Abstract
Over the past decade, vitamin D has generated considerable interest as potentially having important effects on the vasculature and the kidney. Animal and human data indicate that vitamin D suppresses the activity of the renin-angiotensin system and improves endothelial function. Observational studies in humans suggest that low 25-hydroxyvitamin D (25[OH]D) levels are associated with a higher risk of hypertension. However, findings from randomized trials of vitamin D supplementation (with cholecalciferol or ergocalciferol) to lower blood pressure are inconsistent, possibly stemming from variability in study population, sample size, vitamin D dose, and duration. Supplementation with activated vitamin D (i.e., 1,25-dihydroxyvitamin D or analogues) in patients with chronic kidney disease reduces urine albumin excretion, an important biomarker for future decline in renal function. These studies are reviewed, with special emphasis on recent findings. Definitive studies are warranted to elucidate the effects of vitamin D supplementation on mechanisms of hypertension and kidney disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D has long been known to be an important factor for normal calcium metabolism and skeletal health. In the past decade, resurging interest and new research have implicated vitamin D deficiency as a potential contributor to the pathophysiology of many extraskeletal conditions, including vascular diseases such as high blood pressure and kidney disease [1]. Recent experimental animal studies and observational human studies have repeatedly suggested that supplementation with vitamin D metabolites may lower the risk for hypertension and kidney injury, but definitive human trials favoring the adoption of vitamin D therapy for the primary or secondary prevention of these conditions are still pending.
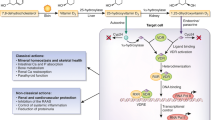
One of the many challenges in evaluating the biologic role of vitamin D in influencing blood pressure and renal function is deciphering which circulating vitamin D metabolites to measure in cross-sectional studies (i.e., 25-hydroxyvitamin D or 1,25-dihydroxyvitamin D) and what forms of vitamin D therapy to use for interventional studies (oral vitamin D2, oral vitamin D3, oral vitamin D receptor agonist, or ultraviolet radiation to promote cutaneous synthesis). The typical clinical barometer of human vitamin D status is 25-hydroxyvitamin D3 (25[OH]D), which is converted to the active vitamin D metabolite 1,25-dihydroxyvitamin D3 (1,25[OH]2D) in a tightly regulated manner by the 1-alpha-hydroxylase enzyme. Although 25(OH)D is a stable steroid metabolite in blood and is easier to quantify and interpret than 1,25(OH)2D, it is 1,25(OH)2D and not 25(OH)D that activates the vitamin D receptor (VDR) at the end-organ level. Thus, observational studies that measure 25(OH)D as a marker of overall vitamin D status may not always represent the full scope of biologic action. Moreover, the inferences drawn from trials that provide conventional over-the-counter vitamin D2 or D3 supplements may differ from those drawn from trials that bypass the tightly regulated 1-alpha-hydroxylase reaction and provide 1,25(OH)2D (or other VDR agonists) to elicit end-organ effects.
Another challenge is designing an investigation to elucidate the potential mechanisms whereby vitamin D could impact blood pressure and kidney function. To date, the most intensely investigated mechanism of action by which vitamin D could prevent or improve vascular diseases is the proposed negative regulation of the renin-angiotensin system (RAS). However, several other biologic pathways were implicated by prior studies.
The following discussion reviews mechanistic studies that have led to human investigations, evaluates the human data with a specific focus on the limitations of their conclusions, and proposes future directions that may definitively ascertain the role of vitamin D in the pathophysiology of hypertension and kidney disease.
Vitamin D and Hypertension
Mechanisms of Association
The association of vitamin D with blood pressure and hypertension has been described for over a quarter of a century [2]. The most notable mechanism implicating vitamin D with hypertension is its role as a negative regulator of the RAS [3]; inappropriately elevated RAS activity is known to contribute to human hypertension and cardiovascular risk [4–6].
The development of VDR null mice has facilitated numerous experiments that have shed light on the relationship between vitamin D, the RAS, and hypertension [7]. Li et al. reported that VDR null mice had significant elevations in renin activity and circulating plasma angiotensin II concentrations [3] and exhibited increased activity of the local cardiac-tissue RAS [3, 8]. These mice displayed a phenotype of hypertension and cardiac hypertrophy that was attenuated when RAS antagonists were administered. A distinct mouse model of 1-alpha-hydroxylase deficiency also exhibited a phenotype of enhanced RAS activity, hypertension, and cardiac hypertrophy, which was attenuated by treatment with 1,25(OH)2D or RAS antagonists [9]. The findings of these experiments were further consolidated with the demonstration that 1,25(OH)2D acts to suppress the expression of renin [10, 11], suggesting that the vitamin D–VDR complex may function as a negative regulator of the RAS and could thereby exert protective downstream effects on blood pressure and cardiac tissue.
