accompanying modulators [14•, 15]. PINK1 is a serine/threonine kinase mitocondrial imported and degraded by the rhomboid protease PARL in the organelle in normal conditions. Changes in mitochondrial membrane potential (depolarization) and other stress situations result in accumulation of PINK1 on the outer membrane, and PINK1 phosphorylates numerous proteins as PARKIN, an E3 ubiquitin ligase. Activated PARKIN results in activation of the ubiquitin-proteasome system that allows degradation of a number of membrane proteins external (MFN 1 and 2), thus enhancing mitochondrial fragmentation. This enables mitophagy and prevents re-fusion between healthy and damaged mitochondria. BNIP3 is a protein that interacts with Bcl-2, and NIX (BH3-only Bcl-2 family protein) are proteins of mitochondrial outer membrane necessary for autophagy, which have proved their importance for mitochondrial elimination during cell maturation erythroid cells. NIX has a domain of interaction with LC3, which allows engulfment of labeled mitochondria in autophagosomes. These recognized modes of capture by the autophagy machinery operate at different efficiencies, from partial to complete elimination of mitochondria [14•, 15].
Taking together, mitochondria are essential for the maintenance of normal physiological function of tissue cells and mitochondrial dynamics is a highly regulated process that controls mitochondrial density in the cells and may be changed depending on the physiological cell state. However, mutations in mtDNA or nuclear mitochondrial genes, bioenergetics defects, changes in dynamics of the mitochondria such fusion or fission, changes in size and morphology, alterations in trafficking or transport, and altered movement of mitochondria can lead to mitochondrial dysfunction.
Crosstalk Between Energy Metabolism and Mitochondrial Dynamics in Obesity
Mitochondrial dynamic is affected by metabolic needs, changes, and alterations, and thus [16], both fusion and fission machineries adapt in response to metabolic signals. Metabolic status can affect the number, form, and function of mitochondria, which consequently influences the organ function. Conversely, organ metabolism is affected by changes in mitochondrial dynamics. Mitochondrial fusion is associated with increased ATP production, while inhibition of this process is associated with impaired OXPHOS and ROS production [17•]. The balance between fission and fusion is regulated by changes in nutrient availability and metabolic demands, causing mitochondria adaptation to changing conditions. Nutrient depletion activates mitochondrial fusion which protects against autophagy and cell death and appears to depends on SPFH family scaffold protein stomatin-like protein 2 (SLP2) [18]. Nutrient depletion also blunts fission, which mediated PKA-mediated phosphorylation of DRP1 [19•]. In turn, excess of glucose causes mitochondrial fragmentation and increased ROS production which depends of DRP1 [19•]. Mitochondrial 8 and reduction in fusion have been observed in overweight rodents fed a high-fat diet. In these animals, high-fat diet causes mitochondria fragmentation and reduction in MFN2 expression in hypothalamic neurons [20••]. Obesity and nutrient status also modulate mitochondrial dynamics in humans [19•, 21••]. Diminished MFN2 expression in skeletal muscle has been observed in obese and type 2 diabetes individuals, which increases after weight loss. It appears that MFN2 expression in skeletal muscle is directly proportional to insulin sensitivity and is inversely proportional to the body mass index [21••].
Mitochondrial Dynamics in Organs Involved in Energy Metabolism
There are numerous evidences showing modifications of mitochondrial dynamics in organs involved in energy metabolism. In fact changes in the pancreas, liver, skeletal muscle, and white adipose tissue mitochondrial dynamics could be of relevance for the development of insulin resistance, obesity, and type 2 diabetes. In this sense, we have previously established a mitochondrial pathophysiological role linked to insulin resistance, where higher levels of ROS are detrimental to cells, having the potential to trigger both mitochondrial-mediated cell death and the degradation of the mitochondrial DNA [22••].
Pancreas alterations and insulin secretion play a crucial role in the development of insulin resistance and diabetes. Furthermore, alterations of mitochondrial dynamics seem to participate in altered insulin production and secretion by pancreatic beta cells [23]. In fact, ablation of OPA1 causes mitochondrial fragmentation and death of pancreatic beta cells. This is associated with reduced insulin secretion and impaired systemic glucose homeostasis [24]. OPA1 is required for an adequate function of the respiratory chain in beta cells. Thus, defects in glucose-stimulated mitochondrial ATP production in pancreatic beta cells deficient in OPA1 may result from OXPHOS alterations, which could be responsible for the mentioned effects on insulin secretion.
