Abstract
The growing recognition of the burden of neurologic disease associated with HIV infection in the last decade has led to renewed efforts to characterize the pathophysiology of the virus within the central nervous system (CNS). The concept of the AIDS-dementia complex is now better understood as a spectrum of HIV-associated neurocognitive disorders (HAND), which range from asymptomatic disease to severe impairment. Recent work has shown that even optimally treated patients can experience not only persistent HAND, but also the development of new neurologic abnormalities despite viral suppression. This has thrown into question what the impact of antiretroviral therapy has been on the incidence and prevalence of neurocognitive dysfunction. In this context, the last few years have seen a concentrated effort to identify the effects that antiretroviral therapy has on the neurologic manifestations of HIV and to develop therapeutic modalities that might specifically alter the trajectory of HIV within the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neurologic impact of HIV infection extends across the disease course from early infection through end-stage disease (Table 1). HIV RNA can be identified in the cerebrospinal fluid (CSF) of individuals infected with HIV as early as 8 days after estimated initial viral exposure [1•]. A subset of approximately 10 % of individuals experience neurologic symptoms in the setting of seroconversion and the acute retroviral syndrome. Evidence of neurologic injury can be seen in the evaluation of CSF biomarkers as early as three months of infection [2], and the increase in these markers tends to correlate with progression of disease as evidenced by decreasing CD4 count [3•].
The prevalence of clinically measurable HIV-associated neurocognitive disorder (HAND) increases with advancing systemic disease stage and has been defined by formal criteria as asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and an advanced form, HIV-associated dementia (HAD) [4]. Studies have shown that even mild HAND can have a significant impact on quality of life and function in daily activities [5–8]. Moreover, new manifestations of CNS HIV-associated disease that may be outside of the typical spectrum of HAND have recently been described, including symptomatic CSF HIV escape [9, 10] and CD8 encephalitis [11, 12]. With the more sophisticated understanding of these processes has come an effort to measure the clinical impact associated with HIV in the CNS and to determine if treatment can mitigate or reverse neurologic damage.
Measuring the Clinical Effects of Treatment with cART
While the AIDS Dementia Complex was characterized at the beginning of the epidemic in the 1980s, the definitions of a broader spectrum of HAND have been formalized within the last decade [4] and the method of studying neurocognitive manifestations of HIV disease in HIV-infected populations has changed. This has made it difficult to compare the prevalence of HAND across populations before and after the widespread availability of cART.
Neuropsychological (NP) testing remains the mainstay for identifying the presence of HAND and evaluating the impact of treatment on the disease course. NP testing batteries for HAND include assessment of a number of neurocognitive domains including verbal/language, attention/working memory, abstraction/executive functioning, memory (learning and recall), speed of information processing, sensory-perceptual skills, and motor skills. In clinical practice, detailed testing batteries are often impractical due to time and resource constraints, and so abbreviated batteries can sometimes be used to identify abnormalities in the clinical setting. The patient’s own experience is also of value in differentiating the milder forms of HAND, as the primary difference between ANI and MND relates to the impact that the disorder has on everyday functioning.
Descriptive Epidemiology of Neurocognitive Impairment in the cART Era
A recent review sought to determine whether antiretroviral therapy improves neurocognitive dysfunction in individuals with HIV infection, and suggested that overall there is longitudinal benefit on the individual level [13], with participants in two-thirds of the included studies demonstrating significant improvement in neurocognitive status with initiation of cART. Of note, in many cases there was incomplete resolution of baseline impairment, suggesting that treatment initiation may not completely reverse neurocognitive and neuropsychological abnormalities. Despite what appears to be a benefit of initiating treatment, the effect of cART on the overall prevalence of neurocognitive impairment is less clear.
Broadly speaking, the widespread use of cART has resulted in a significant decrease in the prevalence of HAD, the most severely impairing manifestation of HAND. It has been estimated that the prevalence of HAD was at least 16 % in the pre-cART era [14], but HAD now occurs in up to 5 % of HIV-infected patients [15]. Despite this improvement in the rates of severe neurocognitive impairment in the cART era, the morbidity and mortality of HIV-infected individuals with severe impairment still exceeds that which is seen in control populations with unrelated severe neurocognitive deficits [16•].
While the most severe manifestations of HAND have declined in the cART era, it is unclear whether the prevalence of milder forms has decreased, persisted, or in fact increased in the setting of evolving definitions, earlier diagnosis, and more rigorous surveillance. While one might expect a significant decline in HAND with the advent of cART, this has not been the case in clinical studies. Initial work found high rates of neurocognitive abnormalities in groups at high risk for impairment in both time periods, but no significant differences in prevalence between the eras [17]. Another study showed a similar finding in pre-cART and cART groups [18], and more recent work again found rates of neurocognitive impairment to be similar across both eras [19•].
