Abstract
Purpose of the Review
T-cells and natural killer (NK) cells share the same ontogeny, and lymphomas derived from them are clinically diverse, occurring in nodal and extranodal sites. In addition to inherent properties of these lymphomas, their microenvironment also impacts on pathogenesis and response to therapy. An understanding of the milieu of T-cell and NK cell lymphomas has important implications on treatment.
Recent Findings
Components of the microenvironment include tumour-associated macrophages (TAM), non-neoplastic T-cells and B-cells, eosinophils, dendritic cells, endothelial cells and blood vessels. TAM typically undergoes M2 polarization, promoting angiogenesis and inhibiting anti-tumour cellular immunity. In lymphomas of follicular helper T-cell derivation, increased B-cell proliferation occurs, which may in turn enhance neoplastic T-cell growth. The expression of immune checkpoint ligands on TAM, dendritic cells or lymphoma cells induces an immunosuppressive environment conducive to neoplastic proliferation. Strategies against this complex cellular and immunologic microenvironment have shown promises. These include the use of signal transduction inhibitors, monoclonal antibodies against chemokines or non-neoplastic microenvironmental cells, immunomodulatory drugs and immune checkpoint blockade. As T-cell and NK cell lymphomas are highly heterogeneous, clinical trials to demonstrate efficacy of a given therapeutic approach requires careful design aiming at enriching patient populations who will best respond.
Summary
Targeting of the immunologic milieu in T-cell and NK-cell lymphomas offers exciting challenges and opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T-cells and natural killer (NK) cells share the same ontogeny, with a lymphoid progenitor common to both lineages [1]. Distinct patterns of transcription factors are involved in lineage commitment, with expression of NOTCH and RUNX members required for T-cell development, and expression of ID2 and E4BP4 required for NK-cell development [1]. Because of common developmental pathways, neoplastic T-cells and NK cells share many similarities in histopathological features, treatment approaches and outcome [2].
In the 2017 World Health Organization classification, lymphomas derived from mature T-cells and NK cells are grouped into 19 distinct categories (Table 1). Conceptually, these neoplasms can be considered to be derived from the innate and adaptive immune systems [3]. The innate immune system provides the first line of barrier defence against infections. It is generally considered not to possess memory, and is not target-specific. T-cells expressing γδ T-cell receptor (γδT-cells) and NK cells are considered part of the innate immune system, and they reside mainly in the skin, mucosal areas and the reticuloendothelial systems (liver, spleen). Therefore, lymphomas derived from γδT-cells and NK cells are mainly extranodal (skin, mucosal surface, liver, spleen). Interestingly, they also have a propensity to affect children and younger individuals.
The adaptive immune system provides immunological defence. It possesses memory and is target-specific. T-cells expressing the αβ T-cell receptor (αβT-cells) are considered part of the adaptive immune system, and they reside mainly in lymph nodes. Therefore, lymphomas deriving from αβ T-cells are mainly nodal, with the three main types being angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL) and peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS). These lymphomas occur predominantly in adults, and childhood cases are exceptional.
Geographical Variations of and Genetic Susceptibility to T-Cell and NK Cell Lymphomas
T-cell and NK cell lymphomas are rare as compared with B-cell lymphomas. Some of them show unique geographic variations. Extranodal NK/T-cell lymphomas have a predilection for Asian and South American populations [4], and are invariably associated with clonal episomal infection of the neoplastic cells with Epstein-Barr virus (EBV). Recent studies have shown that susceptibility to NK/T-cell lymphoma may be related to a genetic polymorphism affecting the peptide-binding groove of HLA-DPB1, with potential impacts on antigen presentation and hence clearance of EBV [5]. Similarly, other EBV-related T-cell lymphomas, including systemic EBV+ T-cell lymphoma of childhood and hydroa vacciniforme-like lymphoproliferative disorder, are also more common in these populations. Whether the same or similar genetic polymorphisms may underlie the susceptibility to these lymphomas requires further investigations.
Adult T-cell leukemia/lymphoma (ATLL) has a distinct geographic distribution, coinciding with the endemicity of infection with the human T-lymphotropic virus I (HTLV-1), which causes the lymphoma. Studies of HLA polymorphisms have also shown haplotype differences in HLA-A, B and DR loci that affect the development of ATLL and other complications of HTLV-1 infection (HTLV-1-associated myelopathy/tropical spastic paraparesis) [6].
