Abstract
Nonalcoholic fatty liver disease (NAFLD) is entering a new era in terms of diagnosis and conceptualization. The term NAFLD is considered to not reflect current knowledge. Metabolic dysfunction-associated fatty liver disease (MAFLD) has been suggested as a more appropriate overarching term by experts in this field. Regarding NAFLD progression, most patients die from non-liver-related diseases, even patients with advanced fibrosis. Liver biopsy is essential for the diagnosis of nonalcoholic steatohepatitis (NASH); it is the only procedure that reliably differentiates NAFLD from NASH. Recently, various noninvasive methods for diagnosing steatosis and fibrosis have been developed. Ultrasound attenuation measurements and proton density fat fraction with magnetic resonance imaging (MRI) have been developed as imaging tools for predicting steatosis. Fibrosis-4 index and NAFLD fibrosis score are complex scores for predicting fibrosis in patients with NAFLD. In addition, elastography based on ultrasound and MRI has been developed as an imaging tool for predicting fibrosis. There is a strong correlation between values from various real-time shear wave elastography devices and transient elastography, which is the gold standard for ultrasound-based measurements of liver stiffness. In conclusion, NAFLD is at a turning point in terms of its conceptualization, terminology, and diagnostics. It is now time to reconfirm the role of ultrasonography for the assessment of NAFLD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
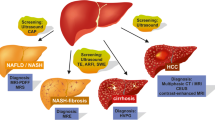
Suppression and elimination of chronic hepatitis B virus and hepatitis C virus infection have become realistic goals. Non-B non-C hepatocellular carcinoma (HCC) now accounts for one-third of all cases of HCC in Japan [1]. The main etiology of non-B non-C HCC is fatty liver disease, which is caused by alcohol consumption, lifestyle-related diseases, or both [1]. In the past, fatty liver disease associated with low alcohol consumption was called nonalcoholic fatty liver disease (NAFLD). A change in nomenclature from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed [2]. NAFLD with progression of fibrosis is a leading cause of liver disease-related mortality (HCC, liver failure, or esophageal variceal hemorrhage) and liver transplantation [3]. In this review article, we describe new trends such as new terminology and noninvasive imaging assessment in NAFLD and nonalcoholic steatohepatitis (NASH), especially in Japan. In addition, we describe the new role of and problems associated with ultrasonic examinations in NAFLD and NASH practice.
Definitions and risk factors
NAFLD is characterized by excessive hepatic fat accumulation related to insulin resistance. NAFLD is defined by the presence of steatosis in > 5% of hepatocytes based on histological analysis or a proton density fat fraction (PDFF) > 5.6% [4] based on magnetic resonance imaging (MRI). In most patients with NAFLD, this disease is commonly associated with metabolic comorbidities such as obesity, type 2 diabetes mellitus, and dyslipidemia. NAFLD can be categorized histologically into nonalcoholic fatty liver (NAFL) or NASH. NAFL is defined as the presence of steatosis in > 5% of hepatocytes without evidence of hepatocellular injury in the form of hepatocyte ballooning. NASH is defined as the presence of steatosis in > 5% of hepatocytes and inflammation with hepatocyte injury (e.g., ballooning), with or without any fibrosis. Definitive diagnosis of NASH requires a pathological finding with liver biopsy. The diagnosis of NAFLD requires the exclusion of secondary causes such as drug-related NAFLD as well as daily alcohol consumption < 30 g for men and < 20 g for women [5]. Alcohol consumption above these limits indicates alcoholic liver disease. The relationship between alcohol intake and liver injury depends on several cofactors such as types of alcoholic beverages consumed, drinking patterns, duration of drinking habit, and individual or genetic susceptibility. In particular, patients who consume moderate amounts of alcohol may still be predisposed to NAFLD if they have metabolic risk factors. Of note, the overall impact of metabolic risk factors on the occurrence of steatosis appears to be higher than that of alcohol in these patients [6].
New terminology
Although pharmacotherapies for NAFLD are in development, response rates appear modest. The heterogeneous pathogenesis of metabolic fatty liver diseases and inaccuracies in terminology and definitions necessitate a reappraisal of nomenclature to inform clinical trial design and drug development. Recently, a group of experts sought to integrate the current understanding of patient heterogeneity captured under the acronym NAFLD and suggest terminology that more accurately reflects pathogenesis to help with patient stratification for management. These experts reached the consensus that NAFLD does not reflect current knowledge. MAFLD was suggested as a more appropriate overarching term [7]. This recommendation will help the research community update the nomenclature and sub-phenotype the disease to accelerate the development of new treatments.
