Abstract
Research studying the role of inflammation in hypertension and cardiovascular disease has flourished in recent years; however, the exact mechanisms by which the activated immune cells lead to the development and maintenance of hypertension remain to be elucidated. The objectives of this brief review are to summarize and discuss the most recent findings in the field, with special emphasis on potential therapeutics to treat or prevent hypertension. This review will cover novel immune cell subtypes recently associated to the disease including the novel role of cytokines, toll-like receptors, and inflammasomes in hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As recently reviewed [1–4], the importance of immunity and inflammation in hypertension and vascular disease has been appreciated for decades, yet progress has been slowed by limited experimental tools and conflicting results. Recently, with the advent of more robust experimental methods, significant progress has been made to elucidate the mechanisms linking inflammation and immunity to hypertension and cardiovascular disease. This review focuses upon recent experimental observations that may provide therapeutic targets.
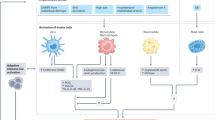
Novel Immune Cell Subtypes Associated with the Development of Hypertension
Tregs and Th17 Cells
Experimental studies have focused upon the pathophysiological role of individual T cell subsets. Specific experimentation is elucidating the functions of two T cell subtypes distinct from the classical Th1 and Th2 paradigm—regulatory T cells (Tregs) and Th17 cells. The development, differentiation, and plasticity of these cell types are still under intense scrutiny among immunologists, but many researchers hypothesize therapeutic benefit for inflammatory disorders by altering the dynamics of Tregs and Th17 cells. In the past few years, studies have shown that Tregs attenuate hypertension and target organ damage, while Th17 cells exacerbate the pathology.
Regulatory T cells are characterized by high expression of the transcription factor forkhead box P3 (FOXP3) and the ability to suppress inflammatory signaling of immune and non-immune cells [5]. Tregs are essential for immunological self-tolerance, and deficiency of this cell type leads to autoimmune disease [6]. Tregs are touted as a possible therapy to quell the abhorrent inflammatory milieu thought to mediate target organ damage in many hypertensive models. Many types of Tregs have been described according to cell surface marker expression and/or cytokine profile in the human immune system (CD8+ Tregs, Tr1, natural killer Tregs, etc.) [7]; however, the main focus in the field of hypertension has been on natural Tregs, or CD4+CD25+FOXP3+ T lymphocytes. The mechanisms by which Tregs suppress inflammatory signaling continue to be elucidated, although it is widely thought that IL-10 and TGFβ play an important role in Treg-mediated immunosuppression [8].
Th17 cells are a recently described subset of T cells characterized by the expression of the master transcription factor retinoic acid-related orphan receptor (ROR)γt and by the production of interleukin 17 (IL-17) [9]. In contrast to the anti-inflammatory role of Tregs, Th17 cells are pro-inflammatory and exacerbate tissue damage and disease in conditions of chronic inflammation and autoimmunity [10]. This appears to be the case in hypertensive pathology as well, and blunting Th17 signaling may alleviate the inflammation associated with hypertension and target organ damage. For instance, the consequences of angiotensin II (AngII) infusion in mice—hypertension, vascular dysfunction, vascular inflammation, oxidative stress, aortic stiffening, and collagen deposition—are attenuated in IL-17a null mice (IL-17A−/−) [11, 12]. Moreover, administration of recombinant IL-17 in C57BL/6 mice decreases NO-dependent vascular relaxation via Rho-kinase signaling and causes hypertension [13••]. Similarly, IL-17 has been demonstrated to have deleterious cardiovascular effects in rodent models of deoxycorticosterone acetate (DOCA)-salt hypertension [14] and preeclampsia [15].
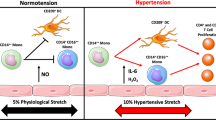
In contrast, Tregs are proposed to be protective. AngII-induced hypertension and endothelium-dependent vascular dysfunction in mice are attenuated by adoptive transfer of Tregs, possibly by reducing oxidative stress and/or increasing nitric oxide bioavailability in the vasculature [16, 17]. Additionally, in vitro studies showed that incubating resistance vessels with culture media from activated Tregs restored endothelium-dependent vasodilation and reduced vascular NADPH oxidase activity via an IL-10 dependent pathway [18]. Tregs have also been implicated in the protection from hypertension experienced by females, which has been attributed to sex-specific hormones [19]. Sex hormones have been shown to modulate immune function [20], and recent studies have investigated sex-dependent differences in immune system characteristics. For instance, in spontaneously hypertensive rats (SHR), Tregs represent a greater percentage of infiltrating T cells in the kidneys of female rats compared to males [21]. This finding correlates with increased number of IL-10+ renal cells and lower IL-17+ cells in female SHR compared to males [22•], suggesting that Tregs may mediate the protection against hypertension in females. Moreover, transaortic constriction in the mouse results in hypertension, infiltration of immune cells in the heart, and cardiac fibrosis. Adoptive transfer of Tregs in this model has no effect on blood pressure but significantly blunts accumulation of immune cells in the heart, blunts cardiac hypertrophy, and attenuates left ventricular fibrosis [23]. Other studies have shown blood pressure-independent protection via Tregs and damage via Th17 cells of cardiovascular organs, suggesting a greater role for Tregs and Th17 cells in cardiovascular disease.