The liver also plays an important role in glucose homeostasis and development of metabolic alterations. Furthermore, mitochondrial function participate in insulin signaling and consequently in systemic glucose homeostasis. In fact, deletion of Mfn2 in the liver of mice is associated with decreased mitochondrial fusion and increased mitochondrial fission [25]. These alterations of mitochondrial remodeling result in increased hepatic glucose production and impairment of insulin signaling and occur in the absence of body weight modifications [25]. In these mice with impaired fusion, reduction of ROS availability enhanced insulin sensitivity and suppressed endoplasmic reticulum stress [21••]. Thus, it appears that the hepatic and insulin sensitivity changes could be a consequence of reduced mitochondrial fusion and increased fission in the liver and even from MFN2 effects on the contact and interactions between mitochondria and the endoplasmic reticulum [26••]. Interrelationships between mitochondria and the endoplasmic reticulum have been also demonstrated in mice with Drp1 deletion in the liver [27]. These animals present reduced mitochondrial fission and enhancement of fusion, as well as disruption of the spatial relation between mitochondria and endoplasmic reticulum [21••]. Drp1-deleted mice in the liver are protected from diet-induced obesity, lacking of increased adiposity accumulation, most probably due to increased body energy expenditure [19•]. These proposed changes in hepatic mitochondrial dynamics could be of physiological relevance in the development of obesity and suggest that changes and/or manipulation of both fission/fusion equilibrium in the liver could affect insulin signaling and sensitivity [28].
Skeletal muscle is a key organ for glucose homeostasis and plays a significant role in the development and maintenance of systemic insulin sensitivity. Alterations in several signaling metabolic pathways have been observed in models with deletion or overexpression of factors involved in mitochondrial dynamics in skeletal muscle. In fact, aberrant mitochondrial fission plays a pivotal role in the pathogenesis of insulin resistance in the skeletal muscle. In isolated myocytes from skeletal muscle deletion of Mfn2 causes mitochondrial fragmentation impairing the glucose/insulin signaling [29]. In mice lacking Mfn2 in skeletal muscle, systemic glucose homeostasis, body weight, and adiposity have been shown to be impaired when the animals are submitted to a high-fat diet. In a similar way to Mfn2 ablation consequences for skeletal muscle, Drp1 overexpression also alters mitochondrial dynamics and impairs skeletal muscle glucose metabolism [30]. It has to be mentioned that in most of these situations, metabolic abnormalities occur in the absence of altered mitochondrial bioenergetics and contractile oxidative functions.
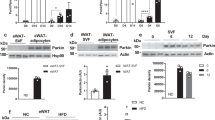
Obesity and insulin resistance produces numerous alterations in the white adipose tissue (WAT) of both humans and rodents, and there are evidences showing that alterations in mitochondrial dynamics are related with these alterations [19•, 20••]. In a recent study in overweight rats on a high-fat diet, we observed reduced WAT protein expression of main factors involved in mitochondrial biogenesis SIRT1, AMPK, PGC1α, and the transcription factor NRF1. This reduced biogenesis was accompanied by alterations of the fusion/fission processes as shown by diminished expression of OMA1 and Yme1L1 and an increased expression of OPA1 and DRP1. Collectively, these results suggest that high-fat-induced overweight in rats is accompanied by reduced mitochondrial biogenesis and fusion processes together with increased fission. These overweight rats in a high-fat diet presented insulin resistance and enhanced WAT expression of TNF alpha, suggesting a possible association of alterations in mitochondrial dynamics with metabolic alterations in WAT (see Fig. 1).