The proposed criteria for a positive diagnosis of MAFLD are based on histological (biopsy), imaging, or blood biomarker evidence of fat accumulation in the liver (hepatic steatosis), in addition to one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus, or evidence of metabolic dysregulation. Metabolic dysregulation is defined by the presence of at least two metabolic risk abnormalities: waist circumference ≥ 102/88 cm in Caucasian men and women (or ≥ 90/80 cm in Asian men and women); blood pressure ≥ 130/85 mmHg or specific drug treatment; plasma triglycerides ≥ 150 mg/dL (≥ 1.70 mmol/l) or specific drug treatment; plasma high-density lipoprotein cholesterol < 40 mg/dL (< 1.0 mmol/L) for men and < 50 mg/dL (< 1.3 mmol/L) for women or specific drug treatment; prediabetes (i.e., fasting glucose levels 100–125 mg/dL (5.6–6.9 mmol/L), or 2-h post-load glucose levels 140–199 mg/dL (7.8–11.0 mmol) or HbA1c 5.7–6.4% (39–47 mmol/mol)); homeostasis model assessment-insulin resistance score ≥ 2.5; plasma high-sensitivity C-reactive protein level > 2 mg/L. In addition, MAFLD concomitant with other liver diseases was suggested as follows: "exclusion of alcohol-associated fatty liver disease based on current criteria for alcohol use disorder, viral infections (human immunodeficiency virus, hepatitis B virus, hepatitis C virus), drug-induced liver injury, autoimmune hepatitis either at baseline or at follow-up is not a prerequisite criterion for diagnosis. Patients who meet the criteria to diagnose MAFLD as described above and who also have one of these concomitant conditions should be defined as having dual (or more) aetiology fatty liver disease."
Epidemiology, especially in Asia
The prevalence of NAFLD pooled across Asian countries was estimated to be 27.4% (95% confidence interval [CI], 23.3–31.9%) [8]. In Japan, the prevalence of NAFLD ranges from 24.6 to 29.7% [9, 10], similar to the prevalence in China and South Korea. NASH is present in at least 20% of obese adults and children and at least 5% of overweight adults and children [11]. The prevalence of NASH pooled across Asian countries in patients with biopsy-proven NAFLD is 63.5% (95% CI 47.7–76.8%) [8]. NASH has emerged as the most common cause of cryptogenic cirrhosis and HCC worldwide. A study from India showed that NAFLD accounts for about 63% of all cases of cryptogenic cirrhosis [12]. In Japan, cirrhosis is now the fourth most common cause of death (4.7%) in patients with type 2 diabetes mellitus, and HCC is the leading cause of cancer-related death (8.6%) [13].
The estimated prevalence of NAFLD in the United States is approximately 23.5%. The prevalence of NASH is estimated to be between 1.5 and 6.5% [14, 15]. The pooled estimated incidence for NAFLD in Western countries is 28 per 1000 person-years (95% CI 19.3–40.6 per 1000 person-years) [14, 16].
Most NAFLD cases worldwide are related to metabolic comorbidities, suggesting a bidirectional association. Metabolic comorbidities are risk factors for NAFLD and NASH, and the prevalence of NAFLD and NASH is high in patients with metabolic comorbidities. In a recent meta-analysis of patients with diabetes, the prevalence of NAFLD was 57.8% (95% CI 53.9–61.6%), whereas the prevalence of NASH was 65.3% (95% CI 51.7–76.7%) and the prevalence of advanced fibrosis (fibrosis ≥ F3) was 15.1% (95% CI 8.2–26.1%) [17]. In addition to type 2 diabetes mellitus, most morbidly obese patients undergoing bariatric surgery have NAFLD, 20–30% have NASH, and 10% have advanced fibrosis [18].
We recently evaluated clinical risk factors for progression of liver fibrosis in 1562 middle-aged (36–64 years) patients with NAFLD and less severe liver fibrosis (Fibrosis-4 [FIB-4] index < 1.3) [19]. During follow-up, 186 patients progressed to advanced fibrosis (FIB-4 index > 2.67). The 3-year, 5-year, 7-year, and 10-year cumulative incidences of progression to advanced fibrosis were 4.4%, 6.7%, 11.0%, and 16.7%, respectively. Univariate analysis showed that age, albumin concentration, and type 2 diabetes mellitus were significantly associated with progression to advanced fibrosis. Multivariate analysis with adjustment for age, smoking, body mass index, albumin, estimated glomerular filtration rate, dyslipidemia, type 2 diabetes mellitus, and steatosis showed that age ≥ 50 years (hazard ratio [HR], 2.121; 95% CI 1.462–3.076; p < 0.001), albumin concentration < 4.2 g/dL (HR, 1.802; 95% CI 1.285–2.528; p < 0.001), and presence of type 2 diabetes mellitus (HR, 1.879; 95% CI 1.401–2.520; p < 0.001) were independently associated with progression to advanced fibrosis. Conversely, degree of steatosis was not associated with progression to advanced fibrosis. The respective 3-year, 5-year, 7-year, and 10-year cumulative incidences of progression to advanced fibrosis were 3.6%, 5.0%, 8.2%, and 12.9% in patients without type 2 diabetes mellitus (n = 1,077) and 6.1%, 10.4%, 16.7%, and 24.0% in patients with type 2 diabetes mellitus (n = 485; p < 0.001). Therefore, we concluded that type 2 diabetes mellitus was associated with progression to advanced liver fibrosis in middle-aged patients with NAFLD, even in those with less severe liver fibrosis.