Corollary human physiology studies have generally supported this evidence from animals. About 25 years ago, Resnick et al. observed lower plasma renin activity with increasing 1,25(OH)2D [12]. More recently, human mechanistic studies have shown that lower levels of 1,25(OH)2D and 25(OH)D are associated with higher plasma renin and angiotensin II concentrations [12, 13•, 14•], and that lower 25(OH)D levels are associated with higher systemic vascular-tissue RAS activity [15].
Alternatively, other investigators have proposed a nongenomic effect of vitamin D on the RAS and blood pressure. Resnick and colleagues hypothesized that vitamin D was involved in regulating the flux of calcium into vascular smooth-muscle cells, thereby influencing intracellular calcium concentrations, vascular tone, and blood pressure [2, 12, 16, 17] and decreasing renin secretion from juxtaglomerular cells [18, 19].
Other vascular-protective pathways also have been implicated in the association of vitamin D with hypertension. In vitro experiments have shown that vitamin D reduces the deleterious effect of advanced glycation products on the endothelium, improves activity of the nitric oxide system, reduces inflammatory parameters, and enhances prostacyclin production [20–23]. In conjunction with these basic findings, human studies exploring mechanisms of vitamin D in the pathophysiology of hypertension reported that concentrations of vitamin D metabolites were associated with improved endothelial function and oxidative stress [24–28] as well as circulating concentrations of adipocytokines implicated in blood pressure control [29–33].
Summary of Human Clinical Data
Most human clinical studies evaluating the role of vitamin D on blood pressure have been cross-sectional analyses. The majority of these were consistent with the animal data in showing an inverse association between vitamin D and blood pressure [34–36] or the prevalence of hypertension [37, 38]. In contrast, at least two large cross-sectional studies demonstrated no detectable association between vitamin D and blood pressure or the prevalence of hypertension, but in addition to the traditional limitations of cross-sectional analyses, these results may be biased by the fact that the study populations had relatively high 25(OH)D concentrations and prevalent use of antihypertensive drugs, both of which could obscure a potential association [39, 40].
Prospective studies have produced similarly mixed results. In a longitudinal analysis of men from the Health Professionals’ Follow-Up Study and women from the Nurses’ Health Study followed for 4 to 8 years, Forman et al. observed a pooled adjusted relative risk for incident hypertension of 3.18 (95% CI, 1.39–7.29) when comparing individuals with lower (<15 ng/mL) versus higher (≥30 ng/mL) concentrations of 25(OH)D [41]. In a subsequent nested case-control analysis of normotensive women from the Nurses’ Health Study II, they observed an adjusted odds ratio for incident hypertension of 1.66 (P-trend 0.01) when comparing those with 25(OH)D levels in the lowest quartile versus the highest quartile [42]. The longitudinal Michigan Bone Health and Metabolism Study evaluated the risk for systolic hypertension in over 500 Caucasian women who had 25(OH)D and blood pressure assessments in 1993 and again 14 years later, in 2007 [43]. Although they observed no cross-sectional association between 25(OH)D concentrations and concurrent blood pressure at baseline in 1993, 25(OH)D concentrations lower than 32 ng/mL at baseline were associated with a significantly increased risk for systolic hypertension in 2007 (adjusted OR, 3.0; 95% CI, 1.01–8.7). In contrast, Jorde et al. reported conflicting observations from the Tromso study, which followed individuals naïve to antihypertensive therapy from 1994 to 2008 [44]. They did note an inverse association between systolic blood pressure and quartiles of 25(OH)D at baseline in 1994, but these baseline 25(OH)D concentrations did not predict incident hypertension or future blood pressure. Regardless of whether the disparity in these findings was due to the narrow range of 25(OH)D concentrations within the study populations or other, unrecognized confounders, it underscored the need for definitive interventional studies.