Quantitative analyses of protein levels by western blot for a sirtuin 1 (SIRT1), b peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), c nuclear respiratory factor type 1 (NRF1), d ATP-independent metalloprotease OMA1, e optic atrophy 1 (OPA1), and f dynamin-related protein 1 (Drp1) in white adipose tissue of control rats (c) and in overweight rats on a high-fat diet (HFD) for 12 weeks. Data are expressed as mean ± SEM (n = 10 animals per group). *p < 0.05 vs. C
Relevance of Mitochondrial Dynamics for Brain Control of Energy Intake and Expenditure
Mitochondrial dynamics and the factors involved in its regulation are critical for neuronal development, survival, and function [31]. It has been demonstrated that neurons are very sensitive to alterations of mitochondrial morphology, function, and availability. In fact, experimental models and clinical observations in patients have shown that defects and mutations in factors such as Mfn2, Drp1, Opa1, and others are accompanied by neurological disorders affecting a variety of functions related with intake regulation energy balance and expenditure and whole-organism metabolism [20••, 26••, 32••]. Experiments in mice demonstrated that manipulation of neurons from hypothalamic arcuate nucleus (ARC) that regulates feeding behavior was accompanied by evident changes in mitochondrial shape and number [33•].
Neurons from either agouti-related peptide (AgRP) or pro-opiomelanocortin (POMC) circuits positively and negatively regulates feeding behavior, respectively [33•]. AgRP neurons produce neuropeptides that increase appetite, while decreasing both energy expenditure and metabolism. It appears that changes in mitochondrial fission leading to reduction of AgRP neurons can produce feeding diminution, while stimulation of mitochondrial fusion in these neurons results in increases of food intake [33•]. In mice, high-fat diet increases the activity of AgRP neurons leading to increased food intake and imbalance of systemic energy metabolism. It appears that high-fat diet feeding induces mitochondrial fission in AgRP neurons [20••]. Furthermore, this also suggests that reduced mitochondrial fusion in these neurons contributes to the neural regulation of whole-body energy metabolism and feeding behavior. In addition, deletion of Mfn2 in these neurons was able to prevent the observed adverse behavioral and metabolic effects. This was associated with insulin sensitivity amelioration and normalization of circulating glucose levels, fat mass reduction, and overweight reversion [32••].
Conversely, stimulation of POMC neurons, which is accompanied by reduction of appetite, food intake, and body weight, is suppressed by a high-fat diet [33•]. However, it is important to note that stimulation of POMC neurons in insulin- and leptin-resistant states is associated with increased food intake and weight gain. Contrary to the effects in AgRP neurons, deletion of Mfn2 in POMC neurons causes overeating, reduced energy expenditure, generalized metabolic dysregulation, and severe obesity [2, 33•]. This appears to suggest that preserved mitochondrial fusion in POMC neurons could prevent increase food intake, positive energy balance, and obesity. However, it has to be noted that deletion of Mfn1 in POMC neurons was not able to disrupt body energy homeostasis, which argues against the notion that dysfunctional mitochondrial fusion could be a feasible cause of the mentioned energy metabolism alterations.
Mitochondrial Dynamics and Hypertension
Activation of the sympathetic nervous system has been considered as crucial point in the pathogenesis of hypertension among obese individuals also playing a key role in cardiac remodeling associated with hypertension [34]. In this regard, norepinephrine triggers cardiomyocyte hypertrophy through the activation of specific signaling pathways, including the Ca2+-activated protein phosphatase calcineurin [35]. Cardiomyocyte hypertrophy related to hypertension further modifies the phenotype and functionality of cardiomyocytes also promoted changes on their energetic metabolism. In this regard, it is important to remind that heart function is very dependent of mitochondrial activity. Indeed, cardiomyocytes are cells containing high mitochondria density probably because they need large and constant supply of ATP to maintain both their repetitive contraction and functionality of several ions transporters. On this point, hypertension-related cardiac hypertrophy has been associated with changes in metabolic substrate utilization, dysfunction of the electron transport chain and ATP synthesis [36]. In this line of evidence, our research group reported, using a proteomic approach, that in hypertrophic left ventricles of spontaneous hypertensive rats, proteins involved in mitochondrial oxidative phosphorylation were overexpressed whereas the α-subunit of the mitochondrial precursor of ATP synthase was down-expressed [37•]. These findings may be in accordance with the reduced ATP levels reported in the hypertrophied hearts [38], further suggesting changes on the mitochondrial energetic metabolism in the hypertrophic ventricle.