Few studies have evaluated the incidence of obesity and NAFLD in Asia. The annual incidence of obesity in 2008 was 0.70% in Chinese subjects aged 35–74 years. The incidence was higher in women (0.77%) than men (0.61%), in northern (0.93%) than in southern China (0.51%), and in rural (0.73%) than in urban areas (0.65%) [20]. The incidence of obesity in Japanese subjects, aged 40–69 years-old and non-obese at baseline, was 0.3–1.1% in men and 0.6–1.2% in women living on the main islands, and 0.8–3.7% in men and 1.4–3.1% in women living on Okinawa, between 1993 and 2003 [21]. The incidence of NAFLD in Japan was 52.3 (95% CI 28.3–96.8) per 1000 person-years in 2005 [8]. Among non-obese Chinese, 8.9% developed NAFLD in the 5 years from 2006 to 2011 [22].
NAFLD progression
Patients with histologic NASH, especially those with some degree of fibrosis, are at the greatest risk for progression to cirrhosis and liver-related mortality [23]. However, the most common cause of death in patients with NAFLD and NASH is cardiovascular disease; liver-related disease is among the top three causes of death [14, 23]. A meta-analysis suggested rates of liver-related and overall mortality to be 0.8 (range 0.3–1.8) per 1000 and 11.8 (range 7.1–19.5) per 1000 person-years among patients with NAFLD, and 15.4 (range 11.7–20.3) per 1000 and 25.6 (range 6.3–103.8) per 1000 person-years among patients with NASH, respectively [8]. With an increasing number of patients with cirrhosis, NAFLD has become one of the most common underlying causes of HCC and the second to third most common indication for liver transplantation [14]. These data show that progressive NAFLD and NASH make up an increasing proportion of patients with HCC and patients listed for liver transplantation [24, 25]. The progression of NASH is nonlinear, with some patients experiencing progression and others experiencing spontaneous regression [23]. This complex pattern requires noninvasive diagnostic and prognostic biomarkers that can help clinicians identify patients at the highest risk for progressive liver disease. Clinically, patients with NAFLD and metabolic comorbidities are at the greatest risk for progression [26,27,28]. In addition, patients with fibrosis stage ≥ 2 on liver biopsy are at risk for liver-related and non-liver-related mortality [29, 30]. Some patients with metabolic comorbidities may not meet the pathologic criteria for NASH, such as patients with cryptogenic cirrhosis or severe steato-fibrosis [14, 31]. Both groups are excluded from clinical trials of NASH, but they experience poor long-term prognosis, similar or worse to the prognosis of patients with cirrhosis secondary to NASH [31]. In clinical practice, all patients with advanced fibrosis should be considered in a similar fashion.
Recently, we evaluated all-cause mortality in 4073 patients with NAFLD based on the NAFLD fibrosis score (NFS) [32]. Of the 4073 patients, 179 died during follow-up, but only nine deaths were due to liver-related diseases. Of the 170 patients who died due to non-liver-related diseases, 83 (48.8%), 42 (24.7%), and 45 (26.5%) patients died due to malignancy, cerebrovascular or cardiovascular disease, and benign diseases (excluding cerebrovascular and cardiovascular disease), respectively. Figure 1 shows the cumulative incidence of liver-related and non-liver-related disease mortality using the competitive risk method. Multivariate analysis showed that intermediate and high NFS were independently associated with each disease category: malignancy, HR 2.163 (95% CI 1.354–3.457) and HR 4.814 (95% CI 2.323–9.977); cerebrovascular and cardiovascular disease, HR 2.265 (95% CI 1.141–4.497) and HR 8.482 (95% CI 3.558–20.220); and benign disease, HR 3.216 (95% CI 1.641–6.303) and HR 5.558 (95% CI 1.923–16.070), respectively. Conversely, steatosis severity was not associated with risk of mortality in the multivariate analysis. Therefore, we concluded that progression of liver fibrosis severity is associated with mortality from various non-liver-related causes in patients with NAFLD.
Pathogenesis: lifestyle and genes
A high-calorie diet, excess saturated fat intake, refined carbohydrates, sugar-sweetened beverages, high fructose consumption, and a Western diet [33] have all been associated with weight gain and obesity and, more recently, with NAFLD. High fructose consumption may increase the risk of NASH and advanced fibrosis, although the association may be confounded by excess calorie intake, unhealthy lifestyle, or sedentary behavior [34], which are more common in patients with NAFLD [35].
