Abstract
Hypertension is extremely common in patients with end-stage renal disease who are receiving hemodialysis, and cardiovascular disease remains the leading cause of death in these patients. However, optimal blood pressure management strategies in this high-risk population are still controversial. This review first discusses the complex association of systolic blood pressure with clinical outcomes in patients on hemodialysis, with a focus on several recent studies. Next, it updates the reader on issues related to optimal timing and methods of blood pressure measurement, appropriate blood pressure targets, and pharmacologic and nonpharmacologic hypertension treatment strategies for patients on hemodialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is ubiquitous in patients with end-stage renal disease (ESRD) on hemodialysis, with prevalence estimates of up to 90% [1]. In the general population, the risk of adverse cardiovascular events increases linearly with increasing systolic blood pressure (SBP) [2]. However, the relationship among SBP and clinical outcomes in patients on hemodialysis is much more complex, owing to issues such as wide fluctuations of volume status and sodium balance. The purpose of this review is to update the reader about ongoing controversies in relation to patients on hemodialysis, including blood pressure (BP) measurement, targets, and treatment, with recent advances highlighted. This review focuses on SBP. Pulse pressure, vascular calcification, and vascular stiffness, though important concepts in ESRD, are beyond the scope of this review, and the reader is directed to recent articles on these topics [3, 4].
Blood Pressure and Mortality in Hemodialysis
Observational studies of over one million persons in the general population have consistently demonstrated that the risk of adverse outcomes such as myocardial infarction and stroke increases linearly with increasing SBP [2]. However, the same linear relationships have not been seen in studies of patients with ESRD (Table 1). Foley et al. followed a cohort of 432 patients with ESRD for an average of 41 months, and showed that although higher predialysis mean arterial pressure was associated with the development of left ventricular hypertrophy and heart failure, each 10 mmHg lower predialysis mean arterial pressure was associated with a 36% higher risk of death [5]. Subsequent observational studies over the past decade in a variety of ESRD cohorts have confirmed the “U-shaped” or “reverse J-shaped” relationship between BP and mortality, with the highest risk of death at lower predialysis and postdialysis SBP (generally <130 mmHg) and only a slight increase, if any, in the risk of death at the highest ranges of SBP (>180 mmHg) [6–9].
Further complicating matters is the observation that the association of SBP and mortality changes over time. Stidley et al. [10] demonstrated that in the first 2 years after initiation of hemodialysis, predialysis SBP less than 120 mmHg was associated with a twofold to threefold higher risk of mortality compared with predialysis SBP of 140 to 149 mmHg, but this association was no longer observed in years 3 and 4 of hemodialysis. Similarly, Mazzuchi et al. [11] found that higher predialysis SBP (>160 mmHg) was associated with an increased risk of mortality only after 5 years of follow-up in a cohort of prevalent hemodialysis patients in Uruguay.
Not only does time modify the association of BP and mortality in hemodialysis, but age and diabetes mellitus status may as well. Myers et al. [12••], in a cohort of 16,283 incident patients on hemodialysis followed for a median of 1.5 years, showed that lower predialysis SBP (<140 mmHg) was associated with an increased mortality risk in the overall cohort, consistent with previous studies. However, when they stratified the cohort by decade of age, the association of lower predialysis SBP with higher mortality risk held true only for patients older than 50 years. Myers et al. also found that the association of lower predialysis SBP with higher mortality risk was stronger in patients with diabetes mellitus than for patients without diabetes mellitus. This study demonstrates that a “one size fits all” approach to BP management may not be appropriate for patients on hemodialysis.
Given the observational nature of the previous studies, reducing SBP cannot be assumed to cause adverse clinical outcomes. Instead, lower SBP may act as a potent marker of concomitant diseases predisposing to death, and recent publications have tried to extend the findings of previous studies by better adjusting for residual confounding. Chang et al. [13], using data from the Hemodialysis (HEMO) study, improved case-mix adjustment by including time-varying comorbidity assessments along with time-varying SBP assessments in the analyses, but still demonstrated that lower predialysis SBP (<120 mmHg) was associated with a twofold higher risk of all-cause mortality compared with the reference group (SBP 140–159 mmHg). In contrast, higher predialysis SBP (≥180 mmHg) had no significant association with mortality. A mortality rate of 10% per year is a suggested “threshold” above which an independent effect of higher SBP on mortality is difficult to demonstrate [14], and two recent studies were conducted in healthier ESRD populations with lower overall mortality rates. Bos et al. [15] examined incident dialysis patients without cardiovascular disease in the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) cohort; Molnar et al. [16] examined dialysis patients with polycystic kidney disease (PKD), who had lower mortality rates than the non-PKD ESRD patients (6.4% per year vs. 12.3% per year, respectively). In both of these studies, however, lower predialysis SBP (<120 mmHg), and not higher predialysis SBP, was again associated with risk of mortality that was higher than in the reference groups.
