Abstract
Background
Studies have suggested that vitamin D may be important for both insulin sensitivity and insulin secretion, and that supplementation with vitamin D may subsequently prevent development of type 2 diabetes.
Aim of the study
The objective of the current study was to test the hypothesis that supplementation with vitamin D would improve glycaemic control in subjects with type 2 diabetes.
Methods
Thirty-six subjects with type 2 diabetes, treated with metformin and bed-time insulin, were randomised to supplementation with cholecalciferol (40,000 IU per week) versus placebo for 6 months. Thirty-two subjects participated throughout the entirety of the study. Fasting blood samples were drawn before and at the end of the 6 month study without the previous bed-time insulin injection. The insulin and metformin doses were not changed throughout the study.
Results
After 6 months, the fasting glucose, insulin, C-peptide, fructosamine, and HbA1c levels were not significantly different from baseline values. In addition, changes in these parameters (values at 6 months minus values at baseline) did not differ between the vitamin D and the placebo group.
Conclusions
We were not able to demonstrate that vitamin D supplementation had a significant effect on glucose metabolism in subjects with type 2 diabetes but without vitamin D deficiency. Further studies are needed in larger groups of subjects with type 2 diabetes or impaired glucose tolerance, who also exhibit low serum 25-hydroxyvitamin D levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D increases intestinal calcium absorption and is of vital importance for skeletal health. In addition, vitamin D appears to have a number of extra-skeletal effects, as low serum levels of 25-hydroxyvitamin D (25(OH)D) have been associated with cardiovascular diseases, cancer, and immunological diseases [28, 29].
There are also indications that vitamin D plays a significant role in glucose metabolism. Thus, we have recently performed a study where measures of calcium metabolism were related to insulin sensitivity, as evaluated with a hyperglycaemic clamp [12]. In a multiple regression model, we found serum 25(OH)D to be a stronger predictor of insulin sensitivity than all other variables tested, including BMI. This is in accordance with other glucose clamp studies where insulin sensitivity has been related to serum levels of 25(OH)D [7, 15]. In addition, in vitro and animal studies have found a reduced insulin secretion when the 25(OH)D level is low, followed by a normalization after vitamin D supplementation [6, 21].
Therefore, it is plausible that supplementation with vitamin D might increase both insulin secretion and sensitivity in humans. This could be of particular interest for subjects with type 2 diabetes, as most of these subjects have a combination of reduced insulin secretion and insulin resistance [14]. In accordance with this insight, it was recently reported that serum 25(OH)D levels in a group of 524 non-diabetic subjects were inversely associated with glycaemia and insulin resistance 10 years later [8].
However, as pointed out in a review by Pittas et al. [25] the available evidence is currently only indicative of a clinical role for vitamin D supplementation in the prevention or treatment of type 2 diabetes. As a result, intervention studies with vitamin D are highly needed [27]. In the present study, we randomised a group of subjects with type 2 diabetes to supplementation with vitamin D (40,000 IU per week) versus placebo for 6 months, with improvement in haemoglobin A1c (HbA1c) as the primary objective.
Methods
Subjects
Males and females with type 2 diabetes, age 21–75, with disease duration for at least 1 year, on treatment with medium long-acting insulin at bed-time in addition to metformin during the day, with HbA1c levels stable in the range of 7.0–9.5% for the last 3 months, and serum calcium <2.55 mmol/L, were recruited from the outpatient clinic at the University Hospital of North Norway. Subjects with a history of coronary infarction, angina pectoris, stroke, renal stone disease, or sarcoidosis were not included.
Protocol
At inclusion all previous supplementations with vitamin D (including cod liver oil) were discontinued. There was no wash-out period. Subjects were randomised into two groups, stratified by gender and smoking status: the vitamin D group was to take two capsules of vitamin D (20,000 IU cholecalciferol per capsule (Decristol®, Jenapharm, Jena, Germany)) per week, while the placebo group was to take two placebo capsules per week. The intervention or treatment period lasted 6 months. The placebo capsules, purchased from Hasco-lek (Wroclaw, Poland) were identical to the vitamin D capsules in regards to size and shape. The subjects were supplied with a new supply of medication after 3 months. Unused capsules were returned and counted. The subjects were classified as current smokers or non-smokers. During the trial, the subjects were instructed not to change their insulin or metformin dosages.