Several genetic modifiers of NAFLD severity have been identified [36]. The best-characterized genetic association is PNPLA3, initially identified in genome-wide association studies and confirmed in multiple cohorts and ethnicities as a modifier of NAFLD severity across the entire histological spectrum [37, 38]. Recently, the TM6SF2 gene has been reported as another disease modifier [39, 40] that may have clinical utility for risk stratification in liver-related versus cardiovascular morbidity. The PNPLA3 rs738409 variant also confers susceptibility and affects the histological pattern of NAFLD and fibrosis in obese children and adolescents [41]. A NASH risk score based on four polymorphisms has been validated in obese children with elevated levels of liver enzymes [42].
Liver-related complications
Like other chronic liver diseases, NAFLD and NASH induce fibrosis progression in some patients, eventually leading to cirrhosis and its complications. However, due to the close association between NAFLD and metabolic syndrome, most patients die of cardiovascular disease or cancer rather than liver-related complications. However, given the high number of patients with NAFLD, many would still develop liver-related complications even if they only represent a small proportion of all patients with NAFLD. Therefore, it is not surprising that NAFLD and NASH represent an important cause of HCC and end-stage liver disease in the Western world [24, 43].
Since NAFLD has not been a research focus in Asia until recently, clinical outcome data are scarce. In a retrospective study of 6508 Japanese patients with NAFLD diagnosed with ultrasonography, only 16 (0.25%) patients developed HCC during a median follow-up of 5.6 years [44]. In another cohort of 307 patients with biopsy-proven NAFLD in Hong Kong, two (0.65%) developed HCC and one (0.33%) developed hepatorenal syndrome and hepatic encephalopathy during a median follow-up of 49 months [45]. In another cohort of 612 patients with clinical indications for cardiac catheterization and, therefore, a high metabolic burden, only two (0.33%) patients developed primary liver cancer during 3679 patient-years of follow-up. No other patients developed liver decompensation [46]. Taken together, liver-related complications do not appear to be a major problem in Asian patients with NAFLD in the short-to-intermediate term.
Extrahepatic diseases associated with NAFLD and NASH
A number of studies have demonstrated an association between NAFLD and ischemic heart disease [46, 47], obstructive sleep apnea [48], and colorectal neoplasia [49]. This topic has recently been reviewed and will not be discussed in detail here [50]. Although most of these studies corrected for other metabolic factors with multivariable analysis, there might still be residual confounding factors. A causal relationship between NAFLD and these extrahepatic disorders has not been established. Type 2 diabetes mellitus, obesity, and other components of metabolic syndrome are strongly associated with NAFLD in a dose-dependent manner [51]. Our data on causes of death in patients with NAFLD are presented in the “Progression of NAFLD” section.
Diagnosis
Liver biopsy
Liver biopsy is essential for the diagnosis of NASH. It is the only procedure that reliably differentiates NAFL from NASH, despite limitations due to sampling variability [52]. NAFL encompasses: (1) steatosis alone, (2) steatosis with lobular or portal inflammation and no ballooning, or (3) steatosis with ballooning but no inflammation [53]. The diagnosis of NASH requires the joint presence of steatosis, ballooning, and lobular inflammation [53,54,55]. Other histological features can be seen in NASH but are not necessary for diagnosis, such as portal inflammation, polymorphonuclear infiltrates, Mallory-Denk bodies, apoptotic bodies, clear vacuolated nuclei, microvacuolar steatosis, and megamitochondria. Perisinusoidal fibrosis is also frequently observed, but it is not part of the diagnostic criteria. The term burned-out NASH describes regression of advanced disease (steatosis, inflammation, or ballooning) in patients with metabolic risk factors.
Noninvasive assessment
Steatosis
In primary care centers, steatosis should be identified with ultrasonography, because it is more widely available and cheaper than the gold standard, magnetic resonance imaging (MRI). However, several limitations of ultrasonography, including operator dependency, subjective evaluation, and limited ability to quantify the amount of fatty infiltration, have raised concerns. Ultrasonography has lower sensitivity for less severe grades of steatosis. In a study of 100 living donors for liver transplantation, Ryan et al. [56] showed that ultrasound could not detect steatosis when it was present in less than 10% of hepatocytes. Ultrasound detected only 55% of patients with steatosis in 10–19% of hepatocytes and 72% of patients with steatosis in 20–29% of hepatocytes [56]. In a study by Dasarathy et al. [57], hepatorenal echo contrast and liver brightness were able to identify ≥ 20% steatosis with a sensitivity of 96.4% and a specificity of 97.8%. The criteria for vascular attenuation had lower sensitivity and specificity (60.7% and 97.8% for portal vein blurring and 92.9% and 95.6% for hepatic vein blurring, respectively). To detect the same degree of steatosis, poor visualization of the diaphragm had 39.3% sensitivity and 93.3% specificity.