Enteropathy-associated T-cell lymphoma (EATL) is a sequel of refractory celiac disease, hence affecting predominantly people of North European descent. HLA is the main genetic determinant of autoimmunity to gluten, which triggers the intestinal inflammation. The HLA haplotypes DQ2.5, DQ2.2 and DQ8 are most commonly involved [7, 8]. Pathogenesis is postulated to be related to inappropriate presentation of deamidated gluten fragments on HLAD2/8 to CD4+ T-cells, triggering the autoimmunity [9]. Prolonged stimulation by CD4+ T-cells towards the resident intraepithelial lymphomas (αβCD8+ T-cells, considered a special form of innate T-cells) subsequently leads to the development of EATL.
The common theme of these T-cell lymphomas is a genetic susceptibility related to inappropriate/abnormal antigen presentation on HLA molecules, resulting in uncontrolled expansion and neoplastic transformation of T-cells. It underlines the importance of the immunologic milieu in the pathogenesis of T-cell lymphoma. Germline predispositions, however, are perhaps less common and important as compared with disturbances in the microenvironment of T-cell lymphomas, which play a key role in lymphomagenesis.
The Microenvironment of T-Cell Lymphomas
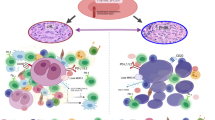
The microenvironment of different T-cell lymphomas varies in the infiltrating cell types, the composition of the stromal cells and hence the secreted chemokines and cytokines. Key constituents of the microenvironment include macrophages, non-neoplastic T-cells and B-cells, eosinophils, other myeloid cells, endothelial cells and blood vessels [10•]. Properties of the lymphomatous T-cells, including inherent genetic alterations, engender the microenvironment. The components of the microenvironment in turn interact with the lymphomatous T-cells to enhance their survival. Thus, the lymphomatous T-cells and their microenvironment together constitute the neoplastic phenotype.
Tumour-Associated Macrophages
Conventionally, macrophages can be dichotomized into two types. Macrophages that produce pro-inflammatory cytokines important for immune defence and tumour cell killing are referred to as M1 macrophages. Alternatively, macrophages that produce anti-inflammatory cytokines suppress immunity against infectious agents and tumour cells, and are referred to as M2 macrophages. M2 macrophages also promote angiogenesis by secreting vascular endothelial growth factor (VEGF), thereby promoting tumour progression and metastasis [11]. Furthermore, M2 macrophages secrete interleukin (IL)-10 and transforming growth factor β (TGF-β), which upregulate the expression of programmed cell death ligand 1 (PDL1) on themselves in an autocrine fashion [11]. Ligation of PDL1 with the inhibitory receptor PD1 expressed on effector T-cells leads to inhibition of T-cell function and hence immunosuppression in the tumour milieu [11].
Non-Neoplastic Lymphoid Cells
Regulatory T-cells (Tregs) are subsets of CD4-positive T-cells that suppress the immune response and maintain immune tolerance. They characteristically express CD25 and forkhead box transcription factor FOXP3 [12]. Tregs suppress immune-mediated anti-tumour activities, downregulate the inflammatory response in the microenvironment and reduce the efficacy of immunotherapy and cancer vaccines [13].
Non-neoplastic B-cells may be found in T-cell lymphomas, particularly those of follicular helper T-cell (TFH) phenotype [14•]. These non-neoplastic B-cells may be EBV-infected. B-cells express inducible costimulator (ICOS) ligand, which on ligation with ICOS expressed on T-cells results in induction of the transcriptional repressor BCL6 that is crucial for TFH differentiation [15]. Therefore, B-cells are integral to the development of T-cell lymphomas of the TFH phenotype.
Eosinophils
Eosinophils are found in different types of T-cell lymphomas, including AITL and cutaneous T-cell lymphomas (CTCL), particularly mycosis fungoides (MF). Eosinophils secrete a variety of cytokines, including IL-4, IL-10 and IL-13 [16], which induce alternative M2 macrophage polarization, thereby promoting tumour progression.
Endothelial Cells and Blood Vessels
Vascular arborization and prominent proliferation of high-endothelial venules are typical histologic features of AITL. Increased angiogenesis is conducive to tumour progression and metastasis. By presenting a complex system of chemokines, vascular endothelial cells provide a docking system for lymphoma cells, leading to their diapedesis across blood vessels and hence systemic dissemination [17].