Measurements
The subjects did not take their bed-time insulin dose the evening before the first and last visits of the 6 month study period, which were performed in the morning in the fasting state. Blood samples were drawn for determination of plasma glucose, serum insulin, C-peptide and serum fructosamine, HbA1c, serum calcium, PTH, 25(OH)D, 1,25-dihydroxyvitamin D (1,25(OH)2D), total cholesterol, triglycerides, HDL- and LDL-cholesterol. After resting for 15 min, blood pressure was measured three times, 2 min apart, with an automatic device (Dinamap Vital Signs Monitor 1846; Criticon Inc, Tampa, FL). The mean of the last two measurements was used in the analyses. Height, weight, and waist and hip circumference were measured wearing light clothing and no shoes. Urine was collected over a 24 h period to calculate the calcium excretion.
Fasting plasma glucose, serum insulin, C-peptide, fructosamine and HbA1c [11], serum calcium, creatinine and PTH [10], and serum total cholesterol, triglycerides, HDL- and LDL-cholesterol [9] were measured as previously described. Serum levels of 25(OH)D3 were determined by immunometry (electrochemiluminescence: ECLIA) using an automated clinical chemistry analyser (Modular E170, Roche Diagnostics®, Mannheim, Germany). According to the manufacturer, this assay has, for total analytical precision, a coefficient of variation ≤7.8% as judged in any of three different concentrations (48.6, 73.8, and 177.0 nmol/L). The cross-reactivity with 25(OH)D2 was <10% and the analytical sensitivity was 10 nmol/L. At present, the laboratory has no reference values for 25(OH)D3, but the manufacturer provides a population based reference range of 27.7–107.0 nmol/L for adults as a guideline. Serum 1,25(OH)2D was measured at the end of the study as previously described [13].
The HOMA method was used to calculate the basal insulin sensitivity [(fasting plasma insulin (pmol/L) × fasting plasma glucose (mmol/L))/135] and secretion [(fasting plasma insulin (pmol/L) × 3.33)/(fasting plasma glucose (mmol/L) 3.5)] [19].
The trial was registered at ClinicalTrials.gov (NCT00400491).
Statistical analyses
The primary outcome was change in HbA1c after 6 months. In the power calculation we used an SD of 0.6% for HbA1c (based on data from our outpatient clinic at the University Hospital of North Norway in subjects with type 2 diabetes having an HbA1c in the range of 7.5–9.5%) and a clinically relevant difference for HbA1c of 0.5%. If the study started with 70 subjects and we assumed that 30 in each group completed the study, we would have an 89% chance of finding a statistically significant (P < 0.05) and clinically relevant difference between the two groups. Secondary endpoints were measures of glucose and calcium metabolism as shown in Table 1.
Comparisons between the groups at baseline levels were done with the Student’s t-test, and comparisons between changes from baseline (delta values = 6 month values minus baseline) were done with ANCOVA using a general linear model with the delta value as the dependent variable and gender, age, BMI, time of year for baseline value, and the baseline value in question, as covariates. All statistical tests were performed two-sided, and P-value < 0.05 was considered statistically significant. The Statistical Package for Social Sciences version 15.0 was used for all statistical analyses (SPSS Inc., Chicago, Ill., USA).
Ethics
The study was approved by the Regional Ethics Committee. All participants gave written informed consent prior to the study.
Results
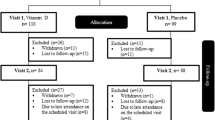
Thirty-six subjects were included in the study. The inclusion period was from June 2006 to January 2007. Four subjects dropped out of the study, all in the vitamin D group. One of them, a 68-year-old female had a serum calcium level of 2.48 mmol/L and serum PTH of 8.2 pmol/L at baseline. After 3 months, the subject had an episode with fever, an increase in serum calcium to 2.66 mmol/L, and exhibited PTH level of 4.7 pmol/L. She was withdrawn from the study, and serum calcium normalised. The baseline characteristics of the remaining 32 subjects are shown in Table 1. Twelve subjects (6 in each group) were included during the summer months. The serum 25(OH)D levels in these 12 subjects were 66.7 nmol/L ± 18.3, while the remaining 20 subjects exhibited levels of 54.8 ± 15.9 nmol/L (P = 0.06). There were no significant differences between the two treatment groups at baseline. At the end of the study, all subjects continued the same bed-time insulin and day time metformin dosage regimen as they had participated in prior to inclusion in this study. Concerning the primary endpoint, changes in HbA1c from baseline (delta value), the two groups did not differ (–0.2 ± 0.9% and –0.2 ± 0.5% for vitamin D group and placebo group, respectively; P = 0.90). Regarding delta values for the secondary endpoints, there were no significant differences between the two groups with the exception of a significant increase in serum 25(OH)D and a significant decrease in serum PTH in the vitamin D group compared with the placebo group. Furthermore, the fasting plasma glucose, serum insulin, C-peptide, fructosamine and HbA1c levels were almost identical to baseline values after 6 months in both treatment groups. There was an increase in the urinary calcium excretion in the vitamin D group, but not significantly different from the placebo group (Table 1). At the end of the study the serum 1,25(OH)2D levels were significantly higher in the vitamin D group than the placebo group (94.5 ± 20.5 and 67.9 ± 20.1 pmol/L, respectively (P = 0.001)). The compliance rate was 98 and 97% in the vitamin D and placebo groups, respectively.
