Abstract
Purpose of Review
The dramatic increase in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) fostered the development and evaluation of non-invasive, imaging based methods for diagnosing NAFLD, NASH, and its complications. We herein review different radiologic modalities in diagnosing steatosis, fibrosis, and liver cirrhosis.
Recent Findings
While routine abdominal ultrasound with hyperechogenic liver structure only detects moderate to severe steatosis, controlled attenuation parameter (CAP), magnetic resonance spectroscopy (MRS), and, especially, MRI-proton density fat fraction (MRI-PDFF) are more sensitive to diagnose and quantify steatosis. MRI-PDFF appears suitable to monitor treatment-related changes in liver fat in clinical trials. Liver fibrosis is related to hepatic and extrahepatic morbidity and mortality in NAFLD. Fibrosis and cirrhosis can be suspected by ultrasound-based elastography techniques (vibration-controlled transient elastography, VCTE; acoustic resonance forced impulse imaging, ARFI; shear wave elastography, SWE), which may be used to screen for fibrosis in high-risk patients. MR elastography (MRE) appears advantageous to quantify and stage fibrosis, while angiographic hepatic venous pressure gradient (HVPG) measurement can confirm portal hypertension in cirrhosis. Screening for hepatocellular carcinoma (HCC) in cirrhotic livers is done by ultrasound; suspicious nodules are followed by multiphasic CT/MRI, contrast-enhanced ultrasound (CEUS), or contrast-enhanced MRI.
Summary
Different radiologic modalities exist to screen, diagnose, stage, and monitor steatosis, steatohepatitis, fibrosis, and HCC, thereby complementing liver biopsy and blood biomarkers in the management of patients with NAFLD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction: Challenges in Diagnosing and Staging Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) has become the major etiology of chronic liver diseases worldwide, affecting 20–30% of the adult population in large parts of the world [1]. The magnitude of affected patients as well as the increasing prevalence of interrelated metabolic comorbidities (e.g., type 2 diabetes, obesity, metabolic syndrome) will soon make NAFLD the leading cause of liver cirrhosis and hepatocellular carcinoma (HCC) and the prime indication for liver transplantation [2]. For the next 15 years, a modest increase in NAFLD cases, but a dramatic increase in liver mortality and advanced liver disease, is being projected for large countries in Europe, Asia, and the Americas [3]. However, unlike for other typical chronic liver diseases, only a minor fraction of patients affected by NAFLD will progress to liver-related complications. In fact, the number one cause of death in NAFLD patients relates to cardiovascular diseases, followed by cancer and liver cirrhosis [1]. This picture is different for the subgroup of NAFLD patients with nonalcoholic steatohepatitis (NASH) and/or liver fibrosis, as liver-related complications dramatically increase in the presence of advanced fibrosis [4•]. Thus, early diagnosis; accurate staging of the disease, especially of hepatic fibrosis; and prognostication are essential for the management of NAFLD [5••, 6].
The current gold standard for an accurate grading and staging of NAFLD is the histological evaluation of a liver biopsy [7•]. However, the procedure is invasive; relatively costly; associated with relevant risks like pain, infections, and bleeding; may not be representative for the whole organ (due to the small sample size); and has a high inter-observer variability [8]. Due to these limitations, it is simply impossible to apply liver biopsy as a screening method for millions of asymptomatic individuals at risk for NAFLD. It also appears unlikely to use liver histology to monitor changes in fibrosis stage over time, because this would require repeated biopsies. Imaging techniques have therefore emerged as attractive methods to assess steatosis, steatohepatitis, fibrosis, cirrhosis, or liver cancer [7•, 9, 10]. In this review, we will provide an overview on non-invasive, imaging-based methods for diagnosing NAFLD, NASH, fibrosis, and complications. Nonetheless, we would like to emphasize that radiologic modalities represent only one part of the assessment of patients with suspected or confirmed NAFLD. The diagnostic and prognostic value of imaging can be greatly enhanced, if findings are combined with clinical risk assessment, disease biomarkers, and genetic predisposition [7•].
Radiologic Modalities for Diagnosing Steatosis
Ultrasound
Ultrasound also called sonography uses high-frequency waves, which are higher than the upper audible limit of human hearing. Ultrasound imaging has the ability to provide real-time images with frame rates exceeding several hundred frames per second. Therefore, this technique is suitable to view anatomical changes as well as to guide minimal invasive procedures [11]. Ultrasound imaging systems have been widely used in the world since the 1960s, and compact systems were available since the early 2000s, while wireless table PC-controllable ultrasound imaging systems were introduced in the early 2010s [12]. These systems are presently used in many clinical applications as a low-cost, portable, and non-invasive imaging modality. In particular, the development of methods for free-hand ultrasound imaging in the 3D domain was a highly innovative step in diagnosis. These systems allow 3D visualization of the entire structure of an organ and its attributes, and they are orientation-independent, less dependent on an operator, are characterized by high accuracy and repeatability, and can be fused with other 3D imaging modalities [13]. In a typical ultrasound exam, a transducer (i.e., probe) is placed on the skin coated with a thin layer of gel allowing the ultrasound waves to transmit from the transducer through the gel into the body. The final ultrasound image is then produced by information resulting from the reflection of the waves, the strength of their amplitude, and the time necessary to take the waves through the body. Although this technology is generally considered safe when appropriately conducted by trained health care providers, there are reports available stating ultrasound energy has the potential to produce unwanted biological effects on the body. These include a slight heating of the tissue analyzed, ultrasound cavitation, production of small pockets of gas in the body fluids, mechanical stress, or other undetermined non-thermal processes [14].