In summary, observational studies in patients on hemodialysis have consistently shown a strong association between lower SBP and higher risk of mortality. In contrast, associations of higher SBP with mortality have been inconsistent and generally weaker than the associations observed for lower SBP.
Blood Pressure Measurement in Hemodialysis
Difficulties with accurate BP measurement may partly explain why higher SBP has not been consistently linked to adverse clinical outcomes in hemodialysis. Accurate BP measurement is fundamental for clinical practice, yet there is still no consensus on which BP measurements to use to define and treat hypertension in patients on hemodialysis. Routinely measured predialysis and postdialysis BP (i.e., measured without any standardization) in the dialysis center are readily available and are therefore most commonly used to guide practice. However, routine predialysis and postdialysis BP measurements overestimate SBP by a variable amount when compared with interdialytic ambulatory BP measurements, often considered the most accurate method of BP measurement [17–19].
Given the inaccuracy of routine predialysis and postdialysis SBP and the practical issues limiting the widespread use of interdialytic ambulatory BP monitoring, alternative BP measurements have been explored. Several studies have examined the performance of standardized in-center predialysis BP measurements (i.e. an average of two or three seated BP measurements taken by trained research nurses after a 5-minute rest). When compared with routine predialysis BP measurements, the standardized SBP was lower by an average of 14.3 mmHg [20], and it also better predicted the presence of left ventricular hypertrophy [21, 22]. However, standardized in-center BP measurements may still be too cumbersome to implement widely. Another recent study demonstrated that the median routinely measured intradialytic SBP from a single mid-week hemodialysis session, which is fairly easy to obtain, was able to diagnose hypertension with reasonable sensitivity and specificity when compared with ambulatory BP measurements [23]. Whether median SBP will perform better than other BP measurements in terms of predicting outcomes and guiding therapy on an individual patient level remains to be evaluated in future longitudinal studies.
A second approach has focused on BP measured at home, rather than in the dialysis center. In a single-center study of 150 patients on hemodialysis, SBP was measured at home several times daily for 1 week and averaged; these patients also had 44-hour interdialytic ambulatory BP monitoring [24]. After a median follow-up of 24 months, higher quartiles of home SBP were associated with a significantly higher risk of all-cause and cardiovascular mortality. In contrast, no significant association was observed with either routine or standardized in-center SBP measurements. This study continued for 4 additional years, and follow-up results with a total of 326 patients followed for a median of 29 months were recently published [25•]. In this study, higher quartiles of home SBP were also associated with higher risk of all-cause mortality, whereas routine in-center SBP had no association with mortality. The lowest mortality risk was associated with a home SBP between 120 and 130 mmHg. Interestingly, the relationship of home SBP and mortality resulted in a W-shape curve, with higher risks of mortality not only at the lower and higher extremes of BP, but also at mid-range SBPs of approximately 150 mmHg. The significance of this unexpected finding could not be elucidated in this cross-sectional study and has yet to be replicated.
In summary, there is no consensus on the optimal method of BP measurement for patients on hemodialysis that balances accuracy with practicality. Though some nephrologists have called for home BP measurements to replace in-center BP measurements for clinical decision making [26], many others feel that these methods of BP measurement are not feasible for most patients [27]. Therefore, current national clinical practice guidelines [28] still focus on routinely measured predialysis and postdialysis BP measurements, though it is recommended that these BP measurements be used “with caution and with the knowledge that these are inferior” [27] to ambulatory or home BP measurements.
Optimal Blood Pressure Targets in Hemodialysis
As noted in the preceding sections, the association of SBP with mortality in hemodialysis is complex, and the best methods and timing for BP measurement are unclear. Given these limitations, it is not surprising that optimal BP targets in hemodialysis remain elusive. National clinical practice guidelines suggest targeting a predialysis BP of less than 140/90 mmHg and postdialysis BP of less than 130/80 mmHg, but they acknowledge that the evidence to support this recommendation is weak [28]. The United Kingdom Renal Association recommended similar predialysis and postdialysis BP targets in 2002 [29]. However, there is some concern that these BP targets, largely extrapolated from observational studies and/or data from non-ESRD patients, could be associated with harm for some patients on hemodialysis. Davenport et al. conducted an audit of BP control and symptomatic intradialytic hypotension and noted that intradialytic hypotension was more frequent in dialysis centers that had a higher percentage of patients achieving the postdialysis BP targets [30]. Intradialytic hypotension, in turn, has been associated with an increased risk of the myocardial stunning phenomenon [31] and death [32–34].