Apart from the 68 year old female who was excluded after 3 months, none of the subjects developed hypercalcaemia (serum calcium > 2.60 mmol/L).
Discussion
In the present study, we found that supplementation with 40,000 IU cholecalciferol for 6 months did not lower the HbA1c levels in subjects with type 2 diabetes, nor was there any improvement in parameters of insulin secretion or resistance.
There are several mechanisms whereby vitamin D may influence insulin secretion and sensitivity. For instance, the insulin producing beta-cells have receptors for 1,25(OH)2D, and they even have the enzyme necessary for hydroxylating 25(OH)D to the active form 1,25(OH)2D [4]. In addition, insulin secretion is a calcium dependent process and can therefore indirectly be influenced by vitamin D, which through its effect on calcium absorption, is an important regulator of the calcium balance. Thus, as suggested by Pittas et al. [25] inadequate vitamin D levels may alter the balance between the extracellular and intracellular calcium pools in the beta-cells, and consequently interfere with normal insulin release.
Regarding insulin sensitivity, it appears that vitamin D may stimulate the expression of the insulin receptor in peripheral tissues and thereby increase glucose transport [18]. Similar to insulin release, insulin mediated processes are calcium dependent [22] and therefore may be indirectly influenced by the subject’s vitamin D status. Thus, it is not surprising that epidemiological studies have found a negative association between serum levels of 25(OH)D and blood glucose both in the fasting state [20] as well as after a glucose challenge [1].
In accordance with this, it was found in the Women’s Health Study, which included 10,066 women 46 years or older, that those with a vitamin D intake below 159 IU per day had a 35% higher prevalence of type 2 diabetes as compared to those with an intake above 511 IU per day [16]. Data from the American Nurses’ Health Study, where 83,779 women were followed for 20 years, also indicated that a high intake of vitamin D may protect against the development of diabetes. Thus, among those with the highest vitamin D intake from supplements there was a relative risk of 0.87 for developing diabetes as compared to those with the lowest vitamin D intake from supplements [24]. A similar observation was also made by Pittas et al. in a post-hoc analysis on blood glucose and insulin levels in 314 subjects randomised to calcium and vitamin D supplementation versus placebo for the treatment of osteoporosis [26]. Among these subjects, 92 had impaired fasting glucose at time of inclusion in the study. After 3 years, those with impaired fasting glucose randomised to calcium and vitamin D had a significantly lower increase in fasting blood glucose in comparison to those randomised to placebo (0.02 mmol/L versus 0.34 mmol/L). Furthermore, there are reports on improvement in glucose tolerance after vitamin D supplementation in vitamin D deficient subjects with impaired glucose tolerance [5].
On the other hand, and in accordance with our results, Ljunghall et al. found no effect on glucose tolerance or insulin secretion in 65 middle-aged men with impaired glucose tolerance and normal serum 25(OH)D levels, when given 0.75 μg alpha-calcidiol daily or placebo for 3 months [17]. Similarly, in a study on 20 subjects with type 2 diabetes by Orwoll et al., 1 μg 1,25(OH)2D for 4 days had no effects on fasting or stimulated glucose, insulin, or C-peptide concentrations [23].
The present study has many limitations and there could be several explanations for the lack of effect of vitamin D on glucose metabolism observed. Firstly, we were only able to include 36 subjects and our power calculation was based on a group of 70 subjects. With the present number included, we would only have a 70% chance of finding a statistically significant difference in HbA1c of 0.5%, which was threshold considered as clinically relevant. The included subjects were treated with insulin and metformin, which might have masked a subtle effect of vitamin D. Although there was a significant decrease in serum PTH in the vitamin D group, the increase in urinary calcium secretion was not significantly higher than in the placebo group, indicating that the effect on calcium metabolism was modest. We did not have data on calcium intake, and calcium supplementation was not given. This could be of importance as there are indications that this is essential if glucose intolerance is to be normalised, especially in cases where vitamin D is depleted [2]. We evaluated the insulin sensitivity and secretion based on fasting levels only, which is not as accurate as the glucose clamp method. Furthermore, we only measured serum 1,25(OH)2D at the end of the study. However, at that time point, the serum 1,25(OH)2D levels were significantly higher in the vitamin D group, and the lack of effect on the glucose metabolism can therefore not be ascribed to a lower conversion to the active form of vitamin D in this group. Finally, the effect of vitamin D supplementation is probably most pronounced in vitamin D deficient subjects, and none of our patients had a serum 25(OH)D level below 25 nmol/L.