Historically, the first paper using ultrasound in diagnosis of hepatic steatosis and steatohepatitis in patients with alcoholic liver disease appeared in 1981 [15]. In this pioneering study, the authors showed that ultrasound is useful in detecting diffuse parenchymal alterations of the liver in patients with alcohol-related disease. More recent studies showed that the performance of ultrasound imaging in respective diseases is relatively good, while the sensitivity of this technique is considerably lower when the overall content of hepatic steatosis is lower than 5–20% [16,17,18]. In particular, the sensitivity and specificity in patients with steatosis exceeding 30% of the hepatocytes were determined to be 92.3 and 77.7%, respectively [19]. Using this imaging technique, it turned out that the prevalence of NAFLD and NASH is significantly higher in the general population than generally assumed [17]. For instance, 29.9% of the general population displayed a hyperechogenic liver pattern (“steatosis”) by ultrasound in a population-based study in Germany [20]. Therefore, several investigators have proposed ultrasound as the first-line diagnostic test in patients with abnormal liver enzymes, including NAFLD patients, and begun to evaluate the potential utility of ultrasound for quantifying hepatic steatosis [21]. A complementary technical advance is contrast-enhanced ultrasound (CEUS), which allows characterizing intrahepatic lesions and the hepatic microcirculation. CEUS applications have consolidated and grown in their application in clinical routine for liver pathologies [22]. However, coexisting conditions such as hepatitis C and other chronic liver diseases have been shown to reduce the sensitivity of ultrasound imaging [23]. In addition, ultrasound-based tests are limited when used in patients suffering from obesity, ascites, or acute inflammation, due to the restricted penetration depth and other technical issues [24].
Controlled Attenuation Parameter
Controlled attenuation parameter (CAP) is a physical parameter based on the ultrasonic properties of the radiofrequency back-propagated signals acquired by the FibroScan® device [25]. The FibroScan® that will be discussed later in this review represents an ultrasound-based vibration-controlled transient elastography (VCTE) device used to asses liver elasticity [26]. Integrated as a software module in FibroScan®, CAP was developed to specifically target the liver. It can be performed by an operator, who does not have any ultrasound imaging skills [25]. Moreover, the implementation of CAP on FibroScan® allows the detection of hepatic steatosis from 10% of fatty hepatocyte degeneration, without being influenced by liver fibrosis or cirrhosis. This fact is important in clinical practice, because 10% of fatty hepatocytes in the liver represents a critical threshold that is of diagnostic and prognostic significance in NASH patients, in which the diagnosis of steatosis is made when lipid content in the liver exceeds 5–10% by weight [27]. Typically, the attenuation of ultrasound signals is determined at the center frequency of the FibroScan® M probe at 3.5 MHz, and the output values are given in decibel per meter [28]. Although exact cutoff values are presently not defined, the values used for adults in many studies for distinct grades for liver steatosis defined by biopsy given in S0-S3 (with S0: no steatosis, S1: ≥ 10% hepatocytes with fat, S2: ≥ 33% hepatocytes with fat, S3: ≥ 66% hepatocytes with fat) are in the range of 214–289 dB/m (S1), 255–311 dB/m (S2), and 281–310 dB/m (S3), respectively [28]. In a large individual patient data meta-analysis, CAP values (in dB/m) were influenced by several covariates like disease etiology, diabetes, and obesity, and correction factors have been proposed for these conditions [29•]. A comparable CAP threshold of 225 dB/m for steatosis in children was proposed in a cross-sectional study analyzing 69 young patients (mean age, 16.0 ± 2.9 years, 62% male) undergoing liver biopsy as part of standard clinical care [30].
Quantification of steatosis by CAP was first introduced and evaluated in 2010 in a collective of 115 patients [25]. This pioneering study showed a significant correlation of CAP and steatosis with the area under receiver operative characteristic curve (AUROC) that was equal to 0.91 and 0.95 for the detection of more than 10% or 33% of steatosis. A later meta-analysis revealed AUROC values of 0.823 and 0.865, respectively [29]. This methodology is potentially less influenced by sampling error than the “gold standard” liver biopsy, because it explores a liver volume approximately 100 times larger. In a prospective study based on 5323 examinations, CAP values were significantly associated with all parameters of metabolic syndrome and with alcohol abuse, suggesting that this method might be particularly useful for the follow-up of NAFLD or alcoholic patients [31]. Likewise, in a more recent prospective cross-sectional study, including 119 adults with and without NAFLD who underwent MRI-based fat quantification (MRI-PDFF) (see below) and CAP, the optimal threshold of CAP for the presence of hepatic steatosis, as defined by a MRI-PDFF equal or larger 5%, was determined to be 288 dB/m [32]. However, it should be critically mentioned that liver stiffness and CAP increases after a meal across all stages of fibrosis, which might be a potential diagnostic pitfall and pose a risk in misclassification of patients with higher stages of fibrosis [33].
MRI-Based Fat Quantification
Magnetic resonance imaging (MRI) is a non-invasive technology producing detailed 3D soft tissue contrast images of high spatial resolution without the use of radiation [34]. Principally, the MRI scanner first forms a powerful magnetic field around the area to be imaged provoking a polarization of hydrogen nuclei of water in order to be polarized in the direction of the magnetic field. Thereafter, a radiofrequency is pulsed through the patients, resulting in stimulation of protons within the tissue. These protons then spin out of equilibrium and strain against the pull of the magnetic field. After deactivation of the radiofrequency, a sensor detects the energy released as the protons realign with the magnetic field. Based on the time used for realignment and amount of energy released, the chemical nature and environment of individual molecules can be determined [35]. To generate images with different contrast, the relaxation properties of the hydrogen atoms can be varied by setting of different pulse sequences. However, when the images are acquired with high resolution, the imaging time can significantly increase, limiting the number of subjects that can be examined daily [36]. The main considerations of image quality are signal-to-noise ratio, spatial resolution, and scan time [35]. Intravenous-administered gadolinium(III)-based contrast agents shortening the time necessary to realign the protons with the magnetic field can be administered to acquire an enhanced and clearer picture of the area being imaged and improving the overall visibility of internal body structures [37].