Specific BP targets for patients on hemodialysis were removed in the United Kingdom’s 2007 update to the clinical guidelines [29]. In 2009, a Kidney Disease: Improving Global Outcomes (KDIGO) conference of 50 international experts met to address the ongoing controversies regarding BP management in dialysis [27]. Aside from recommending that predialysis SBP greater than 200 mmHg be treated aggressively, the KDIGO conference did not provide more specific BP targets, citing the lack of evidence. Clearly, future prospective randomized trials of BP targets in hemodialysis are needed to help inform clinical practice.
Antihypertensive Medications in Hemodialysis
Despite the association of lower SBP with higher risk of mortality from observational studies, lower SBP has not been proven to cause adverse clinical outcomes, and antihypertensive treatment should not be withheld from patients on hemodialysis. In fact, several recent systematic reviews and meta-analyses have demonstrated a benefit of treatment with antihypertensive medications in patients with ESRD on dialysis. Tai et al. conducted a meta-analysis that examined angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in hemodialysis [35]. Eight randomized clinical trials were included in their analysis; three studies examined cardiovascular events as the primary outcome, and five studies examined left ventricular mass index as the primary outcome. Although ACEI and ARB use was associated with reduced left ventricular mass, it was not associated with a lower risk of cardiovascular events in the pooled analysis. However, the included studies, being relatively small and of limited duration, may have been underpowered to detect these differences.
Agarwal and Sinha [36•] identified five eligible randomized clinical trials of various antihypertensive agents in 1,202 patients on hemodialysis (including the three studies that examined cardiovascular events in the meta-analysis by Tai et al. [35]) and found that antihypertensive therapy reduced the risk for cardiovascular events, compared with controls (HR, 0.62; 95% CI, 0.45–0.86). There was also a suggestion of benefit for all-cause mortality with active treatment, although the results were not statistically significant (HR, 0.77; 95% CI, 0.56–1.04). Notably, none of the five studies included in this meta-analysis had point estimates that suggested harm with active treatment. Heerspink et al. [37•] also conducted a meta-analysis with five of the same studies used by Agarwal and Sinha [36•], plus three additional studies, one of which was in patients on peritoneal dialysis. The meta-analysis by Heerspink et al. likewise demonstrated a lower pooled risk of cardiovascular events, but it also showed a significantly lower risk of all-cause and cardiovascular mortality in treated patients versus control patients. Overall, the SBP in the active treatment groups was 4.5 mmHg lower than the SBP in the control groups.
In summary, treatment with antihypertensive medications in patients on hemodialysis appears to be beneficial. However, it is important to note that none of the studies targeted a specific SBP, nor did any of the studies compare different levels of SBP control. Rather, the patients in the included randomized clinical trials were started on the antihypertensive medications largely for their putative cardioprotective effects.
Nonpharmacologic Treatment of Hypertension
Although most patients on hemodialysis receive antihypertensive medications, nonpharmacologic treatments are equally important. To achieve BP control, current national guidelines recommend limiting interdialytic fluid accumulation by having regular counseling by dietitians, emphasizing low sodium intake, and by employing several dialytic strategies, including increased ultrafiltration and longer and/or more frequent dialysis sessions [28]. However, each of these strategies, though conceptually straightforward, can be challenging to implement in practice.
Daily sodium intake estimates in ESRD range from 4 to 7 g per day, well above the recommended daily limits of 2 to 3 g per day [38]. There have been a few studies of the effects of dietary sodium restriction on BP control in hemodialysis, but most have had relatively small sample sizes, did not have a control group, or were of short duration [38]. For example, in a 2-week crossover study, Maduell and Navarro asked 15 patients on hemodialysis to ingest a low-salt diet [39]. The mean sodium intake decreased from approximately 4 g to 2.8 g per day, which correlated with a lower predialysis SBP and lower interdialytic weight gain. Long-term studies of the effect of salt restriction in hemodialysis on BP control or on hard outcomes such as mortality or cardiovascular morbidity have not yet been done.