Observational studies have suggested higher blood pressures in winter months and at latitudes further from the equator, thus implicating insufficient ultraviolet radiation exposure and decreased cutaneous synthesis of vitamin D3 as potential culprits in vascular disease [45]. Interventional studies to evaluate the effect of cutaneous vitamin D3 synthesis with ultraviolet radiation exposure have produced interesting but mixed results. Krause et al. randomized 18 hypertensive subjects to receive total body ultraviolet radiation with either UVA or UVB and observed that those receiving UVB had significant increases in 25(OH)D concentrations with concomitant decrements in 24-hour ambulatory systolic and diastolic blood pressures (−6 mm Hg) [46]. In a similar randomized study design, Scragg et al. evaluated 119 normotensive individuals but observed no changes in blood pressure despite significant rises in 25(OH)D concentrations [47]. The findings of these studies may be limited by their relatively small sample sizes, short durations of follow-up (6 and 12 weeks, respectively), and focus on distinct hypertensive or normotensive study populations.
To date, more than 10 interventional studies have evaluated the effect of oral vitamin D therapy on blood pressure, although a minority of these trials were designed specifically to evaluate blood pressure effects [48–61]. Most demonstrated no effect of oral vitamin D supplementation on blood pressure or incident hypertension. The largest of these studies was the Women’s Health Initiative (N = 36,282), designed to evaluate fracture and cancer risk in a population of largely 25(OH)D-insufficient women receiving either a small dose of vitamin D3 (400 IU/day) with calcium supplementation, or placebo [55]. After 7 years of follow-up, no changes in blood pressure or incident hypertension were observed. The interpretation of these results was limited by the fact that the dose of vitamin D3 was modest and was not expected to significantly raise 25(OH)D levels [62–64]. In addition, the rate of medication noncompliance was high (about 40%), and about 60% of women in the placebo group also received supplemental vitamin D during the course of the study. The second largest of these randomized studies (N = 438), designed to evaluate the effect of vitamin D3 supplementation on weight loss, included overweight and obese individuals and did not exclude the use of antihypertensive medications [60]. No changes in blood pressure were observed after 1 year of therapy with either a placebo or vitamin D3 at a dosage of 40,000 IU/week or 20,000 IU/week. Because 25(OH)D levels in this study rose from approximately 20 ng/mL to higher than 50 ng/mL in the 40,000 IU/week group, these data argued that reasonable elevations in 25(OH)D did not influence blood pressure. On the other hand, it is debatable whether a 1-year follow-up was sufficient to detect blood pressure outcomes, or whether a largely obese population with heterogeneous use of antihypertensive medications was the ideal study population.
Only two randomized trials to date have been designed to evaluate blood pressure as the primary end point in a population maintained free of antihypertensive therapy [50, 51]. The first showed that, when compared with placebo, a single dose of vitamin D3 100,000 IU in a 25(OH)D deficient population (N = 189) did not lower blood pressure after 5 weeks [50]. The conclusions of this study are limited by its short duration of follow-up, relatively small sample size, and the modest 7 ng/mL rise in 25(OH)D levels (from a baseline of 13 ng/mL), all of which may have hampered the detection of a potential effect. In the second study, elderly 25(OH)D-deficient women who received vitamin D3 800 IU/day for 8 weeks exhibited a small but significant decline in systolic blood pressure (−7 mm Hg) when compared with those who received placebo [51]. Again, the conclusions of this positive study were limited by its short duration of follow-up, small sample size (N = 148), and modest effect size.
Given the lack of well-designed, large-scale intervention studies evaluating the influence of oral vitamin D supplementation on blood pressure, several meta-analyses have attempted to aggregate prior study findings but have produced inconclusive results [65, 66•, 67, 68•]. Witham et al. were able to detect a blood pressure lowering effect associated with vitamin D only when limiting their analyses to those few studies that focused on hypertensive individuals [65], whereas Pittas et al. were able to detect this effect only when limiting their analyses to studies that used higher doses of vitamin D supplementation (>1000 IU/day) [66•]. Burgaz and colleagues detected reduced odds of hypertension when comparing the highest category of 25(OH)D concentration versus the lowest (OR, 0.73; 95% CI, 0.63–0.84) [67], but Elamin et al. detected no blood pressure lowering effect in a meta-analysis evaluating pooled cardiovascular outcomes [68•].