In the time period before the widespread availability of cART, the prevalence of neurocognitive impairment increased with successive disease stages [19•]. In the cART era, however, impairment has become commonly recognized in the medically asymptomatic state of HIV infection. A number of studies have also suggested that the overall patterns of neurocognitive impairment may have shifted in the cART era [18, 19•]. While high rates of mild neurocognitive impairment (NCI) persist at all stages of infection, it appears that the characteristics of impairment have shifted from primarily motor and cognitive/verbal impairment in the pre-cART era to impairment primarily in memory and executive function in the cART era [13, 19•, 20].
Recognizing the CNS as an HIV-infected Compartment
The detection of HIV DNA in perivascular brain macrophages, microglial cells, and astrocytes [21–23] and the compartmentalization of HIV quasi-species in CNS tissues [24, 25] suggests the existence of a CNS reservoir of infection that may lead to neurologic injury and create a sanctuary for ongoing viral replication. Understanding the clinical importance of infection within the CNS compartment has critical implications for HIV treatment and eradication strategies.
With the recognition that the CNS can serve as a site for viral persistence has come a targeted effort to optimize the delivery of antiretroviral agents to the CNS, which is segregated from the plasma compartment by a number of barriers that complicate drug delivery. These include the blood-brain, blood-CSF, and CSF-brain barriers. The ability of antiretroviral drugs to penetrate these barriers and achieve therapeutic concentrations in the CNS is determined by a number of characteristics, including their molecular weight and lipophilicity, the extent to which they are bound to protein in the plasma, and whether they are candidates for active transport across the endothelial cells comprising the barrier. For example, a study of the nucleoside reverse transcriptase inhibitor (NRTI) tenofovir, a common component of cART regimens worldwide, demonstrated that CSF concentrations of the drug were only 5 % of those found in the plasma, and that lower CSF concentrations of the drug were associated with detectable CSF HIV RNA [26]. Similarly, recent work studying the pharmacokinetics of efavirenz has demonstrated overall poor penetration of the drug into the CSF. Unlike the results of the tenofovir study, however, the efavirenz levels achieved in the CSF still exceeded those needed to inhibit viral replication [27]. A case of resistance to the integrase inhibitor raltegravir within the CNS compartment has also been reported [28].
The issues with CNS compartmentalization and the blood-brain barrier have led to the development of scoring systems aimed to predict or estimate the exposure and impact of a given cART regimen in the CNS. The CNS penetration-effectiveness (CPE) index represents an effort to quantitatively estimate the relative ability of each antiretroviral agent to penetrate the CNS and interfere with CSF HIV replication. Each agent is assigned a “CPE score,” and a total regimen score can be calculated by summing the scores for individual agents [29].
Some studies have shown that antiretroviral regimens with higher CPE scores tend to be more successful at achieving HIV RNA suppression in the CNS [29, 30]. However, while more potent HIV RNA suppression in this compartment might be expected to lead to better neurocognitive outcomes and more effective treatment of HAND, this has not necessarily been the case. Observational studies have suggested that the initiation of regimens with higher CPE scores may produce a cognitive benefit in patients with HIV-related neurological disease [31, 32], or that lower CPE scores were more likely to be associated with clinical deterioration as measured by serial neuropsychological testing [33]. Other studies have shown that HIV-infected individuals treated with regimens with higher CPE scores actually exhibit poorer neurocognitive performance despite suppression [30] or only benefit if they are on more than three drugs, which is the standard for most cART regimens [34]. Still others show no effect of CPE score [35]. A prospective study of individuals on long-term cART and those starting cART found similar rates of neurocognitive impairment in both groups, and a trend toward lower CPE scores being associated with poorer performance that can likely be attributed to a subset of subjects on monotherapy. This study again suggested that nadir CD4 count was significantly associated with neurocognitive impairment in both groups in a multivariate model [36].
More recently, in vitro drug efficacy data has been used to derive a monocyte efficacy score based upon the expected effectiveness of antiretroviral drugs within monocytes and macrophages. In a prospective study of a cohort of subjects on antiretroviral therapy, higher monocyte efficacy score correlated with better neuropsychological testing performance [37].