Data regarding the role of Tregs and Th17 cells in human hypertension are sparse, although an intriguing study by Kleinewietfeld et al. [24••] showed that differentiation of naïve human T cells to the Th17 phenotype in culture is greatly enhanced when in the presence of elevated concentrations of NaCl [24••]. Due to high levels of NaCl consumption in developed countries and the argued correlation between high salt intake and increased blood pressure, the direct effect of NaCl on T cell differentiation could have drastic effects on organ damage and blood pressure regulation. Ultimately, much work needs to be done to fully appreciate the role of Tregs and Th17 cells in hypertension and target organ damage. Studies thus far indicate that understanding the mechanisms by which these cells elicit their phenotypic effects may lead to novel therapeutic targets for the treatment of hypertension.
Dendritic Cells
Dendritic cells (DCs) are bone marrow-derived, professional antigen-presenting cells (APC) that play a key role as modulators of the inflammatory response. Four subsets of DCs have been characterized, including classical DCs (cDCs), plasmacytoid DCs (pDCs), monocyte-derived inflammatory DCs (Mo-DCs), and Langerhans cells [25, 26]. DCs form a dense network in most human and animal tissues, including areas important in the regulation of blood pressure, such as arteries [27], kidneys [28], and brain [29]. These immature, resident DCs are thought to maintain tolerance and organ homeostasis by patrolling the environment for self- and non-self-antigens. In pathological conditions such as atherosclerosis, chronic kidney disease, or pulmonary hypertension, DCs become activated and induce activation of T lymphocytes.
Immature or precursor DCs can also be found in the bloodstream, surveying for potential antigens. The amount of circulating precursor DCs is indicative of the immune status of the organism, and decreased blood precursor DC levels have been reported in inflammation-related cardiovascular disorders. Reduced circulating precursor DC numbers in patients with atherosclerosis, myocardial infarction, or stage 3 chronic kidney disease have been associated with enhanced activation and recruitment of mature DCs in vascular lesions, infarcted areas, or renal tissue [30–32]. Once in the tissue, DCs act as mediators of cardiovascular disease. These reports highlight the potential use of circulating precursor DCs as new cardiovascular biomarkers to predict the development of cardiovascular disease.
In experimental hypertension, interesting new research has shown that AngII-induced superoxide production in DCs is associated with the accumulation of products of free radical-mediated lipid peroxidation, known as isoketals, in these cells. Isoketals can cross-link lysine residues on proteins, rendering them immunogenic. In turn, DCs present these modified proteins to T lymphocytes, triggering T cell activation and hypertension [33••]. The use of isoketal scavengers in mice prevented activation and immunogenecity of DCs and attenuated hypertension in response to a subpressor dose of AngII. These results identify isoketal scavengers as a new potential therapeutic approach to the prevention of hypertension in humans. These data, together with reports by Nahmod et al. [34] on the importance of AngII receptor type 1 (AT1) on DCs’ differentiation and functionality, emphasize the role of DCs on the development of AngII-induced hypertension.
Recent reports also highlight the role of the mineralocorticoids aldosterone and DOCA, well-known inducers of hypertension, on promoting DC-induced polarization of T cells towards the pro-inflammatory phenotype Th17 [35]. As explained above, Th17 cells have been strongly implicated in the development of hypertension. In these studies, treatment with the mineralocorticoid receptor inhibitors spironolactone and epleronone prevented the stimulatory effects of aldosterone on DCs and inhibited polarization of T cells into Th17 cells [35]. These studies suggest the potential use of mineralocorticoid receptor inhibitors as immunomodulator therapy to treat hypertension.
Naïve T cells require two signals for activation: interaction of the T cell receptor with the processed peptide presented by the APC and simultaneous interaction of additional receptors on the T cell surface with B7 ligands on the APC surface. Due to this required dual activation, inhibition of the B7-mediated T cell activation pathways has also been proposed as new therapy for the treatment of hypertension. Vinh et al. [36] demonstrated that in an experimental model of hypertension, blockade of B7-dependent co-stimulation by CTLA4-Ig reduces the development of hypertension in response to AngII and DOCA, opening a new promising avenue for the development of therapies against the disease. In short, further research is required to fully understand the role of DCs in hypertension, especially regarding the role of these cells in other kinds of hypertension, such as salt-sensitive hypertension.