Properties of Neoplastic T-Cells that Determine the Lymphoma Microenvironment
The putative cell of origin and the genetic alterations of T-cell lymphomas play critical roles in determining the lymphoma microenvironment. AITL is derived putatively from TFH cells [18]. TFH cells secrete a variety of cytokines and chemokines, including CXCL13 (chemotactic for B-cells), VEGF (endothelial cell proliferation and vascular arborization), IL-5 (eosinophilic proliferation), and IL-10 and TGF-β (suppression of other effector T-cell function) [14•]. Expression of CXCL13 is of special interest, because B-cells attracted to the vicinity express ICOS-L, which by binding to ICOS on TFH cells provide a positive-feedback enhancement of lymphoma growth. Mutations of the TET2, IDH2 and DNMT3 genes are frequent in AITL [18]. It has recently been shown in some cases of AITL that B-cells harbour TET2 mutations identical to those of the neoplastic T-cells [19], suggesting a more complex interaction of the lymphoma with its microenvironment, some components of which might even be derived from common neoplastic progenitors.
In PTCL-NOS, expression of the T-cell transcriptional factor GATA-binding protein 3 (GATA-3) occurs in about 45% of cases [20••]. GATA-3 regulates the production of T helper (Th)-2 associated cytokines and IL-10, which promote alternative M2 macrophage polarization. PTCL with GATA-3 expression is therefore associated with a microenvironment that promotes tumour proliferation. Accordingly, amongst PTCL-NOS, those expressing GATA-3 have an inferior prognosis [20••].
ALCL with the NPM-ALK fusion gene has distinctive clinicopathological features. The NPM-ALK protein is a multi-functional oncoprotein, with signal transducer and activator of transcription 3 (STAT3) being its important downstream mediator. NPM-ALK induces via STAT3 the expression of IL-10 and TGF-β, and the expression of PDL1 on lymphoma cells, [21], resulting in an immunosuppressive microenvironment. Acting via hypoxia-inducible factor 1-alpha (HIF-1α), NPM-ALK also induces VEGF expression and hence vascular proliferation, thus increasing tumour growth and metastasis.
The most common form of cutaneous T-cell lymphoma (CTCL) is MF, derived putatively from non-recirculating skin resident effector memory T-cells [22]. Sezary syndrome (SS) is another similar but distinct lymphoma, deriving putatively from central memory T-cells [22]. MF cells have lost the molecules C-C chemokine receptor 7 (CCR7) and CD62L that are required to exit high endothelial venules, but express CCR4 and cutaneous lymphocyte-associated antigen (CLA), which are skin-homing molecules. This pattern of antigen expression results in MF cells localizing to the skin. On the other hand, SS cells express strongly CCR7 and CD62L, consistent with their central memory T-cell and circulating nature. However, expression of chemokine receptors changes with disease state. In disseminated MF, lymphoma cells acquire CCR7, whereas in SS with skin involvement, CCR4 is expressed. In the skin lesions of MF and SS, lymphoma cells may display FOXP3+ Treg, Th2 or Th17 phenotypes. With the FOXp3+ Treg phenotype, IL-10 and TCG-β are produced, whereas with the Th2 phenotype, IL-10 and IL-13 are produced. In both states, an immunosuppressive microenvironment is produced. Furthermore, the pathogenesis of CTCL is regarded to involve prolonged antigen stimulation. Therefore, infiltrating effector T-cells in these skin lesions might exhibit an “exhausted” phenotype, involving the expression of immune checkpoint proteins that impair their cytotoxic function [23].
In EBV-infected lymphomas where immunogenic EBV-associated antigens are expressed, a potential escape mechanism from T-cell immunosurveillance is by expression of PDL1, which interacts with the immune checkpoint PD1 on effector T-cells to inhibit their function [24]. In EBV+ T-cell lymphomas, typically NK/T-cell lymphomas, PDL1 is confirmed to be highly expressed [25, 26], suggesting that lymphoma cells induce an immunosuppressive microenvironment beneficial to their survival.
Microenvironmental Factors Affecting Lymphoma Survival
TAMs play a key role in the lymphoma milieu. Alternative M2 macrophage polarization occurs to the majority of TAMs in PTCL-NOS, AITL, MF and ATLL [27, 28]. Important pro-lymphoma survival consequences include tissue matrix remodelling, angiogenesis and expression of PDL1. Similarly, in gene expression profiling studies, PTCL with a monocytic gene-expression signature had significantly inferior prognosis [29], confirming TAMs to be an important element impacting on outcome.