Several studies have assessed hepatic steatosis based on ultrasound attenuation measurements obtained using an ultrasound scanner [58,59,60]. The controlled attenuation parameter (CAP) in FibroScan (Echosens, Paris, France) is currently used in practice. de Lédinghen et al. [58] reported that for the diagnosis of > 10% hepatic steatosis, > 33% steatosis, and > 66% steatosis, the area under the receiver operating characteristic curve (AUROC) for CAP was 0.79 (95% CI 0.74–0.84), 0.84 (95% CI 0.80–0.88), and 0.84 (95% CI 0.80–0.88), respectively, in 440 patients who underwent liver biopsy. However, FibroScan is not an imaging modality and it requires a dedicated probe.
Recently, several attenuation imaging methods, such as attenuation imaging (ATI; Canon Medical Systems, Tokyo, Japan), ultrasound-guided attenuation parameter (UGAP; GE Healthcare Japan Co., Ltd., Tokyo, Japan), and attenuation coefficient (ATT; Hitachi, Tokyo, Japan), have been developed as new ultrasound-based methods for the assessment of hepatic steatosis [61,62,63,64,65,66,67,68].
We recently investigated the diagnostic ability of ATI to detect histologically diagnosed steatosis in 148 patients with chronic liver disease [61]. We found that ATI values increased significantly with increasing steatosis grade (p < 0.001). The AUROCs of ATI for diagnosing steatosis grades ≥ 1, ≥ 2, and 3 were 0.85 (95% CI 0.72–0.88), 0.91 (95% CI 0.84–0.97), and 0.91 (95% CI 0.82–0.99), respectively. Additionally, ATI values increased significantly with increasing steatosis grade (p = 0.002), even in obese patients. The diagnostic ability of ATI for steatosis grades ≥ 1, ≥ 2, and 3 in obese patients was 0.72 (95% CI 0.54–0.90), 0.72 (95% CI 0.55–0.90), and 0.78 (95% CI 0.55–1.00), respectively. Furthermore, ATI values increased significantly with increasing steatosis grade (p < 0.001) in patients with NAFLD. The AUROCs of ATI for diagnosing steatosis grades ≥ 1, ≥ 2, and 3 in patients with NAFLD were 0.77 (95% CI 0.61–0.94), 0.88 (95% CI 0.77–0.99), and 0.86 (95% CI 0.69–1.00), respectively.
In addition, we recently investigated the diagnostic ability of these coefficients to detect steatosis identified by PDFF on MRI in 126 patients with non-B non-C chronic liver disease [66]. We found that the correlation coefficient (r) for PDFF values and attenuation coefficient values was 0.746 (95% CI 0.657–0.815; p < 0.001), corresponding to a strong relationship. The AUROCs of attenuation coefficients for diagnosing steatosis grades ≥ 1, ≥ 2, and 3 as determined by PDFF were 0.922 (95% CI 0.870–0.973), 0.874 (95% CI 0.814–0.934), and 0.892 (95% CI 0.835–0.949), respectively. The r for PDFF values and attenuation coefficient values was 0.559 (95% CI 0.391–0.705; p < 0.001) in patients with mild or no steatosis (grade ≤ 1). In addition, the r for PDFF values and attenuation coefficient values was 0.773 (95% CI 0.657–0.853; p < 0.001) in obese patients (body mass index ≥ 25 kg/m2). The AUROCs of attenuation coefficients for diagnosing steatosis grades ≥ 1, ≥ 2, and 3 as determined by PDFF were 0.884 (95% CI 0.792–0.976), 0.863 (95% CI 0.778–0.947), and 0.889 (95% CI 0.813–0.965), respectively.
PDFF measurement is an MRI-based method for noninvasive quantitative assessment of hepatic steatosis. MRI-determined PDFF values were reported to have good correlation with histologically determined hepatic steatosis grades in patients with hepatic steatosis [69,70,71,72,73,74]. Imajo et al. [4] reported that the diagnosis of liver steatosis grade using MRI-determined PDFF measurements was superior to CAP in patients with NAFLD who underwent liver biopsy. They reported that the AUROCs for PDFF and CAP were 0.96 (95% CI 0.92–1.00) and 0.88 (95% CI 0.80–0.95; p = 0.048), respectively, for detecting grade ≥ 1 steatosis; 0.90 (95% CI 0.82–0.97) and 0.73 (95% CI 0.64–0.81; p < 0.001) for detecting grade ≥ 2 steatosis; and 0.79 (95% CI 0.65–0.94) and 0.70 (95% CI 0.58–0.83; p = 0.015) for detecting grade 3 steatosis. In addition, MRI-determined PDFF was reported to be a more accurate diagnostic tool than biopsy-based histologic assessment of hepatic steatosis because of the spatial variability in steatosis and the invasiveness of liver biopsy [63]. Therefore, MRI-determined PDFF is considered to be a novel, precise, and accurate noninvasive imaging biomarker for the diagnosis of hepatic steatosis.