Immunosenescent CD8+ Cells
Chronic antigen stimulation leads to gradual accumulation of late differentiated CD8+ T cells, characterized by critically shortened telomeres, loss of the costimulatory receptor CD28, gain of CD57 receptor expression, and increased production of inflammatory cytokines and chemokines [37]. These T cells have been traditionally called immunosenescent, but, contrary to their name, CD8+CD28−CD57+ T cells maintain the ability to proliferate under certain activation conditions [37]. Accumulation of these cells occurs with aging and in chronic inflammatory states such as cancer or autoimmune diseases. Given the role that inflammation plays in hypertension, it has also been proposed that these cells could play a role in the development of disease. Youn et al. [38••] demonstrated that hypertensive patients have an increased fraction of circulating immunosenescent CD8+ T cells and elevated circulating levels of C-X-C chemokine receptor type 3 (CXCR3) chemokines and serum granzyme B compared to healthy, age-matched control subjects. CXCR3 chemokines are well-known tissue-homing chemokines for T cells. On the other hand, granzyme B is a protease used by cytotoxic cells to induce cell death in target cells, and it is elevated during T cell-driven inflammation. Of note, CD8+CD28−CD57+ T cells are known to be highly cytotoxic. Although this study has some limitations, this report is the first implicating the involvement of T cells in human hypertension and specially highlights the importance of immunosenescent CD8+ T cells in the disease.
The Role of Cytokines in the Development of Hypertension
Recent attention has been focused on exploring the pathways and inflammatory mediators that immune cells use to drive high blood pressure and end-organ damage. When immune cells become activated and/or are recruited to a target organ, they produce cytokines that determine the local inflammatory response. Chemokines, special kinds of cytokines, are chemoattractants that direct migration of immune cells into tissues. Some of the inflammatory cytokines and chemokines that have been studied for their involvement in hypertension are TNF-α, IL-17, MCP-1, and IL-6.
Contributions of TNF-α to the development of high blood pressure have been demonstrated by pharmacological or genetic approaches in animal models of AngII-induced hypertension, lupus, metabolic syndrome, and preeclampsia, as reviewed by Ramseyer and Garvin [39]. In these cases, blockade of the TNF-α pathway led to decreased blood pressure and inflammation. However, contrasting results were reported in other models of hypertension like DOCA-salt hypertension or a human Ang/renin double transgenic rat model of AngII hypertension [39]. These opposite observations may be explained by the different type of TNF-α receptor that is activated in each model. To date, two different TNF-α receptors have been described: TNFR1 and TNFR2, but complete understanding of their functions is still lacking. Most pro-inflammatory effects of TNF-α are associated with activation of TNFR1, and, in humans, high serum TNFR1 levels strongly correlate with diseases associated with hypertension, like end-stage renal disease and type 2 diabetes [40]. Other reports, however, indicate that genetic deletion of TNFR1 leads to increased blood pressure in response to AngII [41]. On the other hand, some reports link TNFR2 activation to increased vascular inflammation [42], while others suggest that it has beneficial roles in the cardiovascular system [43]. It is clear from these contradictory reports that further investigation of the role of TNF-α and its receptors in hypertension is needed to develop better therapies.
In recent years, the pro-inflammatory cytokine IL-17 has been implicated in the development of hypertension. This cytokine is produced by Th17 cells, macrophages, dendritic cells, and natural killer cells in response to immune activation [44]. Elevated IL-17 correlated with hypertension in subjects with type 2 diabetes [11] and in patients with preeclampsia and lupus [45], diseases associated with elevated blood pressure. Madhur et al. [11] reported that IL-17 is required for the maintenance of AngII-induced hypertension and vascular dysfunction. Another group recently demonstrated that this effect of IL-17 on vascular function is mediated by promoting NOS3 phosphorylation, thus decreasing enzyme activity and NO production in endothelial cells [13••]. This cytokine could also be important in salt-sensitive hypertension, since recent studies indicate that naïve T cells increase expression of serum glucocorticoid kinase 1 (SGK1; a known salt-sensor protein) and polarize to Th17 cell phenotype in the presence of high salt. These studies demonstrate that SGK1 is critical for the induction of Th17 cells and could hint to a mechanism by which high salt may trigger Th17 development and IL-17 production and promote tissue inflammation [46••].
By activating the CCR2 receptor, chemokine MCP-1 (also known as CCL2) leads to the activation and migration of monocytes and leukocytes to sites of inflammation. Production of MCP-1 can be stimulated by AngII [47] and endothelin-1 [48], fundamental players in the development of hypertension and end-organ damage. Moreover, the use of Ang receptor blockers reduces MCP-1 levels both in experimental models and in hypertensive patients [49]. In addition, genetic deletion of the MCP-1 axis or blockade of CCR2 in experimental models decreases blood pressure and reduces vascular and renal inflammation [50, 51]. These results highlight the potential of MCP-1 axis inhibition for treating hypertension; however, in-depth clinical studies are still needed.