Targeting Treatment Toward the CNS
Along with the recognition that the CNS might represent a viral reservoir or sanctuary site has come an effort to specifically target this compartment when developing treatment regimens for patients with HIV. A recent evaluation of the effect of targeted CCR-5 inhibition with maraviroc in early SIV-infected macaques demonstrated markedly lower SIV RNA and proviral DNA in the CNS in the maraviroc group, suggesting decreased viral replication in these animals. Treatment with maraviroc also lowered monocyte and macrophage activation and decreased amyloid precursor protein immunostaining, suggesting a potential neuroprotective effect [38].
The results of CNS-targeted therapy in humans have been less clear. A recent randomized controlled trial designed to evaluate whether neurocognitive outcomes differ between CNS-targeted or non-targeted regimens was terminated early due to slow accrual and low likelihood of detecting a difference between the two groups. The 16-week follow-up data did not show evidence of neurocognitive benefit for a CNS-targeted strategy, though the study accrual did not reach the sample size calculated as necessary to detect a difference between the planned outcome measures [39].
Because of the difficulties related to penetrating the barriers to the CNS, there has been some effort to explore alternative methods of drug delivery to this compartment. Recent work studying zidovudine has demonstrated that solid lipid microparticles may represent a potential carrier system for drug delivery to CNS via nasal administration [40]. It is unclear whether similar methods could be used to deliver other drugs, including those with poorer plasma-to-CSF penetration, to the CNS.
Treatment in Early Infection
Numerous studies have demonstrated that CD4 nadir is an important predictor of neurocognitive impairment in both eras [19•, 41]. In a study from the CHARTER cohort explicitly addressing this question, higher CD4 nadir was associated with lower odds of neurocognitive impairment and HAND [42]. Similar observations of associations between symptomatic CSF HIV escape and nadir CD4 count have been made in much smaller case series and reports [9, 10]. Other predictors of neurocognitive impairment in the pre-cART era, including duration of infection (combining on- and off-treatment periods) and CSF viral suppression, no longer appear to correlate with neurocognitive abnormalities in treated subjects [19•].
The importance of nadir CD4 as a predictor of neurocognitive impairment and clinical neurologic syndromes which develop despite cART suppression suggests that early initiation of treatment might have a significant impact on the neurocognitive outcomes of individuals with HIV infection. Pathologic processes within the CNS associated with development of neurologic damage in HIV, including viral invasion, immune activation, and compartmentalization of HIV variants are initiated during acute and early infection [1•, 24, 43•]. Recent studies have suggested that these initial processes morbidly impact the nervous system, in that levels of CNS immune activation directly associate with elevation of CSF markers of neuronal injury and neuropathy detected in subjects studied during this early period [2, 44]. However, it is unknown whether early treatment will ameliorate these processes or protect the nervous system from subsequent injury. Neuropsychological performance and mood are abnormal in subjects with early HIV infection compared with those in HIV-uninfected control subjects or the general HIV-uninfected population [45–47], with patterns of neuropsychological impairment paralleling those described in chronic infection, including processing speed and learning deficits. Some of these deficits may be attributable to comorbidities which are prevalent in those at risk for HIV acquisition, including substance abuse and co-infections such as syphilis and hepatitis C [48], suggesting that interventions besides cART alone may be important in addressing CNS injury.
A recent cross-sectional study of 200 HIV-infected subjects and 50 HIV-uninfected comparison subjects engaged in the US military is a first study to strongly suggest that early diagnosis and management of HIV infection may ameliorate or prevent neuropsychological impairment. These HIV-infected subjects had low levels of confounding substance abuse, and the majority initiated treatment within an estimated three years of HIV acquisition, resulting in rates of neurocognitive impairment similar to those seen in matched HIV-uninfected controls [49•].
Measuring the Biological/Neuroimaging Correlates of Treatment with cART
As a result of the challenges associated with neuropsychological testing, including training effects and the frequency of neuropsychological comorbidities found in patients with HIV, efforts have been made to identify biological markers of neurocognitive injury and impairment in individuals with HIV infection and to determine if these markers correlate with clinically relevant aspects of neuropsychological function.
From a cellular perspective, it is thought that the activity of HIV within the central nervous system is primarily related to neuroinflammation rather than direct viral activity. Neurons lack the requisite surface receptors for viral entry, but are subject to the downstream effects of a neuroinflammatory cascade involving microglia and macrophages, which begins within the first year of infection. Understanding the cellular and systems-level events that occur over the time course of HIV infection has become just as important as clinical outcomes as the field of HIV shifts its focus to viral eradication.