In most T-cell lymphomas, there is infiltration by non-neoplastic T-cells. A relevant issue is the pathogenetic part these non-neoplastic T-cells play. Effector T-cells interact with TAM and dendritic cells, which act as antigen-presenting cells. An emerging and recurring theme is that the lymphoma microenvironment is immunosuppressive. This involves the expression on TAMs, dendritic cells and other myeloid cells of ligands for inhibitory receptors on effector T-cells. These ligands include PDL1, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin domain 3 (TIM-3) [30]. These immune checkpoint proteins are dynamically expressed, and may be regulated by Th2 cells, M2 macrophages and STAT signaling [30]. Immune checkpoint axes are particularly relevant in T-cell lymphomas with a rich non-neoplastic cellular infiltration, such as AITL and NK/T-cell lymphomas.
Therapeutic Targeting of the Microenvironment in T-Cell and NK-Cell Lymphomas
Treatment outcome of T-cell and NK-cell lymphoma remains unsatisfactory in comparison with B-cell lymphomas. While chemotherapy targets the neoplastic cells, it does not affect the microenvironment to a significant extent. Hence, innovative treatment strategies targeting the lymphoma microenvironment have been explored (Table 2).
Alternatively M2 polarized TAMs have been proposed as a therapeutic target. Depletion of TAMs led to growth inhibition of a murine model of CTCL, underscoring the importance of these cells in lymphoma survival [31]. An important mechanism of alternative M2 polarization involves IL-10 induced JAK/STAT signalling. Suppression of JAK/STAT signalling with the JAK inhibitor ruxolitinib suppressed M2 polarization ex vivo [20••]. Furthermore, aberrant JAK/STAT signalling is involved in many T-cell lymphomas [32], even in the absence of mutations of the components of the JAK/STAT pathway [33]. Therefore, JAK/STAT targeting is a plausible approach in T-cell lymphoma and requires further investigations.
T-cell lymphomas derived from TFH, with AITL being the most common, have the richest microenvironment that comprises many different cell types. In view of the interaction between B-cells and TFH cells, targeting of the B-cells with the anti-CD20 antibody rituximab had been tested in AITL [34]. No beneficial effect was observed, suggesting the mere targeting of B-cells to be ineffective. However, the immunomodulatory agent lenalidomide exerts pleiotropic effects on the lymphoma microenvironment, including decreasing Th2 cells, reducing Tregs, suppressing angiogenesis and expansion of cytotoxic T-cells [35]. Accordingly, in clinical trials of lenalidomide in relapsed/refractory patients with T-cell lymphomas, AITL appeared to be the most responsive [36]. Lenalidomide was also effective in some cases of relapsed/refractory CTCL [37]. The exact mechanisms of how lenalidomide works in these T-cell lymphomas remain to be elucidated.
Targeting of angiogenesis with the anti-VEGF antibody bevacizumab has been reported anecdotally to be effective in AITL [38, 39]. However, in an unselected cohort of patients with T-cell and NK-cell lymphomas, addition of bevacizumab to conventional chemotherapy failed to show additional benefits [40]. Enrichment of patient populations with T-cell lymphomas showing prominent angiogenesis might be needed in future trials of bevacizumab to investigate its efficacy.
Owing to the critical role CCR4 plays in CTCL, the anti-CCR4 antibody mogamulizumab has been investigated in CTCL and other T-cell lymphomas expressing this chemokine receptor. Preliminary results showed favourable responses [41, 42]. Because FOXP3+ Tregs also express CCR4, their depletion in the lymphoma microenvironment during mogamulizumab therapy may also explain in part the responses observed [43]. Future studies should be directed in defining the patient populations with T-cell lymphomas who will benefit most from mogamulizumab.
Immune Checkpoint Blockade in T-Cell and NK-Cell Lymphomas
Immune checkpoint blockade has assumed a pole position in cancer immunotherapy, due largely to its success in solid tumours. Owing to the presence of TAMs, dendritic cells and other myeloid cells in the T-cell lymphoma microenvironment, which might express PDL1, TIM3 and LAG3 [30], immune checkpoint blockade is an attractive strategy in these malignancies.
In a small unselected cohort of 23 patients with relapsed/refractory T-cell lymphomas, blockade of PD1 with the anti-PD1 antibody nivolumab only resulted in an overall response rate (ORR) of 17%, without the achievement of complete response (CR) [44]. It is unknown if PDL1 was expressed in all these cases, either on the lymphoma cells or cells in the lymphoma microenvironment. Therefore, although the results were only very modest, further enrichment of patients with PDL1-expressing T-cell lymphomas, either on the neoplastic cells or in the microenvironment, is needed.