One of the major criticisms with MRI-determined PDFF assessments is the equipment and cost associated with MRI scanners, along with the technical expertise required to perform and interpret readings. Therefore, this MRI method is far less largely available globally [69]. On the other hand, ultrasound systems are relatively cheap and more widely available. Therefore, it is hoped that ultrasound attenuation measurements will become widely popular as a noninvasive and accurate assessment modality for hepatic steatosis.
Fibrosis
Fibrosis is the most important prognostic factor in NAFLD. The degree of fibrosis is correlated with liver-related outcomes and mortality [75]. The presence of advanced fibrosis identifies patients in need of in-depth hepatological investigation, including, on a case-by-case basis, confirmatory biopsy and intensive therapy. Monitoring of fibrosis progression is also necessary at various time intervals. The 2018 American Association for the Study of Liver Disease Practice Guide recommends four noninvasive tests to evaluate hepatic fibrosis: FIB-4 index, NFS (Table 1), transient elastography (TE, FibroScan), and magnetic resonance elastography (MRE) [76]. Kanwal et al. [77] showed that a FIB-4 index > 2.67 was associated with increased risk of HCC not only in patients with known cirrhosis but also in those without a prior diagnosis of cirrhosis. When utilizing noninvasive tests to stratify patients for HCC screening by risk, a higher threshold is desirable to maximize specificity (90%). TE of 16.1 kPa and MRE of 5 kPa may be considered as cut-off values for noninvasive detection of cirrhosis in HCC screening [76]. In recent years, a two-step diagnostic algorithm that combines these evaluations has become widespread [78,79,80] to identify patients with advanced fibrosis. The simplest, FIB-4 index, is usually the first step, and in the United States TE is usually recommended as the second step [79, 80]. Since the FIB-4 index has a high negative predictive value, it is useful for excluding patients with advanced fibrosis. There is no problem with the FIB-4 index being used as the first step in the primary care setting. However, among hepatologists, the lower cut-off value should be 1.45 [81], 1.3 [82], or 2.0, because the FIB-4 index can overpredict in the elderly [83, 84]. The possibility that the FIB-4 index may be falsely low in patients with diabetes is not controversial, but it is appropriate to use it as the first screening step for the 2 billion patients with NAFLD [78]. On the other hand, TE is not widely used. There are great expectations for serum markers. In Europe, the enhanced liver fibrosis test, consisting of hyaluronic acid, tissue inhibitor of matrix metalloproteinase type 1, and procollagen type III amino-terminal peptide, is commonly used as the second step [85]. A validation study of the efficacy of the enhanced liver fibrosis test was conducted in Japan [86]. In Japan, liver fibrosis markers such as type IV collagen 7S- and Mac-2-binding protein glycosylation isomer are generally used by hepatologists. Elevated type IV collagen 7S, which reflects severe fibrosis [87, 88], is associated with an increased risk of extrahepatic cancer and overall mortality in Japanese patients with biopsy-proven NAFLD [89].
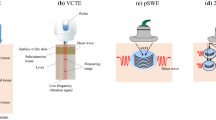
TE is the gold standard for ultrasound-based measurement of liver stiffness. Real-time shear wave elastography (SWE) has also recently emerged as an ultrasound-based technique for noninvasive evaluation of liver stiffness [90,91,92]. This method can easily and accurately assess the degree of liver fibrosis using ultrasound images in clinical practice. Although ultrasound-based SWE devices are being sold by several companies, few studies have directly compared TE with SWE results obtained using various ultrasound devices in the same patient. Many studies have reported the usefulness of TE and SWE for the assessment of liver fibrosis in patients with chronic liver disease. However, the optimal cut-off values vary by device, making it difficult to directly compare measurements obtained using different devices and follow up with patients. Thus, it is essential to establish regression equations to convert SWE values into TE values that are used as the reference standard. Therefore, we investigated correlations to generate regression equations between TE and SWE values and to compare the ability of each method to diagnose liver fibrosis in 109 patients with chronic liver disease who underwent liver biopsy and same-day evaluation of liver stiffness using six ultrasound devices [93]. We found that liver stiffness measured by all six ultrasound devices increased significantly as liver fibrosis stage advanced (p < 0.001). Receiver operating characteristic curve analysis for predicting significant fibrosis (≥ F2) and cirrhosis yielded AUROC values for TE of 0.830 (95% CI 0.755–0.905) and 0.959 (95% CI 0.924–0.995), respectively. The AUROCs for predicting significant fibrosis (≥ F2) and cirrhosis (F4) based on SWE from all five ultrasound devices were above 0.8 and 0.9, respectively. Furthermore, the correlation coefficients between TE values and SWE values from the five ultrasound devices were all above 0.8, indicating a strong relationship.