Similar to ultrasound, MRI scans do not require or emit ionizing radiation occurring in X-ray or computed tomography (CT) imaging. However, the strong and static magnetic field that changes with time during MRI could lead to marginal heating of the body, produce loud knocking noises and attract all kind of magnetic particles (mobile phones, keys, coins etc.), and cause damage to the MRI device. In addition, patients suffering from claustrophobia require special care through the whole examination, while patients with metallic implants or external medical devices are generally unsuitable to MRI scans. Gadolinium-based contrast agents have been found to remain in the body after multiple MRIs, might pass the blood-brain barrier, and their small traces of free forms of these agents are assumed to be toxic. In particular, physiological changes and the integrity and developmental stage of the organs have a strong effect on the dynamics, distribution, and excretion, which may be responsible for Gadolinium contrast agent deposition and toxicity leading to nephrogenic systemic fibrosis and alteration in calcium homeostasis [38]. In addition to these health considerations, it should be mentioned that Gadolinium-based contrast agents are predominantly removed renally without metabolization, thereby posing an environmental risk for aquatic environments [39]. Furthermore, conventional MRI primarily allows only the detection of protons in mobile (unbound) molecules limiting detection of molecules fixed in structures.
Differential electronic shielding of water-bound and triglyceride-bound protons provokes local magnetic field inhomogeneity and subsequent differences in the MR resonant frequency of respective protons. This chemical shift or precessional difference between hydrogen in fat and hydrogen in water can be exploited by emerging chemical shift-encoded MRI (CSE-MRI). This methodology provides the basis for a special type of MRI, allowing the accurate measurement of the proton density fat fraction (PDFF). In respective measurements, PDFF is expressed in an absolute percentage and defined as the ratio of the density of mobile proteins from triglycerides and the total density of protein from mobile triglycerides and mobile water [40].
When comparing the diagnostic performance of MRI-PDFF using a free-drawn region-of-interest measurement of hepatic fat deposition with magnetic resonance spectroscopy (MRS) as the reference method, it turned out that MRI-PDFF is more accurate regarding hepatic fat deposition [41]. This study further revealed that this methodology is particularly useful when hepatic fat deposition is heterogeneous. In a more recent study, comparing conventional ultrasonography signs of hepatic steatosis and MRI-PDFF as a reference method, the authors found a positive correlation between some combination of ultrasound signs and MRI-PDFF values [42]. A great advantage of MRI-PDFF is the short time required for each measurement. This was recently demonstrated in a prospective study investigating 4949 participants (aged 45–73 years), in which a single transverse slice was captured, through the center of the liver superior to the porta hepatis. The acquisition of ten echo times needed for PDFF image construction that were taken during a single expiration breath-hold without given any contrast agents typically took only ≤ 3 min and provided information about liver fat content with an overall success rate of 96.4% with mean intra- and inter-reader correlations of 0.96 (SD = 0.01) and 0.997 (SD = 0.0001), respectively [43]. Moreover, a clear correlation of liver fat deposit and body mass index (BMI) was identified in this study, which were > 20 kg/m2, 0.98%; 20–25 kg/m2, 1.36%; 25–30 kg/m2, 2.54%; 30–35 kg/m2, 4.39%; and < 35 kg/m2, 7.33%. This data suggests BMI over a threshold 25 kg/m2 is clinically useful to stratify people for risk of fatty liver with a negative predictive value of 0.95. The authors were further able to prove by PDFF the fact that the prevalence of high liver fat was approximately 1 in 3 in the higher BMI population defined by a BMI > 30 kg/m2, suggesting PDFF as a screening method for risk patients [43]. MRI-PDFF-based quantifications of hepatic steatosis showed a good agreement with simultaneous histological assessment in prospective trials, including changes in steatosis grade upon pharmacological treatment [44•]. Thus, phase II exploratory studies in NASH occasionally use changes in MRI-PDFF measurements as an efficacy end point [32].
In sum, there are a number of radiologic modalities available for diagnosing steatosis in NAFLD and NASH patients. Each methodology has advantages and disadvantages (Table 1). Critical factors to be considered are patient’s constitution and characteristics (BMI, coexisting conditions), availability of the medical devices, numbers of patients to be examined per day, and budget available for an examination. Moreover, it is important to distinguish whether the method is intended as a screening tool, to establish a diagnosis or to monitor treatment responses.
Radiologic Modalities for Diagnosing Fibrosis in NASH
Transient Elastography (FibroScan®), Shear-Wave Elastography, and Other Ultrasound-Based Elastography Techniques
The measurement of the stiffness of soft tissues, i.e., their viscoelastic properties, may be directly related to the amount of extracellular matrix, i.e., fibrosis [46]. The FibroScan® introduced by Echosens SA (Paris, France) in 2005 works by measuring shear wave velocity of a 50-MHz wave through the liver, which is sent out from a small probe and measured by a transducer. As a result, the operator is able to follow the propagation and velocity of the shear wave (in meters per second [m/s]) in real time. The obtained value is subsequently quantitatively converted by an inversion algorithm into liver stiffness given in kilopascals (kPa), representing a physical parameter for liver’s resistance to deformation [47, 48]. Therefore, this technology is commonly called transient elastography or vibration-controlled transient elastography (VCTE). In a typical setup, the entire system is operated with a computer controlling the low-frequency excitation, typically ranging between 50 and 200 Hz, and the ultrasonic pulsed echo mode sampled at 5 MHz [47, 48]. This non-invasive and painless measurement is fast (~ 5–7 min/scan) and can be performed as a point-of-care testing. Results are instantaneously available and highly repeatable, while side effects or complications were not reported yet [49]. Once the scan has been successfully completed, the degree of hepatic scarring is immediately available. In a multicenter cross-sectional study, AUROCs for advanced fibrosis (F3/4) were 0.831, while VCTE values > 12 kPa (indicating F3/F4 fibrosis) were associated with significantly reduced survival upon long-term follow-up [50•]. This technology has been used in Europe for more than a decade now and was approved in April 2013 by the US Food and Drug Administration [51]. Across different etiologies of liver disease, VCTE is particularly suited to detect cirrhosis by high stiffness, for which the method has specificities about 99% [52]. However, FibroScan® is not applicable in all patients. In particular, the method has main drawbacks and limitations when testing patients suffering from ascites, large amounts of chest wall fat, or in morbidly obese patients [49].