On the other hand, our study also has strength in that we used a high vitamin D dose of 40,000 IU per week, which is considerably larger than in most other studies. This resulted in serum 25(OH)D levels which should be sufficient for an optimal outcome [3].
One of our subjects developed a transient increase in serum calcium after 3 months and was withdrawn from the study. In retrospect, it could be argued that her baseline serum calcium and PTH values indicated a mild form of primary hyperparathyroidism, and accordingly should not have been included in the study. None of the other subjects developed hypercalcaemia, but the study was too small to make a conclusion in regards to safety and the present vitamin D dose.
In conclusion, we were not able to demonstrate an effect on glucose metabolism in subjects with type 2 diabetes after supplementation with vitamin D. However, it should be emphasised that the number of patients was too low to draw definitive conclusions. Therefore, there is a need for larger studies with longer duration and graded doses of vitamin D. In particular, the effect of vitamin D supplementation should be tested at an earlier stage in the diabetes disease process, such as in subjects with impaired glucose tolerance and with low serum 25(OH)D levels.
References
Baynes KC, Boucher BJ, Feskens EJ, Kromhout D (1997) Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia 40:344–347
Beaulieu C, Kestekian R, Havrankova J, Gascon-Barré M (1993) Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes 42:35–43
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, Hewison M (2004) Expression of 25-hydroxyvitamin D3–1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 89–90:121–125
Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ (1995) Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 38:1239–1245
Cade C, Norman AW (1986) Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 119:84–90
Chiu KC, Chu A, Go VL, Saad MF (2004) Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79:820–825
Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ (2008) Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes 57:2619–2625
Iqbal A, Jorde R, Figenschau Y (2006) Serum lipid levels in relation to serum thyroid-stimulating hormone and the effect of thyroxine treatment on serum lipid levels in subjects with subclinical hypothyroidism: the Tromsø Study. J Int Med 260:53–61
Jorde R, Svartberg J, Sundsfjord J (2005) Serum parathyroid hormone as a predictor of increase in systolic blood pressure in men. J Hypertens 23:1639–1644
Jorde R, Hagen T (2006) Screening for diabetes using HbA1c in elderly subjects. Acta Diabetol 43:52–56
Kamycheva E, Jorde R, Figenschau Y, Haug E (2007) Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J Endocrinol Invest 30:126–132
Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R (2008) Serum 1, 25-dihydroxy vitamin D is inversely associated with body mass index. Eur J Nutr 47:87–91
Leahy JL (2005) Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209
Lind L, Pollare T, Hvarfner A, Lithell H, Sørensen OH, Ljunghall S (1989) Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res 11:141–147
Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM (2005) Dietary calcium, vitamin D and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care 28:2926–2932
Ljunghall S, Lind L, Lithell H, Skarfors E, Selinus I, Sørensen OH, Wide L (1987) Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance-a prospective randomized double-blind study. Acta Med Scand 222:361–367
Maestro B, Campión J, Dávila N, Calle C (2000) Stimulation by 1, 25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 47:383–391
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Need AG, O’Loughlin PD, Horowitz M, Nordin BE (2005) Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol 62:738–741
Norman AW, Frankel JB, Heldt AM, Grodsky GM (1980) Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209:823–825
Ojuka EO (2004) Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc 63:275–278
Orwoll E, Riddle M, Prince M (1994) Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr 59:1083–1087
Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB (2006) Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29:650–656
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
Pittas AG, Harris SS, Stark PC, Dawson-Hughes B (2007) The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 30:980–986
Scragg R (2008) Vitamin D and type 2 diabetes: are we ready for a prevention trial? Diabetes 57:2565–2566
Zittermann A (2003) Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 89:552–572
Zittermann A (2006) Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 92:39–48
Acknowledgments
The present study was supported by a grant from the Norwegian Diabetes Association. The superb assistance by Annika Gustafsson and the other nurses at the Clinical Research Unit and by Inger Myrnes and Astrid Lindvall at the Department of Medical Biochemistry, University Hospital of North Norway, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jorde, R., Figenschau, Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr 48, 349–354 (2009). https://doi.org/10.1007/s00394-009-0020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0020-3