Future Directions
To date, in vitro and animal experiments have provided convincing evidence to speculate the involvement of vitamin D in the pathophysiology of hypertension, possibly via its influence on the RAS, but human studies have not consistently supported this hypothesis. Because most human clinical data stem from cross-sectional or interventional studies with notable design limitations, there is a need for definitive, large-scale randomized controlled trials. To detect whether a true association exists between vitamin D and blood pressure control, future interventional studies ideally should be designed with sufficient sample size, long duration of follow-up, higher vitamin D supplementation doses, and restriction of antihypertensive drug use, and potentially should control for confounders of the RAS such as dietary sodium intake.
The Vitamin D and Omega-3 Trial (VITAL) study (NCT01169259) is a large, randomized controlled trial (N = 20,000) in the United States that opened for recruitment in late 2010 and aims to evaluate the impact of higher-dose vitamin D3 supplementation (2,000 IU/day) on cardiovascular and cancer outcomes over 5 years. The size, duration of follow-up, and higher doses in this study design may allow sufficient power to examine the effect of long-term oral vitamin D3 supplementation on blood pressure. In addition, a subset of about 1,000 randomized participants in this trial will also have 24-hour ambulatory blood pressure assessments at baseline and after 2 years of study. This ongoing trial does have some potential limitations: (1) Participants will be older (>50 years of age), and therefore many may already have significant subclinical vascular disease that may not readily respond to a mild therapy; (2) the main trial of 20,000 participants may include patients taking antihypertensive drugs (including those that may influence the RAS), although the 1,000 participants who undergo 24-hour ambulatory blood pressure monitoring will not be taking antihypertensive medication; (3) the placebo group will be allowed to take up to 800 IU/day of vitamin D3 to reflect current standards of care [69], which could potentially attenuate the difference in effect between the intervention and control groups; and (4) whether an intervention that moderately raises concentrations of 25(OH)D (as opposed to direct treatment with a VDR agonist) can provide therapeutic benefit over 5 years is unclear.
Vitamin D and Kidney Disease
Mechanisms of Association
A history of hypertension is frequent in those who develop kidney disease, yet vitamin D has been implicated as being renoprotective independent of its potential effects on blood pressure. This discussion focuses on the novel independent effects that vitamin D metabolites may have on pathways that affect renal function and consequently the progression of kidney disease.
As in the case of blood pressure, the most prominent mechanism explaining the role of vitamin D in kidney disease has been its negative regulation of the RAS in animal models. In addition to elevations in circulating RAS components, VDR-null mice also display increased expression of renal-vascular renin mRNA [3], supporting experiments that suggest that vitamin D may be an inhibitor of renin gene expression [11]. When subjected to a model of renal injury consisting of unilateral ureteral obstruction, these animals demonstrated more severe kidney injury and fibrosis in the obstructed kidney, when compared with wild-type mice [70]. The administration of an angiotensin-receptor antagonist attenuated the observed injury, suggesting that the deficiency of signaling through the VDR resulted in unfavorably high intrarenal RAS activity and obstructive renal injury. Similarly, mice that are deficient in the activity of the 1-alpha-hydroxylase enzyme have exhibited increased activity of the intrarenal RAS that can be ameliorated with 1,25(OH)2D treatment [9]. When mice with diet-induced obesity were treated with doxercalciferol (1-alpha-hydroxyvitamin D2), the expression of intrarenal renin and angiotensin II type 1 receptors was decreased, with concurrent decrements in proteinuria, podocyte injury, mesangial expansion, and inflammation [71].
Nearly identical renoprotective effects of vitamin D signaling were shown in three studies using mouse models of diabetes (streptozocin-induced [type 1 diabetes] and db/db or KK-Ay/Ta [type 2 diabetes]); these animals develop proteinuria and renal injury with elevated intrarenal RAS activity [72–74]. Given alone, a VDR agonist (1,25[OH]2D or paricalcitol) decreased RAS activity in these mice and attenuated the proteinuria and kidney injury. This effect was reproduced with angiotensin-receptor blocker therapy, but there was a synergistic effect on decreasing RAS activity and preventing further kidney injury when both the VDR agonist and angiotensin-receptor blocker were used in combination [72–74]. These experiments further support the RAS as a key mediator of the renal influence of VDR agonism, but they implicated the involvement of two other pathways. VDR agonist treatment was also observed to inhibit the transforming growth factor beta (TGF-β) [72] and extracellular signal-regulated protein kinase (p-ERK1/2) [74] systems in these animals, suggesting a potential antiproliferative or antiinflammatory renoprotective function of VDR agonism in addition to its negative regulation of the RAS.