Acoustic radiation force impulse elastography (ARFI) for the non-invasive evaluation of hepatic fibrosis in NASH is another sonographic technique, which was introduced by Siemens (Siemens Healthineers, Mountain View, CA, USA). In this method, the measuring beam passes over a standardized region of interest (ROI) of the liver, and short-duration acoustic pulses with each lasting less than 1 ms are subsequently generated in the vicinity of the designed ROI [53]. This in turn induces a mechanical excitation of the tissue, resulting in tissue displacement and formation of a shear wave away from the site of excitation, which correlates with the elasticity of the tissue and is expressed in meters per seconds [53]. The position of the echo is determined from the angular position of the transducer, the transit time of the acoustical pulse and its echo, while the resulting gray value of each pixel (i.e., the brightness, B-mode) are displayed on a screen producing a final image [53]. This method was first introduced in 2008 and tested in 20 human volunteers imaged inter-costally to reconstruct the shear moduli of their livers and two volunteers in whom liver stiffness was reconstructed nine times over a 105 day period to demonstrate the reproducibility of measurements [54]. Most importantly, obesity (BMI < 30 kg/m2) or ascites are not considered obstacles in performing shear modulus reconstructions [54, 55]. However, clinical evaluations revealed a rather poor performance of ARFI as well as VCTE to detect fibrosis in morbidly obese patients (BMI > 35 kg/m2) [56].
High-resolution real-time tissue elastography (Hi-RTE) is another diagnostic modality, which is promoted by Hitachi (Hitachi Medical Systems, Tokyo, Japan) [57]. It is an imaging technique to directly reveal the physical property of a tissue with conventional ultrasound probes. The strain and stress of the examined tissue is measured in real time to calculate its elasticity. The final elastography is depicted as a color-coded image over the conventional B-mode image, and final results are given as a video clip or as single images. Typically, blue-colored areas represent hardened tissue, while areas depicted in red or green mark tissue with soft properties [57]. A study analyzing 53 consecutive biopsy proven NASH patients and 20 controls has suggested that RTE could be used as a complementary imaging method to evaluate liver fibrosis in NASH patients [58]. However, determining the stage of liver fibrosis from RTE imaging is challenging, and therefore, ambitions are made to develop sophisticated machine-learning software tools as decision-support systems to improve classification of hepatic fibrosis [59].
Shear wave elastography (SWE) or supersonic shear imaging (SSI) is another ultrasound-based option for real-time visualization of soft tissue viscoelastic properties. The usage of ultrasonic-focused beams allows to remotely generate mechanical vibration sources radiating low-frequency, shear waves inside the tissues, which propagate through the tissue and are progressively distorted by tissue heterogeneities [60]. The information about the propagation stored in small video clips provides information allowing extracting the elasticity of the tissue. A meta-analysis has recently evaluated the diagnostic performance of liver stiffness measurements with SSI for the detection of liver fibrosis. In sum, the study showed that this technique has an overall high diagnostic accuracy for liver fibrosis with a technical success rate of 95.3% [61]. However, data for SWE particularly supports its use for fibrosis detection in hepatitis B, while data in NAFLD patients are rather limited at this time [62].
MR Elastography
Magnetic resonance elastography (MRE) can be considered as an imaging-based counterpart to palpation. It obtains information about the stiffness of a tissue by assessing the propagation of mechanical waves through the tissue with a special magnetic resonance imaging technique [63]. Generation of shear waves in the tissue, acquiring MR images depicting the propagation of the induced shear waves, and processing of images of the shear waves to generate quantitative maps of tissue stiffness are the three essential steps in this technique [63]. There are a number of reports available, which successfully applied MRE for detection of hepatic steatosis and fibrosis [24]. The accuracy of 2D MRE was comprehensively demonstrated in a study comparing the diagnostic utility of this method against eight clinical prediction rules, including the AST/ALT ratio, APRI, BARD, FIB-4, NAFLD fibrosis score, Bonacini cirrhosis discriminant score, Lok Index, and the NASH clinical prediction rule [64]. The obtained data of this study unequivocally showed higher accuracy of MRE than the mentioned algorithms in the diagnosis of advanced fibrosis in NAFLD patients. Moreover, MRE was found superior to VCTE in identifying NAFLD patients with fibrosis in a prospective study that compared both techniques, using histological fibrosis stage as the reference [65•]. This finding has been recently confirmed in an individual patient data meta-analysis comparing MRE and VCTE [66]. MRE has also shown high accuracy in detecting significant hepatic fibrosis in children with chronic liver disease, including severely obese patients [67]. In a recent prospective study, the evaluation of MRE in 49 NFALD patients and 41 healthy controls revealed an area under the ROC curve of MRE in discriminating healthy form NAFLD individuals of 0.964 and a value of 0.928 for distinguishing advanced (F3-F4) from absent/mild fibrosis (F0-F2) [68].
Experimental Imaging Techniques
There are many experimental strategies to develop novel imaging strategies for NASH. Among these strategies, pragmatic approaches attempt to combine different imaging techniques to obtain a comprehensive picture. Multiparametric MRI (LiverMultiScan, LMS) includes proton density fat fraction (PDFF) to assess hepatic triglyceride content, the Liver Inflammation and Fibrosis Score (LIF score) and liver iron content [69]. This multiparametric MRI was found to be associated with clinical outcome in chronic liver diseases [69]. Similarly, DeMILI (detection of metabolic-induced liver injury) is a patented algorithm using two outputs (NASH-MRI and FIBRO-MRI) based on optical analysis of images from standard MR systems. Other experimental approaches evaluate the use of molecular probes against extracellular matrix proteins [10], such as probes against collagen [70] or elastin [71] for MRI-based fibrosis detection.
Diffusion tensor imaging (DTI) is a sensitive MRI method for characterizing the motion of molecular diffusion of water. In an experimental study performed in Sprague-Dawley rats subjected to repetitive dosing of carbon tetrachloride, this method was suitable to demonstrate longitudinal alterations in apparent diffusion coefficient of water and to provide microstructural information in fibrotic liver tissue [72]. The authors of the mentioned study suggested DTI as a valuable method for detecting and characterizing liver fibrosis at early stages [72]. First human studies comparing HCC patients and healthy volunteers testing performance parameters as well as intra- and inter-session repeatability of this method are available [73, 74].