Due to the richness mitochondrial content of cardiac cells, it is not difficult to think about the importance of the mitochondrial dynamics regulation on their functionality. In this regard, alterations in both mitochondrial fusion and fission processes have been associated with several pathological heart conditions. As example, increased mitochondrial fission was reported in models of ischemia–reperfusion and pharmacological inhibition of the mitochondrial fission protein DRP1 reduced the infarct size [39]. Studies using phenylephrine to induce hypertrophy demonstrated a decrease in messenger RNA (mRNA) levels of the mitochondrial fusion protein MFN2 [40]. Moreover, mRNA levels of the fusion proteins MFN 1 and MFN2, and OPA1 were decreased in the heart of hypertensive rats, suggesting that during hypertension, there is a shift towards increased mitochondrial fragmentation. In this regard, it was published that treatment of cultured neonatal rat cardiomyocytes with norepinephrine promotes mitochondrial fission that was associated with a decrease in mitochondrial mean volume and increase in the relative number of mitochondria per cell. It was attributed to an increased cytoplasmic Ca2+ mediated by norepinephrine that activating calcineurin promotes migration of the fission protein DRP1 to mitochondria [41••].
Recruitment of cytosolic DRP1 to the mitochondria during fission is a regulated process involving post-translational modification of DRP1. Phosphorylation by cyclic-AMP-dependent protein kinase A at Ser637 in the GTPase effector domain of DRP1 decreases its GTPase activity reducing mitochondria fission [42]. Incubation of cardiomyocytes with norepinephrine for 48 h decreased DRP1 phosphorylation at Ser637 supporting that norephinephrine-induced mitochondrial fission in cardiomyocytes.
To understand the functional repercussion of these findings, we should look for in the knowledge about the relationship between mitochondrial fission and cardiomyocyte functionality. In this regard, it was reported that transgenic mice with mutated DRP1 developed interstitial fibrosis and the contractile function was significantly decreased [43]. A mutation in the mitochondrial fission gene DRP1 leads to cardiomyopathy suggesting that DRP1-mediated processes are essential for the maintenance of normal cardiac function. Taken together, and as speculation, these findings may even suggest that norepinephrine try to favor mitochondrial fission as compensatory mechanisms to maintain heart contractility under hypertensive conditions and secondly may occurs the thickening of the ventricular wall. Accordingly, it was postulated that reduction in DRP1-mediated mitochondrial fission could prevent detrimental changes in development of cardiac pathologies but the experimental observations also insinuated that a complete loss of DRP1 function could be detrimental [43].
Another important contribution of norephinephrine-induced mitochondrial fission may be the relative to the linkening between mitochondrial fission and both ROS production and cellular apoptosis. In this regard, it is well established that cytochrome c is released through Bax-lined pores at sites of DRP1-mediated mitochondrial fission, which causes cellular apoptosis. Interestingly, in hypertension-related left ventricle hypertrophy both ROS production and cellular myocardial apoptosis were widely postulated as mechanisms involved in the genesis and progression of the disease [44•, 45]. Furthermore, as abovementioned, hypertension-induced mitochondrial alterations was also accompanied by changes in energetic mitochondrial metabolism, including decreased respiration and ATP production. In this regard, it has been suggested that while fusion promotes respiratory efficiency, mitochondria fission was associated with reduced oxidative metabolism [46]. We recently reported the involvement of heat shock protein 70 (Hsp70) and Wilms’ tumor 1 transcription factor (WT-1) in mitochondrial energy metabolism in the kidney and nephrogenesis induction, which could be crucial for the development and maintenance of hypertension [47]. Furthermore, these factors would favor cell survival by WT-1 stabilizing Bcl-2 and would limit the potential for release of cytochrome c from mitochondria [48]. Mitophagy is considered a mechanism to preserve mitochondria quality. In hypertensive hearts, mitophagy is enhanced in obesity and renovascular hypertension. However, other works have suggested that impaired mitophagy contribute to the pathogenesis of vascular diseases including hypertensive heart diseases. Angiotensin II, the main renin-angiotensin system (RAS) effector, plays a crucial role in the development and maintenance of hypertension and stimulates ROS production [22••]. In this regard, it has been shown that valsartan (an angiotensin II receptor blocker) diminished myocardial autophagy and mitophagy, further supporting the role of angiotensin II in changes of mitochondrial dynamics associated with hypertension [49].