MRE is an MRI-based method for noninvasively and quantitatively assessing hepatic fibrosis. MRE values are reportedly strongly correlated with histologically determined hepatic fibrosis grade in patients with chronic liver disease [4, 94, 95]. Imajo et al. [4] reported that MRE was superior to TE for determining liver fibrosis grade in patients with NAFLD who underwent liver biopsy. They reported that the AUROCs for MRE and TE were 0.83 (95% CI 0.72–0.93) and 0.78 (95% CI 0.70–0.87; p = 0.466), respectively, for detecting stage ≥ 1 fibrosis; 0.91 (95% CI 0.86–0.96) and 0.82 (95% CI 0.74–0.89; p = 0.001) for detecting stage ≥ 2 fibrosis; 0.89 (95% CI 0.83–0.94) and 0.88 (95% CI 0.79–0.97; p = 0.426) for detecting stage ≥ 3 fibrosis; and 0.97 (95% CI 0.94–1.00) and 0.92 (95% CI 0.86–0.98; p = 0.049) for detecting stage 4 fibrosis (cirrhosis).
Conclusion
NAFLD is at a turning point in terms of its conceptualization, terminology, and diagnostics. Ultrasonography is inexpensive and widely available, and the latest technology of this modality has enabled noninvasive and objective assessment of hepatic fibrosis and steatosis. It is now time to reconfirm the role of ultrasonography for the assessment of NAFLD.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- NAFLD:
-
Nonalcoholic fatty liver disease
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- PDFF:
-
Proton density fat fraction
- FIB-4:
-
Fibrosis-4
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- NFS:
-
NAFLD fibrosis score
- MRI:
-
Magnetic resonance imaging
- NAFL:
-
Nonalcoholic fatty liver
- CAP:
-
Controlled attenuation parameter
- AUROC:
-
Area under the receiver operating characteristic curve
- ATI:
-
Attenuation imaging
- UGAP:
-
Ultrasound-guided attenuation parameter
- ATT:
-
Attenuation coefficient
- TE:
-
Transient elastography
- MRE:
-
Magnetic resonance elastography
- SWE:
-
Shear wave elastography
References
Tateishi R, Uchino K, Fujiwara N, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol. 2019;54:367–76.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9.
Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–55.
Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–37.
Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84.
Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7.
Eslam M, Sanyal JA, George J, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–95.
Nishioji K, Sumida Y, Kamaguchi M, et al. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011–2012. J Gastroenterol. 2015;50:95–108.
Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–38.
Nayak NC, Vasdev N, Saigal S, et al. End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol. 2010;41:425–30.
Okanoue T, Umemura A, Yasui K, et al. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26:153–62.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
Khan RS, Newsome PN. Non-alcoholic fatty liver disease and liver transplantation. Metabolism. 2016;65:1208–23.
Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205.
Sung K-C, Wild SH, Byrne CD. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol. 2014;60:1040–5.
Tsuneto A, Hida A, Sera N, et al. Fatty liver incidence and predictive variables. Hypertens Res. 2010;33:638–43.
Tada T, Toyoda H, Sone Y, et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34:2011–8.
Li J, Fan S, Li Y, et al. Incidence of obesity and its modifiable risk factors in Chinese adults aged 35–74 years: a prospective cohort study. Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35:349–53.
Matsushita Y, Takahashi Y, Mizoue T, et al. Overweight and obesity trends among Japanese adults: a 10-year follow-up of the JPHC Study. Int J Obes (Lond). 2008;32:1861–7.
Chengfu Xu, Chaohui Yu, Ma H, et al. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai Study. Am J Gastroenterol. 2013;108:1299–304.
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55.
Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–9.
Golabi P, Fukui N, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71:793–801.
Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224–9.
Younossi ZM, Otgonsuren M, Venkatesan C, et al. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352–60.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65.
Younossi ZM, Stepanova M, Rafiq N, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:421–8.
Tada T, Kumada T, Toyoda H, et al. Progression of liver fibrosis is associated with non-liver-related mortality in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:899–910.
Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18:91–112.
Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68:416–23.
Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772–81.
Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44.
Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–17.
Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81.
Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309.
Dongiovanni P, Petta S, Maglio C, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–14.
Valenti L, Alisi A, Galmozzi E, et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology. 2010;52:1274–80.
Nobili V, Donati B, Panera N, et al. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;58:632–6.
Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30.
Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–61.
Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64.
Wong VW, Wong GL, Yeung JC, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology. 2016;63:754–63.
Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65.