CSF HIV
A key marker of antiretroviral activity within the CNS is the quantification of HIV viral RNA after the initiation of therapy. In patients with chronic HIV infection, the pre-cART viral load within the CSF compartment is typically tenfold less than in the plasma compartment [50], although a more marked ratio between CSF and plasma is observed earlier in infection [43•]. Initiation of cART results in a notable decrease in CSF HIV RNA levels [51], although the rate of decay may be slower in individuals with neurocognitive impairment at baseline [50, 52]. Despite the typically brisk response of CSF HIV to initiation of cART, not all subjects achieve or maintain complete viral suppression within the CNS. Recent work has recognized that a subset of individuals on chronic suppressive therapy have elevated CSF HIV RNA levels as compared to plasma, either in the context of neurologically asymptomatic [53] or symptomatic [9, 10] infection. The characterization and clinical significance of CSF “escape” in the setting of cART is a topic of intensive current study.
Further research has suggested that pre-cART viral populations in the plasma and CSF may be identical during acute infection, but subsequently diverge and compartmentalize as the disease progresses from primary to chronic infection [24, 54, 55]. The compartmentalized populations are thought to also derive from different cell lineages trafficking across the BBB, with early compartmentalized HIV apparently replicating in lymphocytes and CNS variants in later stages of disease derived from longer-lived macrophages [25]. This work suggests that in early infection, regimens with high CNS penetration may be less critical, since clearing the infection in the plasma compartment will result in CNS viral decay once the plasma is cleared. In chronic or advanced infection, however, the CNS reservoir may become established and no longer as closely tied to plasma viremia, implying that CNS penetration might be more important at this later time point.
Eggers et al. further studied the dynamics of HIV populations within the CNS in response to therapy by sequencing the env protein V3 loop. In general, env sequences from short-lived cells such as lymphocytes and long-lived cells such as macrophages are reflective of different HIV subpopulations. With cART, virus from fast-replicating cells decays first, which uncovers sub-populations present in slow-replicating cells, presumably macrophages and microglia, likely crucial sources of HIV which will be important to understanding HAND [56].
Soluble CSF Biomarkers of Inflammation and Injury
CSF biomarkers have gained popularity as objective markers of neuronal inflammation and injury in HIV infection, allowing researchers to distinguish static neurological abnormalities from active processes affecting the nervous system. Over the last 10 years, there has been an effort to describe the changes in these biomarkers that occur with different manifestations of HIV infection in the CNS, including HIV-associated dementia, other manifestations of HAND, and CNS opportunistic infections. More recently, dynamic changes in these biomarkers have been explored in the context of antiretroviral therapy.
While viral suppression with antiretroviral therapy leads to a decline in many markers of immune activation and inflammation within the CNS, some remain persistently elevated even in individuals with undetectable viral activity both within and outside of the CNS. Elevations in soluble biomarkers of immune activation, including CSF neopterin, MCP-1/CCL-2, and IP-10/CXCL-10 are detected in patients with HIV prior to cART treatment [57]. CSF neopterin, a biomarker of CNS macrophage activation associated with neuronal injury, can be persistently elevated in subjects on suppressive cART, suggesting that immune activation persists even in the setting of viral control [58].
The light subunit of the neurofilament protein (NFL) is a major structural component of myelinated axons and has been identified as a sensitive marker of axonal injury in HAD, chronic neuroasymptomatic HIV infection, and primary HIV infection. Antiretroviral treatment decreases CSF levels of NFL. However, levels of this marker do not completely normalize with viral suppression even in neuroasymptomatic patients, possibly reflecting ongoing neuronal injury despite the absence of measurable viral replication in the CNS [3•].
Neuroimaging Markers: Persistent Abnormalities on Antiretroviral Therapy
Proton-magnetic resonance spectroscopy (proton-MRS) is a non-invasive imaging modality that has been used to monitor neuronal injury through the analysis of cerebral metabolites. N-acetylaspartate and glutamate are markers of neuronal health that deplete with injury [59, 60]. Research over the last ten years has suggested that reduced brain tissue volumes in cortical and subcortical regions and cerebral metabolite abnormalities persist in individuals on stable cART [61], and may even progressively worsen during cART [62, 63]. Additionally, a recent study employing a positron emission tomography imaging method which putatively quantitates activated microglia similarly suggests ongoing immune activation in patients on stable cART [64].
Some neuroimaging studies have suggested that neuronal injury occurs during primary HIV infection as evidenced by decreased N-acetylaspartate in the frontal cortex of newly infected individuals [65, 66]. A recent study by Sailasuta et al. used proton-magnetic resonance spectroscopy to identify cellular inflammation and found cerebral metabolites suggestive of inflammation in subjects with acute HIV infection prior to initiation of cART, even in the absence of neuronal injury. The markers of injury normalized after initiation of cART in these subjects, suggesting that early cART might be neuroprotective [67].