Levels of the pro-inflammatory cytokine IL-6 are also elevated in hypertensive conditions. Studies by Brands et al. [52] demonstrated that IL-6 is fundamental for the development of AngII-induced hypertension and that activation of the JAK/STAT3 pathway by IL-6 plays a key role in the disease. Moreover, a human study further confirmed these results by showing that plasma levels of IL-6 increase in response to acute AngII infusion and that these levels are exaggerated in hypertensive patients [53•]. IL-6 is also elevated in pulmonary hypertension, and anti-IL-6 antibody therapy has been successfully used in a one-patient clinical trial in Japan [54], indicating the potential therapeutic value of targeting IL-6.
Although CD40L is not considered a cytokine, it is included in this section because of its powerful pro-inflammatory effects. CD40L is part of the TNF superfamily and acts by promoting cytokine and chemokine release. It is derived from activated platelets and has been implicated in thrombosis [55]. Recent research also suggests that AngII promotes and augments the inflammatory activity of the CD40/CD40L system in human vascular cells [56] and that genetic deletion of CD40L improves endothelial dysfunction and decreases aortic inflammation and oxidative stress [57•]. These reports suggest that CD40L mediates many deleterious effects of AngII in the vasculature. In addition, soluble CD40L (sCD40L) is elevated in the plasma of hypertensive patients and significantly decreased after antihypertensive treatment [58]; also, non-dipper hypertensive patients (at increased risk of cardiovascular events) present increased CD40L levels [59]. Based on these reports, CD40L could be a valuable biomarker of cardiovascular disease and a potential therapeutic target.
Toll-Like Receptors: Controllers of the Adaptive Immune Response in Hypertensive Conditions
The involvement of the innate immune response has emerged as an important determinant of hypertension and end-organ damage. Contrary to what was originally believed, the innate immune system not only responds to exogenous pathogens, but it can also be activated by endogenous molecules released by stressed, damaged, or necrotic cells [60]. These molecules, known as damage-associated molecular patterns (DAMPs), are important inflammatory mediators [61]. Examples of DAMPs include high mobility group box 1 (HMGB1), heat shock proteins 60 and 70, AngII, IL-1α, uric acid, DNA fragments, mitochondrial content, HDL, and oxidized LDL [62•, 63]. Interestingly, many of these DAMPs are known to be present in cardiovascular diseases such as atherosclerosis [64], diabetes [65], pulmonary hypertension [66], essential hypertension [67], and preeclampsia [68].
Toll-like receptors (TLRs) are a conserved family of receptors that trigger pro-inflammatory signaling cascades in response to either microbial structures or DAMPs released by injured tissues [62•]. These receptors have a fundamental role in the innate immune response. Eleven different TLRs have been described in humans and thirteen in mice (TLR1-13) [69]. Different signaling cascades are activated by TLRs depending on adaptor protein binding [70]. Examples of adaptor proteins include myeloid differentiation factor 88 (MyD88), MyD88-adapter-like (Mal), IL-1 receptor associated kinase-4 (IRAK4), and TIR-containing adaptor molecule (TICAM).
Recent studies suggest that the innate immune system may be the first step in the pathogenesis of hypertension and that TLRs may be the molecular link between the innate and adaptive immune responses during cardiovascular disease. In fact, expression of TLRs has been described in blood vessels [69], brain [71], renal tubules, podocytes, mesangial cells [65], T lymphocytes, macrophages, and dendritic cells [69, 72]; all key players in the development and maintenance of hypertension. In recent years, TLRs have been implicated in preeclampsia (TLR2, TLR3, TLR4, and TLR9 [73–75]); programming of vascular dysfunction (TLR4 [76]); hypertension induced by AngII (TLR2 and/or TLR4 [77, 78]), obesity (TLR4 [79]), or l-NAME (TLR4, [80]); atherosclerosis (TLR2 [81]); pulmonary hypertension (TLR4 [66]); diabetic nephropathy (mostly TLR4 but also TLR2 [65]); and ischemia-reperfusion renal injury (TLR2 and/or TLR4 [82]). In addition, TLR7 and TLR9 have been shown to be involved in the inflammation typical of lupus [83], a disease also associated to hypertension.
In light of the involvement of TLRs in cardiovascular disease, several novel therapies designed to target these receptors are in the works [84]. By targeting TLRs, modulation of the inflammatory cascade may be achieved at an earlier point and control disease more effectively. The number of TLR antagonists is still very limited, however, and further preclinical research is needed. A powerful anti-TLR2 antibody has been reported as effective in reducing the myocardial infarct area in mouse and pig models of ischemia/reperfusion [85, 86] and to diminish and stabilize atherosclerotic lesions in a mouse model [87]. TLR4-antagonists RsLPS and CXR-526 showed promising results against the development of atherosclerotic plaques [88] and significantly decreased signs of kidney injury in a mouse model of type 1 diabetes [89], respectively. Moreover, treatment with antibodies against TLR4 is efficient in ameliorating hypertension in DOCA-salt and SHR rat models [90, 91]. Additionally, dual blockade of TLR7/9 decreased inflammation in a model of lupus [83]. Alternative approaches, like targeting the adaptor proteins or increasing ubiquitination of TLRs [84], are also being studied.