Not only does excess dietary sodium intake influence sodium balance, but excess dialysate sodium also contributes to net positive sodium balance. A recent cross-sectional study [40] of 1,397 prevalent patients on hemodialysis found that more than half of the patients were dialyzed with a dialysate sodium of 140 mEq/L, even though the mean predialysis plasma sodium concentration was 136.7 mEq/L (±2.9 mEq/L). Moreover, 91% of patients experienced increased postdialysis plasma sodium concentration, which was associated with increased interdialytic weight gain. Manlucu et al. recently completed a pilot study [41] showing that slow, stepwise decreases in dialysate sodium concentration was safe and resulted in significantly lower postdialysis plasma sodium concentrations and lower predialysis SBP.
Maximizing ultrafiltration to achieve dry weight and improve BP control is also a central (but often overlooked) concept in hemodialysis [42]. A recent retrospective cross-sectional study conducted in Turkey [43] emphasized the importance of ultrafiltration by comparing two dialysis centers that had very different BP management strategies. In center A, hypotension and cramps were not necessarily indications to stop ultrafiltration, and antihypertensive medications were used only as a last resort for BP management. All patients in center A were also counseled to restrict dietary salt intake. In center B, patients used antihypertensive medications as needed, and cramps and hypotension were indications that optimal ultrafiltration had been achieved. Although mean predialysis SBP was similar in the two centers, only 7% of patients in center A used antihypertensive medications, compared with 42% in center B. Moreover, the patients in center A had lower left ventricular mass index on echocardiogram and actually had fewer episodes of intradialytic hypotension than the patients in center B (11 vs. 27 episodes per 100 hemodialysis sessions, P = 0.009). However, patients in this Turkish cohort differed from patients on hemodialysis in the United States in terms of their relatively low prevalences of diabetes mellitus (20%) and cardiovascular disease (20%–30%).
Agarwal et al. conducted the Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) study [44], in which 150 patients were randomly assigned to have their dry weights reduced or not. No changes were made to the antihypertensive medication regimen during the study. At the end of 8 weeks, the ultrafiltration-attributable reduction in SBP was 6.6 mmHg (95% CI, −12.2 to −1.0 mmHg). However, patients in the dry weight–reduction group had significantly higher incidences of dizziness, cramping, and other adverse effects.
The DRIP study increased ultrafiltration without increasing the time or frequency of dialysis, which may have accounted for the higher incidence of adverse events. In Tassin, France, hemodialysis sessions lasting 8 h are standard for most patients; in these dialysis units, very few patients require antihypertensive medications and overall rates of intradialytic hypotension are low [45]. The Frequent Hemodialysis Network (FHN) Trial [46••] randomized 245 patients in North America to in-center hemodialysis six times per week (frequent hemodialysis) versus three times per week (conventional hemodialysis). Frequent hemodialysis was associated with benefits in the co-primary composite outcomes of death or an increase in left ventricular mass and death or a decrease in the physical-health composite score. Although the FHN study did not specifically focus on dry-weight reduction, patients receiving frequent hemodialysis had more fluid removed per week than the patients receiving conventional hemodialysis (10.58 L ± 3.83 L vs. 8.99 L ± 3.03 L), but they had fewer episodes of intradialytic hypotension. In prespecified secondary endpoints, frequent hemodialysis was associated with reduced predialysis SBP: the adjusted mean change in predialysis SBP was −9.2 mmHg ± 1.5 mmHg, compared with 0.9 mmHg ± 1.6 mmHg in the patients receiving conventional hemodialysis. The frequent-hemodialysis group also consumed fewer antihypertensive agents (−0.87 ± 1.85 vs. −0.23 ± 1.35) at 12 months.
In summary, nonpharmacologic treatment strategies for BP management in patients on hemodialysis are as important as pharmacologic treatment strategies. Exposure to dialysate with relatively high sodium concentrations is still quite common, but gradual step-wise reduction in dialysate sodium concentration may be a safe and feasible strategy to reduce interdialytic weight gain and BP. Achievement of optimal dry weight is a mainstay in the management of BP in patients on hemodialysis, but it must be carefully balanced against adverse effects. Whether longer and/or more frequent hemodialysis will become the new standard of care remains uncertain.