Genetic of Mitochondria Biogenesis in Hypertension
An important genetic basis has been postulated associated with mitochondrial biogenesis associated with hypertension [50•]. In this regard, in the Framingham Heart Study, complex IV-encoded gene polymorphisms have been correlated with blood pressure. In Korean population, age-dependent polymorphisms in the mitochondria-shaping gene, OPA1, correlates with blood pressure and hypertension [51]. Furthermore, mitochondrial dysfunction caused by mitochondrial tRNAlle 4263A>G mutation is involved in essential hypertension [52]. Moreover, Gly482Ser polymorphisms in PGC-1α are associated with blood pressure and hypertension among Austrian men and white subjects [53]. In addition, maternal heritability of higher blood pressure may implicate the mitochondrial genome [54]. Moreover, polymorphisms in the Opa1 and Mfn2 genes associated with hypertension were also reported.
Endothelial Dysfunction and Mitochondria Dynamics
It is well established that the existence of endothelial dysfunction refers to impairment of endothelium-dependent vasodilatation in the vasculature of essential hypertensive patients compared to normotensive control.
Different to myocardial cells, energy requirements in the endothelium are relatively low and glycolysis is the major source of ATP production. However, it is recognized that endothelial mitochondria play a prominent role in signaling cellular responses through the production of ROS. For example, excess substrate stimulates mitochondrial ROS production to signal a change in endothelial phenotype.
In the endothelium, PGC-1α is activated during energetic demand state increasing the endothelial capacity to produce ATP. However, in the endothelium PGC-1α might play other roles. In this regard, PGC-1α also regulates expression of vascular endothelial growth factor-1 (VEGF-1) and stimulates angiogenesis. Moreover, endothelial-specific overexpression of PGC-1α protects against angiotensin II-induced hypertension. In addition, PGC-1α-induced mitochondrial biogenesis may be a mechanism to protect the endothelial cell against oxidative stress by supplying undamaged mitochondria and then producing less ROS.
In summary, many questions remain unanswered about the relationship between mitochondria biogenesis and hypertension. In this regard, most of the studies associating endothelial dysfunction with mitochondria biogenesis as a cause to develop hypertension were mainly focused on mechanisms related to the production of ROS by mitochondria. Other questions related to the involvement of alterations of mitochondrial dynamics in mechanisms associated with endothelial dysfunction, including changes in the production of nitric oxide, remain to be established.
Probably in the natural history of hypertension, ultrastructural and dynamics mitochondrial damage changes occurs first, before hemodynamic clinical manifestations appear.
Conclusion and Perspectives
After reviewing the previous paragraphs, a question arises: Could alterations of mitochondrial dynamics in white adipose tissue lead to the development and maintenance of hypertension in obesity situations?
It is well known that in overweight and obesity, leptin is overproduced by WAT, being this situation associated with both insulin and leptin resistance in several tissues including the brain. Leptin overproduction by white adipose tissue is associated with alterations of mitochondrial dynamics in the tissue itself [55]. The opposite seems to be also true; leptin can induce mitochondrial dysfunction in several cell types [56, 57]. Thus, it could be hypothesized that mitochondrial dysfunction of WAT could be a feasible cause of leptin overproduction. Leptin, together with insulin, will induce activation of sympathetic nervous system with consequences at renal, vascular, and cardiac levels, driving to sodium retention, hypertension, and left ventricular hypertrophy. On the other hand, both leptin and insulin will induce mitochondrial alterations into arcuate nucleus leading to signals driving to increased food intake and reduced energy expenditure. This, in turn, would perpetuate WAT excess and its well-known metabolic and cardiovascular consequences (see Fig. 2).
Alterations of mitochondrial dynamics in white adipose tissue could lead to the development and maintenance of hypertension, cardiac hypertrophy, sodium retention, and obesity. AgRP agouti-related peptide, Ins insulin, POMC pro-opiomelanocortin, SNS sympathetic nervous system, WAT white adipose tissue
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Logan DC. The mitochondrial compartment. J Exp Bot. 2006;57(6):1225–43.
Wallace DC. The mitochondrial genome in human adaptive radiation and disease: on the road to therapeutics and performance enhancement. Gene. 2005;18(354):169–80.