To date, the only corollary human mechanistic study showed that higher 25(OH)D concentrations were associated with significantly lower renal-vascular RAS activity in normotensive individuals with normal kidney function [75•]. Although this study was cross-sectional in nature, the results strongly support the mechanism of vitamin D–induced RAS inhibition in humans because it controlled for major modulators of the RAS: dietary sodium was fixed and no antihypertensive medications were included. The Modifiable Effectors of Renin System Activation: Treatment Evaluation (MODERATE) study (NCT01320722), currently enrolling subjects in the United States, will further characterize the biologic relationship between vitamin D supplementation and the renal-vascular RAS in humans by evaluating renal-vascular RAS activity and renal plasma flow before and after randomization to 8 weeks of oral vitamin D2 50,000 IU/week, or placebo. Future studies are still needed to evaluate the mechanism of VDR agonists on the RAS, inflammatory pathways, and proteinuria in diabetes.
Summary of Human Clinical Data
Most human clinical studies in this area have investigated the role of vitamin D in patients with established chronic kidney disease (CKD) of varying stages, a condition usually marked by 1,25(OH)2D insufficiency or deficiency, elevated parathyroid hormone levels, and often concurrent hypertension, diabetes, or both. Observational studies have shown cross-sectional associations between lower 25(OH)D concentrations and the prevalence of CKD and proteinuria [76–78]. In an analysis of more than 15,000 individuals in the Third National Health and Nutrition Examination Survey (NHANES III), for example, de Boer et al. observed that those in the lowest quartile compared with the highest quartile of 25(OH)D levels had an adjusted OR for prevalent albuminuria of 1.37 (P < 0.01) [78]. Ravani et al. observed an inverse and independent longitudinal relationship between 25(OH)D concentrations and the 2-year progression of kidney disease in 168 individuals with non–dialysis-dependent CKD (stages 2–5) [79]. Other studies have reached similar conclusions [80, 81].
High-quality, prospective observational and interventional studies evaluating the role of vitamin D2 or D3 in kidney disease are sparse. Kim et al. recently showed that, in patients with diabetes and CKD already treated with conventional RAS antagonists, vitamin D3 supplementation reduced albuminuria and TGF-β, supporting similar observations in diabetic mice treated with paricalcitol [82]. A meta-analysis of 22 studies of low to moderate quality (17 observational and 5 randomized trials) suggested that vitamin D2 or D3 supplementation did not significantly affect serum creatinine or urine albumin excretion, even though supplementation was sufficient to raise 25(OH)D levels and suppress parathyroid hormone [83]. However, most of the studies included in this aggregate analysis were not designed to evaluate outcomes of disease progression, and because CKD is a state of relative 1-alpha-hydroxylase insufficiency, the effectiveness of vitamin D2 or D3 supplementation on any outcome is unclear.
In contrast to investigations that have evaluated the influence of vitamin D2 or D3 in kidney disease, those that have studied the effect of direct VDR agonism have shown more conclusive and consistent findings. To date, there have been at least five notable interventional studies evaluating the renoprotective benefits of VDR agonist therapy in patients with CKD [84–87, 88•] (Table 1). These five studies have provided human clinical evidence to support the aforementioned animal data demonstrating a reduction in surrogate biochemical measures of renal failure (such as proteinuria) [72–74], but they were not designed to examine whether VDR agonist therapy delayed the progression of CKD or whether it may have a role in the primary prevention of kidney disease. Agarwal et al. synthesized the results of 220 patients from three randomized placebo-controlled studies who had CKD (stages 3 or 4) treated with paricalcitol or placebo for up to 6 months [84]. Paricalcitol therapy reduced automated dipstick proteinuria in 50% of the patients (P < 0.01), whereas proteinuria was reduced in only 25% of those who received placebo. Although this study took into account the use of specific anti-RAS hypertensive medications, it may have been limited by the quantification of proteinuria via an automated dipstick assay. In a smaller interventional study, Alborzi et al. randomized 24 individuals with CKD stage 3 to placebo, paricalcitol 1 μg/day, or paricalcitol 2 μg/day for 1 month and observed reductions in C-reactive protein and albuminuria in the group receiving the higher paricalcitol dose [85]. Evaluating only 10 patients with IgA nephropathy who had proteinuria despite using RAS antagonist pharmacotherapy, Szeto et al. observed a 25% decrease in urine protein-to-creatinine ratio (P < 0.01) within 6 weeks of receiving calcitriol 0.5 μg twice weekly [86]. Individuals who experienced lowering of proteinuria in this study also exhibited a proportional reduction in serum TGF-β, thus providing further support for vitamin D–induced inhibition of the TGF pathway [72]. In support of the Agarwal and Alborzi studies, Fishbane et al. also observed a 17% (P < 0.05) reduction in proteinuria among 61 patients with CKD who were randomized to either paricalcitol or placebo for 6 months [87].