Intradialytic Hypertension
For most patients on hemodialysis, SBP decreases during dialysis as fluid is removed by ultrafiltration. However, in recent years more attention has focused on patients who have a paradoxic increase in their SBP during or immediately after the hemodialysis session, a phenomenon known as intradialytic hypertension. Although slightly different definitions have been used, the prevalence of intradialytic hypertension is estimated at 10% to 15% [47]. In a secondary analysis of 443 prevalent hemodialysis patients, patients who experienced an increase in SBP of greater than 10 mmHg had approximately twofold higher odds of hospitalization and death at 6 months of follow-up compared with patients whose SBP fell by at least 10 mmHg during hemodialysis [48]. A subsequent study of 1,748 incident hemodialysis patients, using data from the Dialysis Morbidity and Mortality Wave 2 Study, demonstrated that a 10 mmHg increase in SBP during dialysis was associated with a 6% (95% CI, 1%–11%) increased hazard of all-cause death at 2 years. However, this association was found to interact significantly with baseline predialysis SBP; in stratified analyses, intradialytic hypertension was associated with a higher risk of death only for patients with baseline predialysis SBP less than 120 mmHg.
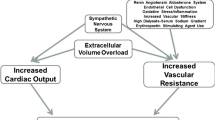
Several mechanisms have been proposed to explain the higher risk of adverse clinical outcomes conferred by intradialytic hypertension, including volume overload, sympathetic overactivity, activation of the renin-angiotensin-aldosterone system, endothelial cell dysfunction, and removal of antihypertensive medications during dialysis, but the exact pathogenesis remains uncertain [47]. Treatment strategies targeting each of these purported causes of intradialytic hypertension are possible, but whether reducing the incidence of intradialytic hypertension will improve clinical outcomes remains to be tested in future studies.
Conclusions
In summary, the association of SBP with clinical outcomes in patients on hemodialysis is complex, with observational studies generally demonstrating the highest risk of cardiovascular events and mortality at lower levels of SBP. SBP in the very highest ranges (>180 mmHg) shows only a weak association with adverse clinical outcomes. Moreover, there are many additional controversies regarding SBP in patients on hemodialysis. First, the optimal timing and method of BP measurement has yet to be defined. Second, although most would agree to treat SBP above 200 mmHg, there is no consensus on appropriate and safe BP targets for patients on hemodialysis. Finally, the use of antihypertensive medications appears to be safe and efficacious for patients on hemodialysis, but larger, more definitive clinical trials need to be conducted. Nonpharmacologic therapies such as dry weight reduction, salt restriction, and perhaps increased duration and/or frequency of hemodialysis are also important aspects of BP management in hemodialysis. With annual mortality rates for patients on hemodialysis approaching 20% [49]—and over half of all these deaths attributable to cardiovascular causes—future clinical trials that will elucidate ways to improve outcomes for these highest-risk patients are desperately needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Agarwal R, Nissenson AR, Batlle D, et al. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115:291–7.
Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13
Kanbay M, Afsar B, Gusbeth-Tatomir P, Covic A. Arterial stiffness in dialysis patients: where are we now? Int Urol Nephrol. 2010;42:741–52.
Guerin AP, Pannier B, Marchais SJ, London GM. Cardiovascular disease in the dialysis population: prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15:105–10.
Foley RN, Parfrey PS, Harnett JD, et al. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49:1379–85.
Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–17.
Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54:561–9.
Tozawa M, Iseki K, Iseki C, Takishita S. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61:717–26.
Li Z, Lacson Jr E, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–15.
Stidley CA, Hunt WC, Tentori F, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol. 2006;17:513–20.
Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58:2147–54.
•• Myers OB, Adams C, Rohrscheib MR, et al. Age, race, diabetes, blood pressure, and mortality among hemodialysis patients. J Am Soc Nephrol. 2010;21:1970–8. In a well-conducted analysis of 16,283 incident hemodialysis patients, Myers et al. demonstrated that lower predialysis SBP was associated with higher risk of death, but more so for older patients and for patients with diabetes mellitus. Although the mortality rate was lower for blacks than for whites, race was not a significant effect modifier of the association of SBP and mortality.
Chang TI, Friedman GD, Cheung AK, et al. Systolic blood pressure and mortality in prevalent haemodialysis patients in the HEMO study. J Hum Hypertens. 2011;25:98–105.
Agarwal R. Hypertension and survival in chronic hemodialysis patients–past lessons and future opportunities. Kidney Int. 2005;67:1–13.
Bos WJ, van Manen JG, Noordzij M, et al. Is the inverse relation between blood pressure and mortality normalized in ‘low-risk’ dialysis patients? J Hypertens. 2010;28:439–45.