Overall, these studies support the involvement of TLRs in the development of hypertension and other related cardiovascular diseases and highlight these receptors as attractive targets for potential new therapies. Despite the existence of several TLR antagonists, further understanding of the roles of TLRs and how they vary in different cardiovascular diseases is needed in order to develop more TLR-targeting therapeutic options.
The Role of Inflammasomes in Hypertension
The nucleotide-binding oligomerization domain (Nod)-like receptor containing pyrin domain 3 (NLRP3, also known as NALP3 or cryopyrin) inflammasome is the most characterized member of the nucleotide-binding domain leucine-rich repeat (NLR) family of pattern recognition receptors (PRRs). Activation of the NLRP3 inflammasome signifies cleavage and activation of a subclass of inflammatory caspases that are responsible for the maturation of inactive pro-inflammatory cytokine precursors like pro-IL-1β or pro-IL-18. NLRP3 inflammasome formation controls the innate immune system activation in response to a wide range of danger signals including pathogen-associated molecular patterns (PAMPs) and DAMPs derived from disease and infection.
With 23 NLR genes identified, there exist other types of caspase-processing inflammasomes, including NLRP1, NLRC4, and AIM2. NLRP1 was the first discovered inflammasome demonstrated to process both caspase-5 and caspase-1 and is primarily activated by bacterial cell wall component muramyl dipeptide (MDP) and Bacillus anthracis lethal toxin [92, 93]. NLRC4 inflammasomes sense various gram-negative bacteria conserved proteins like flagellin, rod, and needle [94, 95], while AIM2 inflammasomes respond to foreign nucleic acids and double-stranded DNA [96]. The NLRP3 inflammasome has gained much attention over recent years due to its growing role in the sterile inflammatory response to DAMPs associated with a number of chronic degenerative diseases [97, 98]. Activation by this diverse range of danger signals results in a cytosolic multiprotein complex formed by the oligomerization of the NLRP3 sensory protein, the adaptor molecule apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD) (ASC), and the cysteine protease caspase-1, causing the maturation of pro-inflammatory cytokines IL-1β and IL-18, thereby contributing to very early initiation of the immune response.
Very recent and exciting literature suggests an important role for NLRP3 inflammasomes in humans and animal models of kidney disease and hypertension. NLRP3 inflammasome involvement has been reported in glomerular and tubulointerstitial injury, where NLRP3 mRNA is significantly increased in renal biopsies of patients with various types of nondiabetic kidney disease, including acute tubular necrosis, focal segmental glomerulosclerosis, and hypertensive nephrosclerosis [99••]. In NLRP3 and ASC-deficient mice, glomerular injury, renal leukocyte infiltration, and T cell activation associated with nephrotoxic serum nephritis was attenuated [100••]. Furthermore, the inflammasome has been shown to contribute to IgA nephropathy, hyperhomocysteinemia-induced glomerular sclerosis, and ischemia-reperfusion injury [101–103]. In mouse models of hypertension, studies have demonstrated protective effects of inflammasome inhibition in the two-kidney, one clip (2K1C) model, where NLRP3 or ASC deficiency prevents blood pressure elevation and lowers plasma renin activity [104]. Additionally, in murine ATP-induced hypertension, ATP infusion resulted in increased salt-sensitive hypertension, caspase-1 activity, IL-1β production, and CD43+ T cell infiltration in the renal medulla [105]. Administration of caspase-1 inhibitor WEHD, however, blocked ATP-induced hypertension, reduced sodium retention, and blunted inflammasome activation and the production of IL-1β. In humans, an intronic 42 base pair variable number of tandem repeat (VNTR) polymorphism in the CIAS1 gene that encodes for NLRP3 has been linked to essential hypertension susceptibility [106••]. Interestingly, the CIAS1 gene is part of the CATERPILLER gene family, which also contains PYPAF5 that encodes the AngII/vasopressin receptor (AVR) implicated in salt-sensitive hypertension in the Dahl SS model [107]. Patients with pulmonary arterial hypertension demonstrated increased NLRP3 inflammasome complex formation and caspase-1 activation in purified monocytes compared to control subjects and also had significantly elevated IL-1β and IL-6 in the serum [108]. Superoxide scavenging experiments suggest that these inflammasome-activating effects may be due to an oxidant/antioxidant imbalance [109]. Together, these animal and human data strongly implicate an important contribution of the NLRP3 inflammasome in the development of hypertension and may potentially serve as an early disease biomarker and therapeutic target.