The largest and most recent interventional study by de Zeeuw et al. provides the strongest translation to date of animal to human evidence. In a multinational, double-blind placebo-controlled trial, de Zeeuw and colleagues randomly assigned 281 patients with type 2 diabetes with albuminuria, who were all on pharmacologic RAS antagonist therapy, to 6 months of placebo, paricalcitol 1 μg/day, or paricalcitol 2 μg/day [88•]. Patients treated with paricalcitol 2 μg/day experienced a steady 18–28% decrease in the urinary albumin-to-creatinine ratio when compared with placebo (the primary end point).
Future Directions
In parallel with studies in diabetic mice showing reductions in albuminuria and renal injury with combined VDR agonists and RAS-antagonist therapy [72–74], the results by de Zeeuw et al. [88•] consolidate prior human data reported by Agarwal, Alborzi, Szeto, Fishbane et al. [84–87] and strongly support the use of VDR agonists to reduce proteinuria in diabetic nephropathy that is already being treated with conventional RAS antagonism. Although these studies evaluated strong surrogate measures of progressive CKD (proteinuria), they have limitations: they did not evaluate long-term outcomes (such as the time to progression of disease or dialysis), and they were not designed to investigate whether the mechanism of the beneficial effects of VDR agonists was incremental RAS antagonism, synergistic inhibition of the TGF pathway, or other biologic mechanisms. A better understanding of these queries could have significant implications for the potential use of vitamin D analogues in the primary prevention of kidney disease, or for their continued use in CKD in combination with other pharmacotherapies. Furthermore, studies have associated the use of VDR agonists among non–dialysis-dependent and dialysis-dependent CKD patients with an improved survival benefit, but these studies were not designed to evaluate the underlying mechanism for improved survival [89–94]. Future studies are needed to ascertain whether vitamin D therapy is effective for the primary prevention of CKD, to delay the progression of CKD stages, to reduce the need for dialysis in CKD, and to improve overall survival in CKD. Mechanistic studies in humans, to further identify the biologic pathways by which vitamin D influences renal function, may help to shape efficient trial designs.
Conclusions
Translational research has produced novel insights into the role of vitamin D in vascular diseases such as hypertension and kidney disease. The role of 1,25(OH)2D as a negative inhibitor of the RAS has been supported by mechanistic animal and human studies, but other potential biologic mechanisms to explain the role of vitamin D on vascular function are also emerging. To date, human clinical studies have ascribed a modest but inconsistent blood pressure lowering effect to oral vitamin D therapy and exposure to ultraviolet radiation. On the other hand, they have demonstrated a consistent decrement in surrogate measures of CKD progression, such as proteinuria, with VDR agonists. Future interventional studies with higher vitamin D doses, larger sample sizes, longer durations of follow-up, and study designs that are suited to measure and recognize the mechanism of action, may provide more conclusive data on whether vitamin D therapy may be beneficial in the primary prevention or the treatment of hypertension and kidney disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Holick MF. Viatamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Resnick LM. Calciotropic hormones in salt-sensitive essential hypertension: 1,25-dihydroxyvitamin D and parathyroid hypertensive factor. J Hypertens Suppl. 1994;12(1):S3–9.
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–38.
Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33–41.
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–53.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–76.
Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–32.
Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–9.
Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, et al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem. 2007;282(41):29821–30.
Li YC, Vitamin D. regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327–31.
Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105(5):649–54.
• Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411(17–18):1354–60. This large cohort study of free-living participants on ambient diets showed associations between lower levels of circulating vitamin D metabolites and higher levels of plasma renin activity, providing human data to support the findings in animal models.
• Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12(3):311–9. This detailed physiologic study of individuals on controlled sodium intake (both high and low) showed associations between lower levels of circulating vitamin D metabolites and higher levels of plasma renin activity, providing human data to support the findings in animal models.
Vaidya A, Forman JP, Williams JS. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens. 2011;25(11):672–8.
Shan J, Resnick LM, Lewanczuk RZ, Karpinski E, Li B, Pang PK. 1,25-dihydroxyvitamin D as a cardiovascular hormone. Effects on calcium current and cytosolic free calcium in vascular smooth muscle cells. Am J Hypertens. 1993;6(12):983–8.
Erne P, Bolli P, Burgisser E, Buhler FR. Correlation of platelet calcium with blood pressure. Effect of antihypertensive therapy. N Engl J Med. 1984;310(17):1084–8.
Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol. 2010;298(1):F1–F11.
Naftilan AJ, Oparil S. The role of calcium in the control of renin release. Hypertension. 1982;4(5):670–5.
Bukoski RD, DeWan P, McCarron DA. 1,25 (OH)2 vitamin D3 modifies growth and contractile function of vascular smooth muscle of spontaneously hypertensive rats. Am J Hypertens. 1989;2(7):553–6.
Talmor Y, Bernheim J, Klein O, Green J, Rashid G. Calcitriol blunts pro-atherosclerotic parameters through NFkappaB and p38 in vitro. Eur J Clin Invest. 2008;38(8):548–54.
Talmor Y, Golan E, Benchetrit S, Bernheim J, Klein O, Green J, et al. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol. 2008;294(5):F1059–64.
Wakasugi M, Noguchi T, Inoue M, Kazama Y, Tawata M, Kanemaru Y, et al. Vitamin D3 stimulates the production of prostacyclin by vascular smooth muscle cells. Prostaglandins. 1991;42(2):127–36.
Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94(10):4023–30.
Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24(5):557–62.
Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57(1):63–9.
Yiu YF, Chan YH, Yiu KH, Siu CW, Li SW, Wong LY, et al. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):E830–5.
Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58(2):186–92.
Vaidya A, Forman JP, Underwood PC, Hopkins PN, Williams GH, Pojoga LH, et al. The influence of body mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin D and adiponectin in Caucasian men. Eur J Endocrinol. 2011;164(6):995–1002.
Vaidya A, Pojoga L, Underwood PC, Forman JP, Hopkins PN, Williams GH, Williams JS. The association of plasma resistin with dietary sodium manipulation, the renin-angiotensin-aldosterone system, and 25-hydroxyvitamin D3 in human hypertension. Clin Endocrinol (Oxf). 2011;74(3):294–9.
Vaidya A, Williams JS, Forman JP. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring). 2012;20(1):186–91.
Thomopoulos C, Daskalaki M, Papazachou O, Rodolakis N, Bratsas A, Papadopoulos DP, et al. Association of resistin and adiponectin with different clinical blood pressure phenotypes. J Hum Hypertens. 2011;25(1):38–46.
Yiannikouris F, Gupte M, Putnam K, Cassis L. Adipokines and blood pressure control. Curr Opin Nephrol Hypertens. 2010;19(2):195–200.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713–9.
Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57(2):298–305.
Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87(1):136–41.
Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62(9):1079–89.
Burgaz A, Byberg L, Rautiainen S, Orsini N, Hakansson N, Arnlov J, et al. Confirmed hypertension and plasma 25(OH)D concentrations amongst elderly men. J Intern Med. 2011;269(2):211–8.
Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Viatamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30(6):1549–55.
Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261(6):558–65.
Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–9.
Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828–32.
Griffin FC, Gadegbeku CA, Sowers MR. Vitamin D and subsequent systolic hypertension among women. Am J Hypertens. 2010;24(3):316–21.
Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55(3):792–8.
Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2 Pt 1):150–6.
Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709–10.
Scragg R, Wishart J, Stewart A, Ofanoa M, Kerse N, Dyall L, et al. No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. J Hypertens. 2011;29(9):1749–56.
Orwoll ES, Oviatt S. Relationship of mineral metabolism and long-term calcium and cholecalciferol supplementation to blood pressure in normotensive men. Am J Clin Nutr. 1990;52(4):717–21.