Studies of cerebral metabolites have shown brain-region specific abnormalities that correlate with a number of disease markers, including nadir CD4 count, in subjects with stable HIV on cART [68, 69], suggesting that delayed initiation of cART may increase vulnerability to neurologic abnormalities. A study comparing virologically suppressed individuals with MND to healthy controls showed micro-structural alterations, including loss of structural integrity and edema in a number of brain regions in the MND subjects [70]. Another study evaluating brain tissue volume of structures on MRI suggested that effective therapy could attenuate the shrinkage of the frontal and temporoparietal cortices, insula, and hippocampus and decreased rapidity of the expansion of the Sylvian fissure, implying less rapid decline in higher-order functions [71].
Peripheral Blood Monocyte HIV DNA
One prevailing theory regarding the cause of continued cognitive impairment in the cART era is that a peripheral blood monocyte HIV DNA reservoir that persists despite treatment serves as a mechanism for the spread of the virus to the brain. In a prospective study of cART-naïve HIV-infected Thai subjects, there was a 14.5 increased odds ratio for HAND for each tenfold increase in HIV DNA copy number. Moreover, HIV DNA levels correlated with the inflammatory marker neopterin in the CSF, as well as proton-MRS markers of neuronal injury and glial dysfunction [72].
Could Antiretroviral Drugs Contribute to HAND?
The toxic effects of treatment with cART within the CNS are also an important consideration in the era of HAND, particularly in the setting of the growing concern that therapeutic concentrations of antiretroviral drugs in the CSF can be associated with neurotoxicity.
From a clinical perspective, the adverse reactions associated with the NNRTI efavirenz are particularly notable and include sleep disturbances, mood disorders, impaired concentration, and in some cases, suicidality. While typically occurring within the first four weeks of treatment, neuropsychiatric effects of efavirenz can persist [73]. The mechanism of neurotoxicity is unknown, but recent work has suggested that it may be related to drug levels due to individual variability in metabolism and is more likely associated with reversible dendritic changes rather than cell death [74]. Recent in-vitro work studying efavirenz metabolites demonstrated a dose-dependent toxic effect of the 8-OH metabolite on neuronal dendrite morphology and viability [75]. Further work in mice has shown that treatment with efavirenz generated increased production and decreased clearance of beta-amyloid through upregulation of beta-secretase activity and down-regulation of microglial amyloid-beta phagocytosis [76]. Additional work in rats and macaques has suggested that antiretroviral agents can result in the accumulation of reactive oxygen species and the induction of neuronal injury [77].
Partly in the setting of concern for the toxic effects of antiretroviral therapy in well-controlled patients has come a movement toward less-drug regimens, which represent an effort to simplify treatment and minimize the costs and adverse reactions associated with these medications. As their name suggests, these regimens typically consist of dual- or mono-therapy and are slightly less systemically efficacious compared with cART. Despite their relative success at suppressing systemic virus replication, there is concern that these regimens would inadequately suppress viral reservoirs such as the CNS. A recent review of studies of less-drug regimens consisting of ritonavir-boosted protease inhibitors suggested that this may not actually be the case. While symptomatic CSF viral escape was observed to occur in subjects on monotherapy who also failed in the plasma, asymptomatic CSF escape was not more common than in standard cART and there were no differences in functional outcomes [78]. This work suggests that less-drug regimens might be a reasonable clinical option in a specific subset of patients, but further prospective work needs to be done to determine whether the benefits of a simplified regimen outweigh the risks of viral relapse and resistance.
Non-antiretroviral Therapies
Prior Trials of Adjunctive Medications
A number of non-antiretroviral therapies have been suggested in an effort to attenuate the inflammatory events that are characteristic of CNS HIV infection and may underlie the pathogenesis of HAND. Studies of memantine, selegiline, and nimodipine have failed to demonstrate any benefit [79].
The neuropsychiatric agents valproic acid and lithium, which affect glycogen synthase kinase-3β, and the selective serotonin reuptake inhibitors citalopram and paroxetine have been hypothesized to downregulate HIV replication and neuroinflammation; however studies of these agents have not demonstrated improvement in neurological outcomes related to HAND [80]. The acetylcholinesterase inhibitor rivastigmine and the NMDA receptor antagonist memantine, both of which are commonly used in Alzheimer’s dementia, also did not show benefit in terms of cognitive performance. Although there were improvements in secondary outcomes (processing speed and executive function) in virally suppressed individuals on rivastigmine, cognitive functioning did not differ between treatment and placebo groups [81]. While there was a suggestion of improvement in aggregate neuropsychological scores at short-term follow-up in the open-label group, there were no differences at the one-year time point [82]. Similarly, the antibiotic minocycline has not shown a benefit in randomized trials [83, 84].