Hang LW, Chen CF, Wang CB, et al. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath. 2017;21:461–7.
Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011;60:829–36.
Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–53.
Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai. China J Hepatol. 2005;43:508–14.
Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906.
Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Bedossa PFLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–75.
Ryan CK, Johnson LA, Germin BI, et al. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–22.
Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–7.
de Lédinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–31.
Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–68.
Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–35.
Tada T, Iijima H, Kobayashi N, et al. Usefulness of attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med Biol. 2019;45:2679–87.
Ferraioli G, Maiocchi L, Raciti MV, et al. Detection of liver steatosis with a novel ultrasound-based technique: a pilot study using MRI-derived proton density fat fraction as the gold standard. Clin Transl Gastroenterol. 2019;10:e00081.
Jeon SK, Lee JM, Joo I, et al. Prospective evaluation of hepatic steatosis using ultrasound attenuation imaging in patients with chronic liver disease with magnetic resonance imaging proton density fat fraction as the reference standard. Ultrasound Med Biol. 2019;45:1407–16.
Bae JS, Lee DH, Lee JY, et al. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol. 2019;29:6499–507.
Dioguardi Burgio M, Ronot M, Reizine E, et al. Quantification of hepatic steatosis with ultrasound: promising role of attenuation imaging coefficient in a biopsy-proven cohort. Eur Radiol. 2020;30:2293–301.
Tada T, Kumada T, Toyoda H, et al. Utility of attenuation coefficient measurement using an ultrasound-guided attenuation parameter for evaluation of hepatic steatosis: comparison with MRI-determined proton density fat fraction. AJR Am J Roentgenol. 2019;212:332–41.
Fujiwara Y, Kuroda H, Abe T, et al. The B-mode image-guided ultrasound attenuation parameter accurately detects hepatic steatosis in chronic liver disease. Ultrasound Med Biol. 2018;44:2223–32.
Tamaki N, Koizumi Y, Hirooka M, et al. Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol Res. 2018;48:821–8.
Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65:1006–16.
Reeder SB, Robson PM, Yu H, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332–9.
Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–9.
Yokoo T, Serai SD, Pirasteh A, RSNA-QIBAPDFF Biomarker Committee. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286:486–8.
Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–25.
Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267:767–75.
Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57.
Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–37.
Sumida Y, Shima T, Mitsumoto Y, et al. Epidemiology, pathogenesis, and diagnostic strategyof diabetic liver disease in Japan. Int J Mol Sci. 2020;21:4337.
Yoneda M, Imajo K, Takahashi H, et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J Gastroenterol. 2018;53:181–96.
Chan WK, Treeprasertsuk S, Goh GB, et al. Optimizing use of nonalcoholic fatty liver disease fibrosis score, fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Clin Gastroenterol Hepatol. 2019;17:2570–80.
Sumida Y, Yoneda M, Hyogo H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2.
Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–12.
McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–51.
Ishiba H, Sumida Y, Tanaka S, Japan Study Group of Non-Alcoholic Fatty Liver Disease (JSG-NAFLD). The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: a multi-center study. J Gastroenterol. 2018;53:1216–24.
Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–8.
Inadomi C, Takahashi H, Ogawa Y, et al. Accuracy of the enhanced liver fibrosis test, and combination of the enhanced liver fibrosis and non-invasive tests for the diagnosis of advanced liver fibrosis in patients with non-alcoholic fatty liver disease. Hepatol Res. 2020;50:682–92.
Ogawa Y, Honda Y, Kessoku T, et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1795–803.
Okanoue T, Ebise H, Kai T, et al. A simple scoring system using type IV collagen 7s and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol. 2018;53:129–39.
Seko Y, Sumida Y, Tanaka S, et al. Predictors of malignancies and overall mortality in Japanese patients with biopsy-proven non-alcoholic fatty liver disease. Hepatol Res. 2015;45:728–38.
Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409.
Tada T, Kumada T, Toyoda H, et al. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: comparison of liver fibrosis indices. Hepatol Res. 2015;45:E122–9.
Aoki T, Iijima H, Tada T, et al. Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J Gastroenterol. 2017;52:104–12.
Iijima T, Tada T, Kumada T, et al. Comparison of liver stiffness assessment by transient elastography and shear wave elastography using six ultrasound devices. Hepatol Res. 2019;49:676–86.
Yin M, Glaser KJ, Talwalkar JA, et al. Hepatic MR elastography: clinical performance in a series of 1377 consecutive examinations. Radiology. 2016;278:114–24.
Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR elastography of liver: a meta-analysis. Radiology. 2017;285:92–100.
Funding
There was no grant support or other financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tada, T., Nishimura, T., Yoshida, M. et al. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: new trends and role of ultrasonography. J Med Ultrasonics 47, 511–520 (2020). https://doi.org/10.1007/s10396-020-01058-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-020-01058-y