Conclusions
Innate and adaptive immunity play a significant role in the pathogenesis of hypertension. Specific immune cell types, cytokines, toll-like receptors, and components of inflammasomes all pose novel targets for antihypertensive therapy. These targets are summarized in Table 1. Further research will remain to elucidate the interrelationship and common mediators of these immune mechanisms.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci. 2014;126:267–74.
Bernardo R-IB. Renal infiltration of immunocompetent cells: cause and effect of sodium-sensitive hypertension. Clin Exp Nephrol. 2010;14:105–11.
Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–40.
Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol. 2014; (in press).
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6.
Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13.
Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis. Arthritis. 2012;2012:805875.
Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–11.
Ivanov I, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgamma t directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33.
Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–11.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–7.
Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2008;114:616–25.
Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. This study demonstrates that IL-17 leads to the deleterious effects on the vasculature by promoting NOS3 phosphorylation and therefore decreasing enzyme activity.
Amador CA, Barrientos V, Peña J, Herrada AA, González M, Valdés S, et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63:797–803.
Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62:1068–73.
Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–76.
Matrougui K, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, et al. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–41.
Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–42.
Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7.
Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–8.
Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012;303:R359–67.
Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension. 2014;64:557–64. With increasing emphasis on the use of female disease models, this group explores some of the inflammatory characteristics that may explain disparities in the presentation of hypertension between males and females.
Kanellakis P, Dinh TN, Agrotis A, Bobik A. CD4(+)CD25(+)Foxp3(+) regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens. 2011;29:1820–8.
Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. In vitro elevation of NaCl concentration can affect T cell differentiation. These findings could have major implications regarding the emerging hypothesis of sodium storage in the skin.
Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–54.
Christ A, Temmerman L, Legein B, Daemen MJAP, Biessen EAL. Dendritic cells in cardiovascular diseases: epiphenomenon, contributor or therapeutic opportunity. Circulation. 2013;128:2603–13.
Bobryshev YV, Lord RS. Ultrastructural recognition of cells with dendritic cell morphology in human aortic intima. Contacting interactions of vascular dendritic cells in athero-resistant and athero-prone areas of the normal aorta. Arch Histol Cytol. 1995;58:307–22.
Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, et al. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–6.
D’Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: biology and pathology. Acta Neuropathol. 2012;124:599–614.
Van Vré EA, Van Brussel I, de Beeck KO, Hoymans VY, Vrints CJ, Bult H, et al. Changes in blood dendritic cell counts in relation to type of coronary artery disease and branchial endothelial cell function. Coron Artery Dis. 2010;21:87–96.
Kretzschmar D, Betge S, Windisch A, Pistulli R, Rohm I, Fritzenwanger M, et al. Recruitment of circulating dendritic cell precursors into the infarcted myocardium and pro-inflammatory response in acute myocardial infarction. Clin Sci. 2012;123:387–98.
Paul K, Kretzschmar D, Yilmaz A, Bärthlein B, Titze S, Wolf G, et al. Circulating dendritic cell precursors in chronic kidney disease: a cross-sectional study. BMC Nephrol. 2013;14:274.
Kirabo A, Fontana V, Davies S, Roberts LJ, de Faria A, Galindo C, et al. Dendritic cell superoxide and isoketals activate T cells and promote angiotensin II hypertension. FASEB J. 2014;28:s1153.2. This study shows that AngII-induced hypertension is associated to isoketal formation and that isoketals scavengers can effectively decreased immunogenicity of DCs and attenuate hypertension.
Nahmod K, Gentillini C, Vermeulen M, Uharek L, Wang Y, Zhang J, et al. Impaired function of dendritic cells deficient in angiotensin II type 1 receptors. J Pharmacol Exp Ther. 2010;334:854–62.
Herrada AA, Contreras FJ, Marini NP, Amador CA, González PA, Cortés CM, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184:191–202.
Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, et al. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–37.
Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32.
Youn J-C, Yu HT, Lim BJ, Koh MJ, Lee J, Chang D-Y, et al. Immunosensescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–33. This is the first study that directly demonstrates the involvement of T cells, specifically immunosenescent CD8+ cells, in human hypertension.
Ramseyer VD, Garvin JL. Tumor necrosis factor α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231.
Saulnier P-J, Gand E, Ducrocq G, Halimi J-M, Hulin-Delmotte C, Llaty P, et al. Association of serum concentration of TNFR1 with all-cause mortality in patients with type 2 diabetes and chronic kidney disease: follow-up of the SURDIAGENE cohort. Diabetes Care. 2014;37:1425–31.
Chen CCA, Pedraza PL, Hao S, Stier CT, Ferreri NR. TNFR1-deficient mice display altered blood pressure and renal responses to AngII infusion. Am J Physiol Ren Physiol. 2010;299:F1141–50.
Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, et al. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–25.
Min W, Wan T, Luo Y. Dissecting TNF-TNFR1/TNFR2 signaling pathways in vasculature. In: Dauphinee S, Karsan A, editors. Endothelial Dysfunction and Inflammation. 2010. pp:137–159.