Adjunctive Medications Still Under Investigation
One class of non-antiretroviral drugs that remains promising is the statin medications, which are inhibitors of the HMG-CoA reductase enzyme and are thought to have widespread anti-inflammatory effects. While an early, small study did not show any appreciable effect on CSF HIV RNA levels or markers of immune activation [85], recent work suggesting a correlation between protease inhibitors, HAND, and cerebral small-vessel disease [86•] could imply that the effects of statins in this patient population remain incompletely explored and warrant further investigation.
Also to be explored in the future is the possibility that, despite the absence of HIV replication achieved by effective cART, infected cells might generate pro-inflammatory viral products such as tat, whose effects on the immune system may require specific targeting by agents beyond antivirals [87].
Non-pharmacologic Approaches
Finally, non-pharmacologic therapy may have a significant role to play in the treatment of individuals with HAND. A recent review of approaches to cognitive rehabilitation within this patient population suggests that a great deal remains unknown about the therapies that might lead to better health outcomes through functional improvement [20]. Other work has suggested that exercise might also have a significant impact on improving neurocognitive outcomes in HIV-infected adults [88, 89].
Conclusion
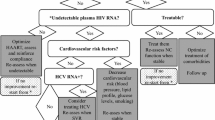
Even in the era of widespread access to antiretroviral therapy, the burden of neurocognitive impairment associated with HIV infection remains significant and continues to evolve. Despite optimal treatment, many individuals experience persistent HAND, CSF viremia, and neuropsychological abnormalities that have a significant impact on everyday functioning and quality of life. Figure 1 summarizes the biological, clinical, and epidemiological impact of antiretroviral therapy within the CNS. While the results of studies on the CNS effects of antiretroviral treatment continue to be somewhat divergent, there are growing data supporting the importance of the CD4 nadir as a marker for neurological risk and suggesting that early initiation of antiretroviral therapy might be the most important factor in reducing neuropsychological morbidity for individuals with HIV. Still, there remains concern that the overwhelming benefits of antiretroviral therapy might be tempered by the risks associated with CNS viral compartmentalization and neurotoxicity, underscoring the need for further investigation aimed at clarifying the mechanisms behind the establishment and persistence of the CNS compartment, the neurobiochemical effects of treatment targeted at this compartment, and the individual and population level impact of antiretroviral therapy in HIV-infected individuals.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82. This study demonstrated the presence of viral particles in the CSF of patients acutely infected with HIV as early as eight days after estimated transmission.
Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207(11):1703–12.
Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. This study demonstrated elevations in neurofilament light chain, a marker of neuronal injury, even in asymptomatic individuals with well-controlled HIV infection.
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99.
Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–31.
Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, Group HIVNRP. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol. 2011;56(1):77–84.
Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, Group HIVNRP. Aging, prospective memory, and health-related quality of life in HIV infection. AIDS Behav. 2012;16(8):2309–18.
Cattie JE, Doyle K, Weber E, Grant I, Woods SP, Group HIVNRP. Planning deficits in HIV-associated neurocognitive disorders: component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol. 2012;34(9):906–18.
Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74.
Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8.
Lescure FX, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57(1):101–8.
Gray F, Lescure FX, Adle-Biassette H, et al. Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment. Brain Pathol. 2013;23(5):525–33.
Joska JA, Fincham DS, Stein DJ, Paul RH, Seedat S. Clinical correlates of HIV-associated neurocognitive disorders in South Africa. AIDS Behav. 2010;14(2):371–8.
McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–52.
Heaton RK, Clifford DB, Franklin Jr DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96.
Lescure FX, Omland LH, Engsig FN, et al. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: a Danish nationwide cohort study. Clin Infect Dis. 2011;52(2):235–43. This large study demonstrated that patients with severe HIV-associated neurocognitive impairment had worse morbidity and mortality outcomes than HIV-uninfected patients with unrelated neurocognitive impairment.
Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8(2):136–42.
Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–7.
Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. This study compared HIV-infected individuals from pre- and cART-era cohorts and further supported earlier observations that neurocognitive impairment is more common in the cART era, but has shifted toward mild impairment.