Chatzi A, Manganas P, Tokatlidis K. Oxidative folding in the mitochondrial intermembrane space: a regulated process important for cell physiology and disease. Biochim Biophys Acta. 2016;1863(6PtA):1298–306.
Chaban Y, Boekema EJ, Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilization. Biochim Biophys Acta. 2014;1837(4):418–26.
Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19(8):1777–83.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359.
• Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science (New York, NY). 2012;337:1062–5. Description and relevance of mitochondrial fussion and fission processes.
Malka F, Guillery O, Cifuentes-Diaz C, Guillou E, Belenguer P, Lombes A, et al. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–9.
•• Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31:2117–33. This study provides the first description of OMA1 and reinforces the importance of mitochondrial quality control for normal metabolic function.
Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–66.
Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–55. The study shows that mammalian cells have multiple pathways to control mitochondrial fusion through regulation of the spectrum of OPA1 isoforms.
• Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–56. The study shows that Drp1 contributes to mitochondrial division in mammalian cells.
van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5:a011072.
• Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. FEBS J. 2016; Jul 27. doi: 10.1111/febs.13820. A review of recent findings that highlight the importance of the mitochondrial biogenesis and underlying molecular mechanisms in energy production and the cellular processes.
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, et al. PINK1-dependent recruitment of PARKIN to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107(1):378–83. doi:10.1073/pnas.0911187107.
Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–48.
• Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. The study describe that mitochondrial dynamics provide a new mechanism linking excess nutrient environment to progressive mitochondrial dysfunction, common to age-related diseases.
Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–600.
• Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):446–52. The review highlights recent findings regarding the functions of mitochondria in adipocytes, in regulating substrate metabolism, energy expenditure, disposal of reactive oxygen species (ROS), and in the pathophysiology of obesity and insulin resistance.
•• Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155:188–99. The study shows the important role for mitochondrial dynamics governed by Mfn1 and Mfn2 in Agrp neurons in central regulation of whole-body energy metabolism.
•• Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27(2):105–17. The review describe the ways in which metabolic alterations convey changes in mitochondrial morphology and how disruption of mitochondrial morphology impacts cellular and organismal metabolism.
•• Manucha W, Ritchie B, Ferder L. Hypertension and insulin resistance: implications of mitochondrial dysfunction. Curr Hypertens Rep. 2015;17(1):504. The review describes that mitochondria possess both a functional RAS and vitamin D receptors and its relevance in hypertension and diabetes.
Baltrusch S. Mitochondrial network regulation and its potential interference with inflammatory signals in pancreatic beta cells. Diabetologia. 2016;59(4):683–7.
Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol Biol Cell. 2011;22:2235–45.
Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, et al. Critical reappraisal confirms that mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc Natl Acad Sci U S A. 2016;113(40):11249–54.
•• Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–43. The study pictures mitochondria as ‘super-organized’ organelle responsible for regulation of cellular needs, as well as its own dysfunction.
Bansal S, Biswas G, Avadhani NG. Mitochondria-targeted heme oxygenase-1 induces oxidative stress and mitochondrial dysfunction in macrophages, kidney fibroblasts and in chronic alcohol hepatotoxicity. Redox Biol. 2013;2:273–83.
Camporez JP, Asrih M, Zhang D, Kahn M, Samuel VT, Jurczak MJ, et al. Hepatic insulin resistance and increased hepatic glucose production in mice lacking Fgf21. J Endocrinol. 2015;226:207–17.
Sebastián D, Hernández-Alvarez MI, Segalés J, Sorianello E, Muñoz JP, Sala D, et al. Mitofusin 2 (Mfn2) links mitocondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–8.
Touvier T, De Palma C, Rigamonti E, Scagliola A, Incerti E, Mazelin L, et al. Muscle-specific Drp1 overexpression impairs skeletal muscle growth via translational attenuation. Cell Death Dis. 2015;6:e1663.
Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2015;11:11–24.
•• Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–87. The results establish MFN2 in POMC neurons as an essential regulator of systemic energy balance by fine-tuning the mitochondrial-ER axis homeostasis and function.
• Zorzano A, Claret M. Implications of mitochondrial dynamics on neurodegeneration and on hypothalamic dysfunction. Front Aging Neurosci. 2015;7:101. The review shows findings in the field of mitochondrial dynamics and their relevance for neurodegeneration and hypothalamic dysfunction.