Onishi RM, Gaffen SL. Interleukin-17 and its targets genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–21.
Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and Il-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93.
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic Th17 cells by inducible salt-sensing SGK1. Nature. 2013;496:513–7. This paper demonstrates the importance of SGK1 expression for the polarization of naïve T cells into the Th17 phenotype, in presence of high salt.
Funakoshi Y, Ichiki T, Shimokawa H, Egashira K, Takeda K, Kaibuchi K, et al. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension. 2001;38:100–4.
Ishizawa K, Yoshizumi M, Tsuchiya K, Houchi H, Minakuchi K, Izawa Y, et al. Dual effects of endothelin-1 (1-31): induction of mesangial cell migration and facilitation of monocyte recruitment through monocyte chemoattractant protein-1 production by mesangial cells. Hypertens Res. 2004;27:433–40.
Fliser D, Buchholz K, Haller H, for the EUTOPIA investigators. Antiinflammatory effects of angiotensin subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–7.
Shen JZ, Morgan J, Tesch GH, Young MJ. CCL2-dependent macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in male mice. Endocrinology. 2014;155:1057–66.
Chan CT, Moore JP, Budzyn K, Guida E, Diep H, Vinh A, et al. Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in DOCA salt-treated mice. Hypertension. 2012;60:1207–12.
Brands MW, Banes-Berceli AKL, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin-6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and JAK2/STAT3 activation. Hypertension. 2010;56:879–84.
Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium and the renin-angiotensin system in humans. Am J Hypertens. 2011;24:1143–8. This study confirms the involvement of IL-6 in the AngII pathway in human hypertensives.
Furuya Y, Satoh T, Kuwana M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int J Rheum. 2010; 2010 Article ID 720305.
Santilli F, Basili S, Ferrori P, Davi G. CD40/CD40L system and vascular disease. Intern Emerg Med. 2007;2:256–68.
Ruef J, Browatzki M, Pfeiffer CA, Schmidt J, Kranzhofer R. Angiotensin II promotes the inflammatory response to CD40 ligation via TRAF-2. Vasc Med. 2007;12:23–7.
Hausding M, Jurk K, Daub S, Kröller-Schön S, Stein J, Schwenk M, et al. CD40L contributes to angiotensin II-induced pro-thrombotic state, vascular inflammation, oxidative stress and endothelial dysfunction. Basic Res Cardiol. 2013;108:386. This paper demonstrates that genetic deletion of CD40L improves endothelial dysfunction and decreases aortic inflammation and oxidative stress.
Derosa G, D’Angelo A, Mudellini A, Pesce RM, Fogari E, Maffioli P. Evaluation of emerging biomarkers in cardiovascular risk stratification of hypertensive patients: a 2-year study. Curr Med Res Opin. 2012;28:1435–45.
Surgit O, Erturk M, Akgul O, Pusuroglu H, Korkmaz AF, Isiksacan N, et al. Assessment of mean platelet volume and soluble CD40 ligand levels in patients with non-dipper hypertension, dippers and normotensives. Clin Exp Hypertens. 2014;27:1–5.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5.
McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC. Toll-like receptors and damage-associated molecular patterns: novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H184–96. Nice review paper on the role of toll-like receptors in hypertension.
Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23.
Falck-Hansen M, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis. Int J Mol Sci. 2013;14:14008–23.
Lin M, Tang SCW. Toll-like receptors: sensing and reacting to diabetic injury in the kidney. Nephrol Dial Transplant. 2014;29:746–54.
Bauer EM, Shapiro R, Zheng Z, Ahmad F, Ishizawar D, Comhair SA, et al. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of toll-like receptor 4. Mol Med. 2012;18:1509–18.
Facholi Bomfim G, Szasz T, Carvalho MH, Webb RC. The Toll way to hypertension: role of the innate immune response. Endocrinol Metabol Syndr. 2011;S8:002.
Iversen A-C. Inflammatory mechanisms in preeclampsia. Pregnancy Hyper. 2013;32:58.
Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45.
Kawai T, Akira S. The role of pattern recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–84.
Mallard C. Innate immune regulation by toll-like receptors in the brain. ISRN Neurol. 2012; 2012 Article ID 701950.
Jin B, Sun T, Yu XH, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol. 2012;2012:836485.
Xie F, von Dadelszen P, Nadeau J. CMV infection, TLR-2 and -4 expression, and cytokine profiles in early-onset preeclampsia with HELLP syndrome. Am J Reprod Immunol. 2014;71:379–86.
Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, et al. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. 2012;7:e41884.
Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in preeclampsia. Clin Sci. 2012;123:429–35.
Thompson JA, Webb RC. The potential role of Toll-like receptors in programming of vascular dysfunction. Clin Sci. 2013;125:19–25.
Hardigan T, Sepulveda MA, Pedrosa NK, Webb RC. Toll-like receptor 2 blockade decreases contractility in angiotensin II-induced hypertensive rat resistance arteries. Hypertension. 2013;62:A189.