In sum, the determination of fibrosis in steatohepatitis and steatosis is challenging. Several techniques and medical devices have been introduced during the last decades, which have their specific “Pros and Cons” (Table 2).
Radiologic Modalities for Diagnosing Complications of Fibrotic NASH
NAFLD patients with advanced fibrosis (stages F3 and F4) have a high risk for liver-related complications, such as decompensation of liver function, portal hypertension, and HCC [4•]. The presence of cirrhosis can be suspected by several routine-imaging methods like ultrasound, CT or MRI, due to findings like a nodular liver surface, coarsened echotexture, blunting of liver edges, and concomitant splenomegaly [82]. Non-invasive techniques that assess the elastic properties of the liver (“liver stiffness”), like transient elastography, shear wave elastography, acoustic radiation force impulse imaging, or MR elastography, have a clear role for diagnosing cirrhosis in NAFLD [7•]. Nonetheless, cutoff values for liver stiffness to diagnose cirrhosis are less well defined for NAFLD compared to viral hepatitis, and obesity may impede the technical accuracy of ultrasound-based elastography techniques [83].
Portal hypertension can be suspected in patients with high values for transient elastography and low platelet counts, and this may guide the subsequent management of the patients such as the need for endoscopy to rule out esophageal varices [84]. Definitive diagnosis of portal hypertension can be made by the invasive angiographic procedure of measuring the hepatic venous pressure gradient (HVPG), which is associated with end points (e.g., risk for bleeding) in patients with liver cirrhosis [85].
Another domain of radiologic modalities is the screening, diagnosis, and staging of HCC. Patients with NAFLD have an increased risk of HCC development, even in the absence of liver cirrhosis. However, the role of imaging methods for HCC surveillance in non-cirrhotic NAFLD patients is currently not well established [86] and, therefore, not generally recommended [6]. Patients with liver cirrhosis, irrespective of the underlying etiology, should undergo HCC surveillance [86, 87]. The American and European guidelines recommend ultrasound, with or without concomitant measurement of α-fetoprotein from blood, as the first-line screening method for HCC in patients at high risk [86, 87]. In general, the sensitivity of an expert ultrasound for HCC detection during screening is around 60–80% with a specificity of more than 90% [88]. However, ultrasound has technical limitations due to the penetration depth, especially in patients with morbid obesity.
In case of suspicious nodules in a fatty and/or cirrhotic liver, multiphasic CT or MRI should be performed [87]. HCC can be confidently diagnosed by these imaging techniques, if the tumor shows a typical intense contrast uptake during the arterial phase followed by contrast washout during the venous phases [89•]. Alternatively, contrast-enhanced ultrasound may be used as well [86]. If a typical vascularization pattern is detected by CT or MRI in nodules between 1 and 2 cm, the specificity for diagnosing HCC is close to 100%, and the sensitivity can reach 71% [89•]. Standardized reporting and data collection of CT and MRI, such as the Liver Imaging Reporting and Data System (LI-RADS) by the American College of Radiology, further improve the diagnostic accuracy of interpreting nodules in cirrhotic and non-cirrhotic liver [89•]. Liver MR imaging has been further advanced by the use of extracellular or hepatobiliary contrast agents (gadoxetic acid or gadobenate dimeglumine). The dynamic plus hepatobiliary phase with gadobenate dimeglumine-enhanced MRI was superior to multiphasic CT and ultrasound in diagnosing HCC [90]. Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) MRI and diffusion-weighted imaging appear exceptionally promising for detecting early HCC and high-grade dysplastic nodules in patients with cirrhosis [91]. Nonetheless, it has to be emphasized that most radiologic methods for detecting HCC have been developed and validated in non-NAFLD patients, leaving a degree of uncertainty to whether their performance will be similarly good in steatotic livers and/or obese patients.
Perspective
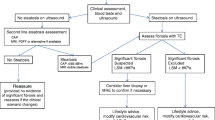
Due to its non-invasive nature, wide availability, and recent technical advances, radiologic modalities have an increasing relevance in screening, diagnosis, staging, and prognostication of NAFLD. Different techniques exist that are capable of quite accurately quantifying steatosis and fibrosis. It can be envisioned that simple and fast techniques such as ultrasound, CAP, or elastography may be implemented to screen for advanced steatosis and fibrosis in clinical routine within the next decade [9], while the more expensive and time-consuming MRI-based methods may be restricted to individuals with suspected NAFLD after using a screening test. Figure 1 summarizes the potential application of the different techniques for screening or diagnostic purposes in the different stages of NAFLD.
Potential application of radiologic techniques in different stages of NAFLD. In order to screen for steatosis, ultrasound and controlled attenuation parameter (CAP) appear suitable, while liver fat quantification for diagnostic purposes or clinical trials is more accurate with magnetic resonance spectroscopy (MRS) and MRI-proton density fat fraction (MRI-PDFF). Fibrosis and cirrhosis can be suspected by ultrasound and ultrasound-based elastography techniques (transient elastography, TE; acoustic resonance forced impulse imaging, ARFI; shear wave elastography, SWE). MR elastography (MRE) may quantify fibrosis, and angiographic hepatic venous pressure gradient (HVPG) measurement can confirm portal hypertension in cirrhosis. Screening for hepatocellular carcinoma (HCC) in cirrhotic livers is done by ultrasound; suspicious nodules are followed by multiphasic CT/MRI, contrast-enhanced ultrasound (CEUS), or contrast-enhanced MRI
Several recent studies directly compared the diagnostic accuracy of these various modalities. In a cross-sectional study from Japan on 142 patients with biopsy-proven NAFLD, hepatic steatosis grade ≥ 2 was identified by CAP with an AUROC curve value of 0.73, while PDFF reached an AUROC curve value of 0.90 (p < 0.001); similarly, MRE performed superior to VCTE to identify patients with fibrosis stage ≥ 2 in this cohorts (AUROC 0.82 vs. 0.91, p < 0.001) [92•]. In a similar cross-sectional single-center study on 104 individuals performed in the USA, MRI-PDFF was superior to CAP for the detection of any steatosis (AUROC 0.99 vs. 0.85, p = 0.0091), while MRE was superior to VCTE in detecting any fibrosis stage 1 or more (AUROC 0.82 vs. 0.67, p = 0.0116) [65•]. However, the diagnostic validity of these different methods in clinical routine of unselected, heterogeneous patient cohorts require further prospective evaluation.