Pan WH, Wang CY, Li LA, Kao LS, Yeh SH. No significant effect of calcium and vitamin D supplementation on blood pressure and calcium metabolism in elderly Chinese. Chin J Physiol. 1993;36(2):85–94.
Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49(9):640–6.
Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633–7.
Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9.
Major GC, Alarie F, Dore J, Phouttama S, Tremblay A. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85(1):54–9.
Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–5.
Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52(5):847–55.
Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7.
Daly RM, Nowson CA. Long-term effect of calcium-vitamin D(3) fortified milk on blood pressure and serum lipid concentrations in healthy older men. Eur J Clin Nutr. 2009;63(8):993–1000.
Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48(6):349–54.
Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26(1):19–27.
Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267(5):462–72.
Vaidya A, Forman JP. Vitamin D and hypertension: current evidence and future directions. Hypertension. 2010;56(5):774–9.
Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008;88(6):1535–42.
Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, et al. Estimation of the dietary requirement for vitamin D in free-living adults >=64 y of age. Am J Clin Nutr. 2009;89(5):1366–74.
Lehtonen-Veromaa M, Mottonen T, Nuotio I, Irjala K, Viikari J. The effect of conventional vitamin D(2) supplementation on serum 25(OH)D concentration is weak among peripubertal Finnish girls: a 3-y prospective study. Eur J Clin Nutr. 2002;56(5):431–7.
Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27(10):1948–54.
• Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152(5):307–14. This recent meta-analysis of randomized trials that evaluated the effects of vitamin D supplementation on blood pressure found significant heterogeneity among trials. The summary effects were not significant, but there was a modest effect of high-dose vitamin D on lowering diastolic blood pressure.
Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–45.
• Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–42. This recent meta-analysis of randomized trials that evaluated the effects of vitamin D supplementation on blood pressure found no overall effect, although there was significant heterogeneity among trials.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21(6):966–73.
Wang XX, Jiang T, Shen Y, Santamaria H, Solis N, Arbeeny C, et al. Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am J Physiol Renal Physiol. 2011;300(3):F801–10.
Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 2010;77(11):1000–9.
Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105(41):15896–901.
Ohara I, Tanimoto M, Gohda T, Yamazaki T, Hagiwara S, Murakoshi M, et al. Effect of combination therapy with angiotensin receptor blocker and 1,25-Dihydroxyvitamin D3 in Type 2 diabetic nephropathy in KK-Ay/Ta mice. Nephron Exp Nephrol. 2011;117(4):e124–32.
• Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55(5):1283–8. This detailed physiologic study of individuals on a controlled sodium intake found that lower levels of 25-hydroxyvitamin D were associated with activation of the intra–renal tissue renin-angiotensin system, providing human data in support of animal studies that found renal effects of vitamin D synergistic with pharmacologic renin angiotensin inhibition.
Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukroun G, et al. Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1128–35.
Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24(5):503–10.
de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2007;50(1):69–77.
Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95.
de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6(9):2141–9.
Ureña-Torres P, Metzger M, Haymann JP, Karras A, Boffa JJ, Flamant M, NephroTest Study Group, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. 2011;58(4):544–53.
Kim MJ, Frankel AH, Donaldson M, Darch SJ, Pusey CD, Hill PD. Oral cholecalciferol decreases albuminuria and urinary TGF-β1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int. 2011;80(8):851–60.
Kandula P, Dobre M, Schold JD, Schreiber Jr MJ, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011;6(1):50–62.
Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68(6):2823–8.
Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52(2):249–55.
Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51(5):724–31.
Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis. 2009;54(4):647–52.
• de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–51. This large randomized trial found that paricalcitol therapy (an analogue of 1,25-dihyroxyvitamin D) reduced albuminuria in patients with type 2 diabetes and nephropathy, providing human data to support findings in animal models.
Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–56.
Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19(8):1613–9.
Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070–8.
Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19(1):179–84.
Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo Jr CA, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115–25.
Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, et al. Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol. 2008;19(7):1379–88.
Acknowledgments
The authors are funded by National Institutes of Health grants F32HL104776 (AV), 5R01HL105440 (JF), and American Heart Association grant 2009A050171 (JF).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaidya, A., Forman, J.P. Vitamin D and Vascular Disease: The Current and Future Status of Vitamin D Therapy in Hypertension and Kidney Disease. Curr Hypertens Rep 14, 111–119 (2012). https://doi.org/10.1007/s11906-012-0248-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-012-0248-9