Molnar MZ, Lukowsky LR, Streja E, et al. Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. J Hypertens. 2010;28:2475–84.
Agarwal R, Lewis RR. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001;60:1982–9.
Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–98.
Fagugli RM, Ricciardi D, Rossi D, et al. Blood pressure assessment in haemodialysis patients: comparison between pre-dialysis blood pressure and ambulatory blood pressure measurement. Nephrology. 2009;14:283–90.
Rahman M, Griffin V, Kumar A, et al. A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226–30.
Agarwal R, Brim NJ, Mahenthiran J, et al. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–8.
Khangura J, Culleton B, Manns B, et al. Association between routine and standardized blood pressure measurements and left ventricular hypertrophy among patients on hemodialysis. BMC Nephrol. 2010;11:13.
Agarwal R, Metiku T, Tegegne GG, et al. Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol. 2008;3:1364–72.
Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–34.
• Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–8. In this cross-sectional study, Agarwal demonstrated that home SBP was superior to routine in-center SBP to predict mortality. Home SBP between 120 and 130 mm Hg was associated with lowest mortality risk.
Agarwal R. Managing hypertension using home blood pressure monitoring among haemodialysis patients—a call to action. Nephrol Dial Transplant. 2010;25:1766–71.
Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a kidney disease: improving global outcomes controversies conference. Kidney Int. 2010;77:273–84.
K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–S153.
Harper J, Nicholas J, Webb L, et al. UK renal registry 12th annual report (December 2009): chapter 11: blood pressure profile of prevalent patients receiving dialysis in the UK in 2008: national and centre-specific analyses. Nephron Clin Pract. 2010;115 Suppl 1:c239–60.
Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2008;73:759–64.
Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–20.
Tislér A, Akócsi K, Borbás B, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2003;18:2601–5.
Tislér A, Akócsi K, Hárshegyi I, et al. Comparison of dialysis and clinical characteristics of patients with frequent and occasional hemodialysis-associated hypotension. Kidney Blood Pres Res. 2002;25:97–102.
Tai DJ, Lim TW, James MT, et al. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol. 2010;5:623–30.
• Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53:860–6. In this well-conducted meta-analysis of five randomized clinical trials of patients on hemodialysis, Agarwal and Sinha demonstrated that treatment with antihypertensive medications reduced cardiovascular events and death in hemodialysis patients
• Heerspink HJL, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–15. In this meta-analysis of eight randomized clinical trials of patients on hemodialysis and peritoneal dialysis, antihypertensive medication treatment was associated with a lower risk of all-cause and cardiovascular mortality and morbidity.
Chazot C. Can chronic volume overload be recognized and prevented in hemodialysis patients? use of a restricted-salt diet. Semin Dial. 2009;22:482–6.
Maduell F, Navarro V. Dietary salt intake and blood pressure control in haemodialysis patients. Nephrol Dial Transplant. 2063;2000:15.
Munoz Mendoza J, Sun S, Chertow GM, et al. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26:1281–7.
Manlucu J, Gallo K, Heidenheim PA, Lindsay RM. Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: a pilot study using a biofeedback software system. Am J Kidney Dis. 2010;56:69–76.
Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1255–60.
Kayikcioglu M, Tumuklu M, Ozkahya M, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24:956–62.
Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–7.
Charra B, Chazot C, Jean G, et al. Long 3 × 8 hr dialysis: a three-decade summary. J Nephrol. 2003;16 Suppl 7:S64–9.
•• FHN Trial Group, Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. In this well-conducted clinical trial, 245 patients were randomly assigned to receive in-center hemodialysis six times per week or three times per week. Patients who received more frequent hemodialysis had benefits in the co-primary outcomes (death or increase in left ventricular mass, and death or decrease in physical composite score), as well as in prespecified secondary outcomes related to hypertension: lower predialysis SBP and fewer antihypertensive medications used.
Inrig JK. Intradialytic hypertension: a less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis. 2010;55:580–9.
Inrig JK, Oddone EZ, Hasselblad V, et al. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71:454–61.
U S Renal Data System, USRDS 2010 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010
Acknowledgments
I thank Drs. Glenn M. Chertow and Jeffry Young for their critical review of this manuscript.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, T.I. Systolic Blood Pressure and Mortality in Patients on Hemodialysis. Curr Hypertens Rep 13, 362–369 (2011). https://doi.org/10.1007/s11906-011-0223-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0223-x