Weber E, Blackstone K, Woods SP. Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: a qualitative review and call to action. Neuropsychol Rev. 2013;23(1):81–98.
Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66(2):253–8.
Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179(4):1623–9.
Gray LR, Cowley D, Crespan E, et al. Reduced basal transcriptional activity of central nervous system-derived HIV type 1 long terminal repeats. AIDS Res Hum Retrovir. 2013;29(2):365–70.
Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84(5):2395–407.
Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5(4):e1000395.
Best BM, Letendre SL, Koopmans P, et al. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr. 2012;59(4):376–81.
Yilmaz A, Watson V, Dickinson L, Back D. Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval. Antimicrob Agents Chemother. 2012;56(9):4583–5.
Mora-Peris B, Mackie NE, Suan D, Cooper DA, Brew BJ, Winston A. Raltegravir resistance in the cerebrospinal fluid. Infection. 2013;41(3):731–4.
Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70.
Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66.
Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52(1):56–63.
Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56(3):416–23.
Vassallo M, Durant J, Biscay V, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28(4):493–501.
Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65.
Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12(6):333–8.
Casado JL, Marin A, Moreno A, et al. Central nervous system antiretroviral penetration and cognitive functioning in largely pretreated HIV-infected patients. J Neurovirol. 2014;20(1):54–61.
Shikuma CM, Nakamoto B, Shiramizu B, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17(7):1233–42.
Kelly KM, Beck SE, Pate KA, et al. Neuroprotective maraviroc monotherapy in simian immunodeficiency virus-infected macaques: reduced replicating and latent SIV in the brain. AIDS. 2013;27(18):F21–F28.
Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58(7):1015–22.
Dalpiaz A, Ferraro L, Perrone D, et al. Brain uptake of a Zidovudine prodrug after nasal administration of solid lipid microparticles. Mol Pharm. 2014;11(5):1550–61.
Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–8.
Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–51.
Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–60. This study characterized the extent of neuroinflammation present in individuals during the first year of HIV infection, even in those with low CSF HIV RNA levels.
Wang SX, Ho EL, Grill M, et al. Peripheral neuropathy in primary HIV infection associates with systemic and CNS immune activation. J Acquir Immune Defic Syndr. 2014;66(3):303–10.
Moore DJ, Letendre SL, Morris S, et al. Neurocognitive functioning in acute or early HIV infection. J Neurovirol. 2011;17(1):50–7.
Atkinson JH, Higgins JA, Vigil O, et al. Psychiatric context of acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: IV. AIDS Behav. 2009;13(6):1061–7.
Gold JA, Grill M, Peterson J, et al. Longitudinal characterization of depression and mood states beginning in primary HIV infection. AIDS Behav. 2014;18(6):1124–32.
Weber E, Morgan EE, Iudicello JE, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19(1):65–74.
Crum-Cianflone NF, Moore DJ, Letendre S, et al. Low prevalence of neurocognitive impairment in early diagnosed and managed HIV-infected persons. Neurology. 2013;80(4):371–9. This cross-sectional study is the first to suggest that early initiation of treatment may mitigate neuropsychological impairment in individuals with HIV.
Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98.
Mellgren A, Antinori A, Cinque P, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10(6):701–7.
Eggers C, Hertogs K, Sturenburg HJ, van Lunzen J, Stellbrink HJ. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17(13):1897–906.
Eden A, Andersson LM, Andersson O, et al. Differential effects of efavirenz, lopinavir/r, and atazanavir/r on the initial viral decay rate in treatment naive HIV-1-infected patients. AIDS Res Hum Retrovir. 2010;26(5):533–40.
Harrington PR, Schnell G, Letendre SL, et al. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23(8):907–15.
Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R. Increased human immunodeficiency virus type 1 (HIV-1) env compartmentalization in the presence of HIV-1-associated dementia. J Virol. 2005;79(16):10830–4.
Eggers C, Muller O, Thordsen I, Schreiber M, Methner A. Genetic shift of env V3 loop viral sequences in patients with HIV-associated neurocognitive disorder during antiretroviral therapy. J Neurovirol. 2013;19(6):523–30.
Cinque P, Brew BJ, Gisslen M, Hagberg L, Price RW. Cerebrospinal fluid markers in central nervous system HIV infection and AIDS dementia complex. Handb Clin Neurol. 2007;85:261–300.
Yilmaz A, Yiannoutsos CT, Fuchs D, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62.
Lentz MR, Kim JP, Westmoreland SV, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235(2):461–8.
Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32(5):1045–53.
Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–33.
Cardenas VA, Meyerhoff DJ, Studholme C, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15(4):324–33.