Plavia V, Pyle C, Mezzetti E, Chen X, Didion S. Toll-like receptor 4 contributes to hypertension and endothelial dysfunction produced by angiotensin II. FASEB J. 2012;26:879.5.
Liang C-F, Liu JTC, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol. 2013;33:777–84.
Sollinger D, Eiβler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, et al. Damage-associated molecular pattern activated toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res. 2014;101:464–72.
Speer T, Rohner L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor 2. Immunity. 2013;38:754–68.
Wu H, Chen G, Wyburn KR. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–59.
Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, et al. TLR recognition of self-nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–41.
Spirig R, Tsui J, Shaw S. The emerging role of TLR and innate immunity in cardiovascular disease. Cardiol Res Pract. 2012; 2012, Article ID 181394.
Arslan F, Keogh B, McGuirk P, Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediat Inflamm. 2010;2010:704202.
Arslan F, Houtgraaf JH, Keogh B, Kazemi K, de Jong R, McCormack WJ, et al. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5:279–87.
Wang X, Lv X, Wang J, Yan H, Wang Z, Liu H, et al. Blocking TLR2 activity diminishes and stabilizes advanced atherosclerotic lesions in apolipoprotein E-deficient mice. Acta Pharmacol Sin. 2013;34:1025–35.
Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol. 2013;216:61–71.
Lin M, Yiu WH, Li RX, Wu HJ, Wong DWL, Chan LYY, et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83:887–900.
Nunes KP, Bonfim GF, Szasz T, Carvalho MH, Webb RC. Treatment with anti-TLR4 antibody lowers blood pressure and improves erectile function in DOCA-salt rats. Hypertension. 2012;60:A254.
Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci. 2012;122:535–43.
Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–4.
Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–24.
Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–80.
Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600.
Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–60.
Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54.
Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259–64.
Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–44. Aside from demonstrating that NLRP3-deficient mice were protected from chronic kidney disease associated from unilateral ureteral obstruction, this study also examined a panel of human non-diabetic kidney diseases and found that NLRP3 mRNA increased with disease prevalence and correlated with impaired renal function.
Andersen K, Eltrich N, Lichtnekert J, Anders HJ, Vielhauer V. The NLRP3/ASC inflammasome promotes T-cell-dependent immune complex glomerulonephritis by canonical and noncanonical mechanisms. Kidney Int. 2014. This study importantly demonstrated that NLRP3 inflammasome inhibition prevents T cell activation and infiltration into the kidney.
Yang SM, Ka SM, Hua KF, Wu TH, Chuang YP, Lin YW, et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic Biol Med. 2013;61:285–97.
Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60:154–62.
Kim HJ, Lee DW, Ravichandran K, O Keys D, Akcay A, Nguyen Q, et al. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2013;346:465–72.
Wang Q, So A, Nussberger J, Tschopp J, Burnier M. Impact of NLRP3 inflammasome on the development of hypertension and renal and cardiac hypertrophy in 2K1C and DOCA/salt mice. Kidney Res Clin Pract. 2012;31(2):A83.
Xia M, Xiong J, Boini KM, Abais JM, Li PL. Characteristics and hypertensive actions of renal medullary NALP3 inflammasomes in mice. FASEB J. 2012;26:879.884.
Omi T, Kumada M, Kamesaki T, Okuda H, Munkhtulga L, Yanagisawa Y, et al. An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet. 2006;14:1295–305. This was the first genetic study to associate the CIAS1 (NLRP3) gene to essential hypertension susceptibility.
Ruiz-Opazo N, Lopez LV, Herrera VL. The dual AngII/AVP receptor gene N119S/C163R variant exhibits sodium-induced dysfunction and cosegregates with salt-sensitive hypertension in the Dahl salt-sensitive hypertensive rat model. Mol Med. 2002;8:24–32.
Shaik R, Kolliputi N, Waxman AB. Activation of inflammasome in pulmonary arterial hypertension. Pulmonary hypertension: biomarkers, genetics, and genomics: insights into pathogenesis, A4865, 2010.
Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, et al. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal. 2013;18:1753–64.
Acknowledgments
This manuscript was partially funded by grants DK96859 and HL116264 to DLM and NIH T32 DK007545 to CDM.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Nathan P. Rudemiller declares that he has no conflict of interest.
Justine M. Abais, Carmen De Miguel and David L. Mattson report grants from the National Institutes of Health.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Antihypertensive Agents: Mechanisms of Drug Action
Rights and permissions
About this article
Cite this article
De Miguel, C., Rudemiller, N.P., Abais, J.M. et al. Inflammation and Hypertension: New Understandings and Potential Therapeutic Targets. Curr Hypertens Rep 17, 507 (2015). https://doi.org/10.1007/s11906-014-0507-z
Published:
DOI: https://doi.org/10.1007/s11906-014-0507-z