Barki-Harrington L, Perrino C, Rockman HA. Network integration of the adrenergic system in cardiac hypertrophy. Cardiovasc Res. 2004;63:391–402.
Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2000;97:1196–201.
Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–51.
Zamorano-León JJ, Modrego J, Mateos-Cáceres PJ, Macaya C, Martín-Fernández B, Miana M, et al. A proteomic approach to determine changes in proteins involved in the myocardial metabolism in left ventricles of spontaneously hypertensive rats. Cell Physiol Biochem. 2010;25:347–58. The study is a proteomic approach showing changes in proteins involved in the metabolism of left ventricles of hypertensive rats.
Shen W, Asai K, Uechi M, Mathier MA, Shannon RP, Vatner SF, et al. Progressive loss of myocardial ATP due to a loss of total purines during the development of the heart failure in dogs. Circulation. 1999;100:2113–8.
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22.
Fang L, Moore XL, Gao XM, Dart AM, Lim YL, Du XJ. Down-regulation of mitofusin-2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci. 2007;80:2154–60.
•• Pennanen C, Parra V, López-Crisosto C, Morales PE, del Campo A, Gutierrez T, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J Cell Sci. 2014;127:2659–71. The results demonstrate the importance of mitochondrial dynamics in the development of cardiomyocyte hypertrophy and metabolic remodeling.
Santel A, Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60:448–55.
Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, et al. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. Plos Genet. 2010;6(6):e1001000.
• López Farré A, Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38:1400–5. The review analyzes the involvement of ROS in the cellular and molecular mechanisms associated with endothelial dysfunction in heart failure.
van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63(3):487–99.
Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292.
Mazzei L, García M, Calvo JP, Casarotto M, Fornés M, Abud MA, et al. Changes in renal WT-1 expression preceding hypertension development. BMC Nephrol. 2016;17:34.
Mazzei L, Docherty NG, Manucha W. Mediators and mechanisms of heat shock protein 70 based cytoprotection in obstructive nephropathy. Cell Stress Chaperones. 2015;20:893–906.
Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, et al. Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension. 2014;64(1):87–93.
• Liu C, Yang Q, Hwang SJ, Sun F, Johnson AD, Shirihai OS, et al. Association of genetic variation in the mitochondrial genome with blood pressure and metabolic traits. Hypertension. 2012;60:949–56. The study provide the first evidence of association of variants in the mitochondrial genome with systolic blood pressure and fasting blood glucose in general population.
Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin stimulated AMPKα1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L844–55.
Wang S, Li R, Fettermann A, Li Z, Qian Y, Liu Y, et al. Maternally inherited essential hypertension is associated with the novel 4263A>G mutation in the mitochondrial tRNAlle gene in a large Han Chinese family. Circ Res. 2011;108(7):862–70.
Andersen G, Wegner L, Jensen DP, Glümer C, Tarnow L, Drivsholm T, et al. PGC-1alpha Gly482Ser polymorphism associates with hypertension among Danish whites. Hypertension. 2005;45(4):565–70.
Bengtsson B, Thulin T, Schersten B. Familial resemblance in casual blood pressure—a maternal effect? Clin Sci (Lond). 1979;57 Suppl 5:279s–81s.
Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114(9):1281–9.
Blanquer-Rosselló MM, Santandreu FM, Oliver J, Roca P, Valle A. Leptin modulates mitochondrial function, dynamics and biogenesis in MCF-7 cells. J Cell Biochem. 2015;116(9):2039–48.
Zhang W, Ambati S, Della-Fera MA, Choi YH, Baile CA, Andacht TM. Leptin modulated changes in adipose tissue protein expression in ob/ob mice. Obesity (Silver Spring). 2011;19(2):255–61.
Authors’ Contributions
All authors contributed to the manuscript writing. The authors have read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Lahera, de las Heras, López-Farré, Manucha, and Ferder declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Lahera, V., de las Heras, N., López-Farré, A. et al. Role of Mitochondrial Dysfunction in Hypertension and Obesity. Curr Hypertens Rep 19, 11 (2017). https://doi.org/10.1007/s11906-017-0710-9
Published:
DOI: https://doi.org/10.1007/s11906-017-0710-9