Data from clinical trials in NASH also report important changes in radiologic findings longitudinally, suggesting that these techniques may be suitable as surrogates for clinical end points. For instance, a relative reduction in liver fat on MRI-PDFF by 29% had been associated with a histological improvement of NASH, defined as a 2-point reduction in the NAFLD Activity Score [93]. Thus, radiologic techniques are now being increasingly used as short-term read-out indicating therapeutic efficacy in early clinical trials [32].
The further advances in radiologic techniques, standardizations of interpretation, and validated algorithms support the expectation that imaging will remain and evolve as a cornerstone in the management of patients with NAFLD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Parikh ND, Marrero WJ, Wang J, Steuer J, Tapper EB, Konerman M, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology. 2017 Aug 17. https://doi.org/10.1002/hep.29473.
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Geier A, Kondili LA, Lazarus JV, Loomba R, Manns MP, Marchesini G, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. https://doi.org/10.1016/j.jhep.2018.05.036
• Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557–65. https://doi.org/10.1002/hep.29085 This meta-analysis established the link between fibrosis and mortality in NAFLD.
•• European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004 This is the first international, multidisciplinary guideline on the management of NAFLD.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367.
• Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Goodman ZD, Chalasani NP, Kowdley KV, George J, Lindor K. Diagnostic modalities for non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and associated fibrosis. Hepatology. 2018;68(1):349–60. https://doi.org/10.1002/hep.29721. https://doi.org/10.1002/hep.29721. An important and recent review article on diagnostic modalities in NAFLD.
Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705–12. https://doi.org/10.1111/j.1478-3231.2008.01691.x.
Loomba R. Role of imaging-based biomarkers in NAFLD: recent advances in clinical application and future research directions. J Hepatol. 2018;68(2):296–304. https://doi.org/10.1016/j.jhep.2017.11.028.
Baues M, Dasgupta A, Ehling J, Prakash J, Boor P, Tacke F, et al. Fibrosis imaging: current concepts and future directions. Adv Drug Deliv Rev. 2017;121:9–26. https://doi.org/10.1016/j.addr.2017.10.013.
Azhari H. Ultrasound: medical imaging and beyond (an invited review). Curr Pharm Biotechnol. 2012;13(11):2104–16. https://doi.org/10.2174/138920112802502033.
Seo J, Kim Y-S. Ultrasound imaging and beyond: recent advances in medical ultrasound. Biom Eng Lett. 2017;7:57–8. https://doi.org/10.1007/s13534-017-0030-7.
Mozaffari MH, Lee WS. Freehand 3-D ultrasound imaging: a systematic review. Ultrasound Med Biol. 2017;43(10):2099–124. https://doi.org/10.1016/j.ultrasmedbio.2017.06.009.
Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Bioeffects Committee of the American Institute of Ultrasound in Medicine Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4):623–34. https://doi.org/10.7863/jum.2012.31.4.623.
Taylor KJ, Gorelick FS, Rosenfield AT, Riely CA. Ultrasonography of alcoholic liver disease with histological correlation. Radiology. 1981;141(1):157–61. https://doi.org/10.1148/radiology.141.1.6270725.
Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52(4):579–85. https://doi.org/10.1016/j.jhep.2010.01.008.
Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–31. https://doi.org/10.1053/j.gastro.2010.09.038.
Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol. 2007;102(12):2716–7. https://doi.org/10.1111/j.1572-0241.2007.01520.x.
Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47(2):239–44. https://doi.org/10.1016/j.jhep.2007.02.007.
Baumeister SE, Völzke H, Marschall P, John U, Schmidt CO, Flessa S, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134(1):85–94. https://doi.org/10.1053/j.gastro.2007.10.024.
Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(22):6821–5. https://doi.org/10.3748/wjg.v20.i22.6821.
Battaglia V, Cervelli R. Liver investigations: updating on US technique and contrast-enhanced ultrasound (CEUS). Eur J Radiol. 2017;96:65–73. https://doi.org/10.1016/j.ejrad.2017.08.029.
Perez NE, Siddiqui FA, Mutchnick MG, Dhar R, Tobi M, Ullah N, et al. Ultrasound diagnosis of fatty liver in patients with chronic liver disease: a retrospective observational study. J Clin Gastroenterol. 2007;41(6):624–9. https://doi.org/10.1097/01.mcg.0000225680.45088.01.
Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65(5):1006–16. https://doi.org/10.1016/j.jhep.2016.06.005.
Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36(11):1825–35. https://doi.org/10.1016/j.ultrasmedbio.2010.07.005.
Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–13. https://doi.org/10.1016/j.ultrasmedbio.2003.07.001.
Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G852–8. https://doi.org/10.1152/ajpgi.00521.2005.
Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - where do we stand? World J Gastroenterol. 2016;22(32):7236–51. https://doi.org/10.3748/wjg.v22.i32.7236.
• Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–30. https://doi.org/10.1016/j.jhep.2016.12.022 This individual patient data-based meta-analysis identified important confounders for CAP measurement.
Desai NK, Harney S, Raza R, Al-Ibraheemi A, Shillingford N, Mitchell PD, et al. Comparison of controlled attenuation parameter and liver biopsy to assess hepatic steatosis in pediatric patients. J Pediatr. 2016;173:160–164.e1. https://doi.org/10.1016/j.jpeds.2016.03.021.
de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026–31. https://doi.org/10.1016/j.jhep.2013.12.018.