Gongvatana A, Harezlak J, Buchthal S, et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J Neurovirol. 2013;19(3):209–18.
Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72.
Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72(17):1465–72.
Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011;17(3):220–9.
Sailasuta N, Ross W, Ananworanich J, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One. 2012;7(11):e49272.
Hua X, Boyle CP, Harezlak J, et al. Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV-infected patients on stable treatment. NeuroImage Clin. 2013;3:132–42.
Harezlak J, Cohen R, Gongvatana A, et al. Predictors of CNS injury as measured by proton magnetic resonance spectroscopy in the setting of chronic HIV infection and CART. J Neurovirol. 2014;20(3):294–303.
Granziera C, Daducci A, Simioni S, et al. Micro-structural brain alterations in aviremic HIV + patients with minor neurocognitive disorders: a multi-contrast study at high field. PLoS One. 2013;8(9):e72547.
Pfefferbaum A, Rogosa DA, Rosenbloom MJ, et al. Accelerated aging of selective brain structures in human immunodeficiency virus infection: a controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35(7):1755–68.
Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8(7):e70164.
Fumaz CR, Munoz-Moreno JA, Molto J, et al. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38(5):560–5.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99.
Tovar-y-Romo LB, Bumpus NN, Pomerantz D, et al. Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther. 2012;343(3):696–703.
Brown LA, Jin J, Ferrell D, et al. Efavirenz promotes beta-secretase expression and increased abeta1-40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND). PLoS One. 2014;9(4):e95500.
Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53.
Ferretti F, Gianotti N, Lazzarin A, Cinque P. Central nervous system HIV infection in “less-drug regimen” antiretroviral therapy simplification strategies. Semin Neurol. 2014;34(1):78–88.
Uthman OA, Abdulmalik JO. Adjunctive therapies for AIDS dementia complex. Cochrane Database Syst Rev. 2008;3, CD006496.
Ances BM, Letendre SL, Alexander T, Ellis RJ. Role of psychiatric medications as adjunct therapy in the treatment of HIV associated neurocognitive disorders. Int Rev Psychiatry. 2008;20(1):89–93.
Simioni S, Cavassini M, Annoni JM, et al. Rivastigmine for HIV-associated neurocognitive disorders: a randomized crossover pilot study. Neurology. 2013;80(6):553–60.
Zhao Y, Navia BA, Marra CM, et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin Trials. 2010;11(1):59–67.
Sacktor N, Miyahara S, Deng L, et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology. 2011;77(12):1135–42.
Nakasujja N, Miyahara S, Evans S, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80(2):196–202.
Probasco JC, Spudich SS, Critchfield J, et al. Failure of atorvastatin to modulate CSF HIV-1 infection: results of a pilot study. Neurology. 2008;71(7):521–4.
Soontornniyomkij V, Umlauf A, Chung SA, et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28(9):1297–1306. This cross-sectional study of the California NeuroAIDS Tissue Network found a correlation between protease inhibitor use and cerebral small vessel disease, suggesting a mechanism by which antiretroviral agents might contribute to HAND.
Johnson TP, Patel K, Johnson KR, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110(33):13588–93.
Mapstone M, Hilton TN, Yang H, et al. Poor aerobic fitness may contribute to cognitive decline in HIV-infected older adults. Aging Dis. 2013;4(6):311–9.
Dufour CA, Marquine MJ, Fazeli PL, et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J Neurovirol. 2013;19(5):410–7.
Compliance with Ethics Guidelines
Conflict of Interest
Michael J. Peluso and Serena Spudich declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peluso, M.J., Spudich, S. Treatment of HIV in the CNS: Effects of Antiretroviral Therapy and the Promise of Non-Antiretroviral Therapeutics. Curr HIV/AIDS Rep 11, 353–362 (2014). https://doi.org/10.1007/s11904-014-0223-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-014-0223-y
Keywords
- HIV
- AIDS
- HIV-associated neurocognitive disorder (HAND)
- Asymptomatic neurocognitive impairment (ANI)
- Mild neurocognitive disorder (MND)
- HIV-associated dementia (HAD)
- AIDS dementia complex
- Cerebrospinal fluid (CSF)
- Central nervous system (CNS)
- Antiretrovirals
- Combination antiretroviral therapy (cART)
- Central nervous system penetration effectiveness (CPE)
- Neopterin
- Neurofilament light chain
- Proton-MR spectroscopy (MRS)
- Peripheral blood monocyte DNA
- Neuroinflammation
- CSF escape
- CD4 T lymphocyte
- Neuropsychological testing
- Neurotoxicity