Caussy C, Reeder SB, Sirlin CB, Loomba R. Non-invasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018 Jan 21;68:763–72. https://doi.org/10.1002/hep.29797.
Kjærgaard M, Thiele M, Jansen C, Stæhr Madsen B, Görtzen J, Strassburg C, et al. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS One. 2017;12(4):e0173992. https://doi.org/10.1371/journal.pone.0173992.
Plewes DB, Kucharczyk W. Physics of MRI: a primer. J Magn Reson Imaging. 2012;35(5):1038–54. https://doi.org/10.1002/jmri.23642.
Handbook of MRI technique, 4th edition. Ed.: C. Westbrook, John Wiley & Sons, 2014. ISBN: 978–1–118-66162-8.
Kim KH, Do WJ, Park SH. Improving resolution of MR images with an adversarial network incorporating images with different contrast. Med Phys. 2018;45:3120–31. https://doi.org/10.1002/mp.12945.
Ibrahim MA, Dublin AB. Magnetic Resonance Imaging (MRI), Gadolinium. 2018 Jan 25. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from http://www.ncbi.nlm.nih.gov/books/NBK482487/
Pasquini L, Napolitano A, Visconti E, Longo D, Romano A, Tomà P, et al. Adolinium-based contrast agent-related toxicities. CNS Drugs. 2018;32(3):229–40. https://doi.org/10.1007/s40263-018-0500-1.
Rogowska J, Olkowska E, Ratajczyk W, Wolska L. Gadolinium as a new emerging contaminant of aquatic environments. Environ Toxicol Chem. 2018;37(6):1523–34. https://doi.org/10.1002/etc.4116.
Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67(4):1348–59. https://doi.org/10.1002/hep.29639.
Kim KY, Song JS, Kannengiesser S, Han YM. Hepatic fat quantification using the proton density fat fraction (PDFF): utility of free-drawn-PDFF with a large coverage area. Radiol Med. 2015;120(12):1083–93. https://doi.org/10.1007/s11547-015-0545-x.
Kim M, Kang BK, Jun DW. Comparison of conventional sonographic signs and magnetic resonance imaging proton density fat fraction for assessment of hepatic steatosis. Sci Rep. 2018;8(1):7759. https://doi.org/10.1038/s41598-018-26019-x.
Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, et al. Characterisation of liver fat in the UK biobank cohort. PLoS One. 2017;12(2):e0172921. https://doi.org/10.1371/journal.pone.0172921.
• Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153(3):753–61. https://doi.org/10.1053/j.gastro.2017.06.005 This study demonstrated agreement between MRI-PDFF and histological steatosis.
Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32(6):902–10. https://doi.org/10.1111/j.1478-3231.2012.02781.x.
Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2018. https://doi.org/10.1016/j.mam.2018.06.003
Sandrin L, Tanter M, Gennisson JL, Catheline S, Fink M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(4):436–46. https://doi.org/10.1109/58.996561.
Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(4):426–35. https://doi.org/10.1109/58.996560.
Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N Y). 2012;8(9):605–7.
• Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65(3):570–8. https://doi.org/10.1016/j.jhep.2016.04.023 This work compared cross-sectional and longitudinal assessments of several serum biomarker tests and elastography in a large cohort of NAFLD patients.
U.S. Food & Drug Administration (FDA): Echosens’s FibroScan® System. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf12/k123806.pdf. last downloaded: September 17, 2018.
Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, et al. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58(5):928–35. https://doi.org/10.1016/j.jhep.2012.12.021.
Jaffer OS, Lung PF, Bosanac D, Patel VM, Ryan SM, Heneghan MA, et al. Acoustic radiation force impulse quantification: repeatability of measurements in selected liver segments and influence of age, body mass index and liver capsule-to-box distance. Br J Radiol. 2012;85(1018):e858–63. https://doi.org/10.1259/bjr/74797353.
Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546–58. https://doi.org/10.1016/j.ultrasmedbio.2007.10.009.
Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55(3):666–72. https://doi.org/10.1016/j.jhep.2010.12.019.
Karlas T, Dietrich A, Peter V, Wittekind C, Lichtinghagen R, Garnov N, et al. Evaluation of transient Elastography, acoustic radiation force impulse imaging (ARFI), and enhanced liver function (ELF) score for detection of fibrosis in morbidly obese patients. PLoS One. 2015;10(11):e0141649. https://doi.org/10.1371/journal.pone.0141649.
Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, et al. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81(3):e325–31. https://doi.org/10.1016/j.ejrad.2011.10.029.
Orlacchio A, Bolacchi F, Antonicoli M, Coco I, Costanzo E, Tosti D, et al. Liver elasticity in NASH patients evaluated with real-time elastography (RTE). Ultrasound Med Biol. 2012;38(4):537–44. https://doi.org/10.1016/j.ultrasmedbio.2011.12.023.
Chen Y, Luo Y, Huang W, Hu D, Zheng RQ, Cong SZ, et al. Machine-learning-based classification of real-time tissue elastography for hepatic fibrosis in patients with chronic hepatitis B. Comput Biol Med. 2017;89:18–23. https://doi.org/10.1016/j.compbiomed.2017.07.012.
Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(4):396–409. https://doi.org/10.1109/TUFFC.2004.1295425.
Deng H, Qi X, Zhang T, Qi X, Yoshida EM, Guo X. Supersonic shear imaging for the diagnosis of liver fibrosis and portal hypertension in liver diseases: a meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12(1):91–8. https://doi.org/10.1080/17474124.2018.1412257.
Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67(1):260–72. https://doi.org/10.1002/hep.29179.
Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23(5):497–511. https://doi.org/10.1002/ca.21006.
Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41(12):1271–80. https://doi.org/10.1111/apt.13196.
• Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607.e2. https://doi.org/10.1053/j.gastro.2016.10.026 This work directly compared MRE and VCTE, demonstrating superior performance of MRE for fibrosis staging.
Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, Hooker J, Tu X, Bettencourt R, Yin M, Sirlin CB, Ehman RL, Nakajima A, Loomba R. Magnetic resonance vs transient elastography analysis of patients with non-alcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2018. https://doi.org/10.1016/j.cgh.2018.05.059.
Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, et al. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr. 2014;164(1):186–8. https://doi.org/10.1016/j.jpeds.2013.07.050.
Costa-Silva L, Ferolla SM, Lima AS, Vidigal PVT, Ferrari TCA. MR elastography is effective for the non-invasive evaluation of fibrosis and necroinflammatory activity in patients with nonalcoholic fatty liver disease. Eur J Radiol. 2018;98:82–9. https://doi.org/10.1016/j.ejrad.2017.11.003.
Pavlides M, Banerjee R, Tunnicliffe EM, Kelly C, Collier J, Wang LM, et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37(7):1065–73. doi. https://doi.org/10.1111/liv.13284.
Farrar CT, Gale EM, Kennan R, Ramsay I, Masia R, Arora G, et al. CM-101: type I collagen-targeted MR imaging probe for detection of liver fibrosis. Radiology. 2018;287(2):581–9. doi. https://doi.org/10.1148/radiol.2017170595.
Ehling J, Bartneck M, Fech V, Butzbach B, Cesati R, Botnar R, et al. Elastin-based molecular MRI of liver fibrosis. Hepatology. 2013;58(4):1517–8. doi. https://doi.org/10.1002/hep.26326.
Cheung JS, Fan SJ, Gao DS, Chow AM, Man K, Wu EX. Diffusion tensor imaging of liver fibrosis in an experimental model. J Magn Reson Imaging. 2010;32(5):1141–8. https://doi.org/10.1002/jmri.22367.
Li X, Liang Q, Zhuang L, Zhang X, Chen T, Li L, et al. Preliminary study of MR diffusion tensor imaging of the liver for the diagnosis of hepatocellular carcinoma. PLoS One. 2015;10(8):e0135568. https://doi.org/10.1371/journal.pone.0135568.
Wong OL, Leung TWT, Lo GG, Yuan J, Li WW, Noseworthy MD. Intrasession and intersession repeatability of diffusion tensor imaging in healthy human liver. J Comput Assist Tomogr. 2017;41(4):578–85. https://doi.org/10.1097/RCT.0000000000000572.
Ajmera V, Loomba R. Can elastography differentiate isolated fatty liver from nonalcoholic steatohepatitis? Semin Liver Dis. 2018;38(1):14–20. https://doi.org/10.1055/s-0037-1618587.
Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51(3):828–35. https://doi.org/10.1002/hep.23425 NA.
Lv S, Jiang S, Liu S, Dong Q, Xin Y, Xuan S. Noninvasive quantitative detection methods of liver fat content in nonalcoholic fatty liver disease. J Clin Transl Hepatol. 2018;6(2):217–21. https://doi.org/10.14218/JCTH.2018.00021.
Praveenraj P, Gomes RM, Basuraju S, Kumar S, Senthilnathan P, Parathasarathi R, et al. Preliminary evaluation of acoustic radiation force impulse shear wave imaging to detect hepatic fibrosis in morbidly obese patients before bariatric surgery. J Laparoendosc Adv Surg Tech A. 2016;26(3):192–5. https://doi.org/10.1089/lap.2015.0396.
Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38(7):1103–8. https://doi.org/10.1016/j.ultrasmedbio.2012.02.032.
Liu H, Fu J, Hong R, Liu L, Li F. Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: a systematic review & meta-analysis. PLoS One. 2015;10(7):e0127782. https://doi.org/10.1371/journal.pone.0127782.
Shiraishi A, Hiraoka A, Aibiki T, Okudaira T, Kawamura T, Imai Y, et al. Real-time tissue elastography: non-invasive evaluation of liver fibrosis in chronic liver disease due to HCV. Hepato-Gastroenterology. 2014;61(135):2084–90.
Choong CC, Venkatesh SK, Siew EP. Accuracy of routine clinical ultrasound for staging of liver fibrosis. J Clin Imaging Sci. 2012;2:58. https://doi.org/10.4103/2156-7514.101000.
Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018;286(3):738–63. https://doi.org/10.1148/radiol.2018170601.
de Franchis R, Baveno VI, Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. https://doi.org/10.1016/j.jhep.2015.05.022.
European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. https://doi.org/10.1016/j.jhep.2018.03.024.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–80. https://doi.org/10.1016/j.jhep.2018.03.019.
Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37–47. https://doi.org/10.1111/j.1365-2036.2009.04014.x.
• Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2 An important recent review on diagnosis and management of liver cancer.
Di Martino M, De Filippis G, De Santis A, Geiger D, Del Monte M, Lombardo CV, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol. 2013;23(4):887–96. https://doi.org/10.1007/s00330-012-2691-z.
Renzulli M, Biselli M, Brocchi S, Granito A, Vasuri F, Tovoli F, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut. 2018;67:1674–82. https://doi.org/10.1136/gutjnl-2017-315384.
• Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–37. https://doi.org/10.1053/j.gastro.2015.11.048 This work directly compared transient elastography- and MRI-based methods for steatosis and fibrosis assessment in biopsy-confirmed NAFLD patients.
Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9(5):692–701. https://doi.org/10.1177/1756283X16656735.
Acknowledgments
The authors sincerely thank Sabine Weiskirchen for preparing the figure for this review article.
Funding
The laboratories of the authors are supported by the German Research Foundation (DFG, SFB/TRR57) and grants from the Interdisciplinary Centre for Clinical Research (projects O3-1, O3-2) within the Faculty of Medicine at the RWTH Aachen University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Frank Tacke has been supported by funding from Tobira Therapeutics, Bristol Myers Squibb, Galapagos, and Allergan.
Ralf Weiskirchen cooperates with Silence Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Fatty Liver Disease
Rights and permissions
About this article
Cite this article
Weiskirchen, R., Tacke, F. The Role of Radiologic Modalities in Diagnosing Nonalcoholic Steatohepatitis (NASH) and Fibrosis. Curr Hepatology Rep 17, 324–335 (2018). https://doi.org/10.1007/s11901-018-0421-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-018-0421-y