The success of this approach of patient enrichment was illustrated in a recent study of patients with relapsed/refractory NK/T-cell lymphomas. Because these lymphomas are EBV+, PDL1 is upregulated on the lymphoma cells [24, 25]. In seven patients who had failed multiple lines of therapy including allogeneic haematopoietic stem cell transplantation, treatment with the anti-PD1 antibody pembrolizumab resulted in an ORR of 100%, and a CR rate of 71% after a median follow up of 6 months [47]. Immunohistochemistry study showed strong PDL1 staining in four patients, three of whom achieved CR, suggesting PDL1 to be an important predictive biomarker of response. Blockade of PD1 with nivolumab appeared also effective anecdotally in NK/T-cell lymphomas [45]. These results show that patient selection is of paramount importance in demonstrating therapeutic efficacy of immune checkpoint blockade in T-cell and NK-cell lymphomas.
The success of immune checkpoint targeting relies on blockade of PD1 on effector T-cells, in order to elicit an anti-lymphoma effect. However, PD1 may also be expressed on the neoplastic T-cells, especially those of TFH derivation. Recently, PD1 has been observed to be a haploinsufficient suppressor of T-cell lymphomagenesis in a murine T-cell lymphoma model [46]. Therefore, the effect of PD1 blockade might have unexpected results in T-cell lymphomas, which are highly heterogeneous, and more experimental and clinical investigations are needed.
Conclusions and Future Directions
Significant advances have been made in defining the genomic alterations in T-cell lymphomas, and how these changes result in oncogenesis. However, how the microenvironment impacts on T-cell lymphomagenesis is relatively less understood. The definition of HLA-related genetic susceptibility to certain types of T-cell and NK cell lymphomas offers the chance of prevention if approach strategies can be formulated. The availability of novel drugs that target both the cellular and cytokine milieu should provide impetus for detailed research in these areas. It is, moreover, imperative that clinical studies be meticulously designed with selection of appropriate patients, so that therapeutic benefits can be demonstrated.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol. 2011;11(10):645–57. https://doi.org/10.1038/nri3044.
Tse E, Kwong YL. Treatment algorithms for mature T-cell and natural killer-cell neoplasms. Future Oncol. 2011;7(9):1101–12. https://doi.org/10.2217/fon.11.84.
Jaffe ES, Campo E, Harris NL, Pileri S, Stein H, Swerdlow SH. Introduction and overview of the classification of lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2017. p. 190–8.
Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. 2017;10(1):85. https://doi.org/10.1186/s13045-017-0452-9.
Li Z, Xia Y, Feng LN, Chen JR, Li HM, Cui J, et al. Genetic risk of extranodal natural killer T-cell lymphoma: a genome-wide association study. Lancet Oncol. 2016;17(9):1240–7. https://doi.org/10.1016/S1470-2045(16)30148-6.
Sonoda S, Li HC, Tajima K. Ethnoepidemiology of HTLV-1 related diseases: ethnic determinants of HTLV-1 susceptibility and its worldwide dispersal. Cancer Sci. 2011;102(2):295–301. https://doi.org/10.1111/j.1349-7006.2010.01820.x.
Romanos J, van Diemen CC, Nolte IM, Trynka G, Zhernakova A, Fu J, et al. Analysis of HLA and non-HLA alleles can identify individuals at high risk for celiac disease. Gastroenterology. 2009;137(3):834–40. https://doi.org/10.1053/j.gastro.2009.05.040.
Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJ, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371(1):42–9. https://doi.org/10.1056/NEJMoa1313977.
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–56. https://doi.org/10.1001/jama.2017.9730.
• Tse E, Kwong YL. T-cell lymphoma: Microenvironment-related biomarkers. Semin Cancer Biol. 2015;34:46–51. A comprehensive review of potential biomarkers for response to treatment based on the microenvironment in T-cell lymphomas.
Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117(11):1583–91. https://doi.org/10.1038/bjc.2017.356.
Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017;17(11):703–71711. https://doi.org/10.1038/nri.2017.75.
Enokida T, Nishikawa H. Regulatory T cells, as a target in anticancer immunotherapy. Immunotherapy. 2017;9(8):623–7. https://doi.org/10.2217/imt-2017-0057.
• Gaulard P, de Leval L. The microenvironment in T-cell lymphomas: emerging themes. Semin Cancer Biol. 2014;24:49–60. a comprehensive review of the components of the microenvironment in T-cell lymphomas.
Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–46. https://doi.org/10.1016/j.immuni.2011.03.023.
Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, et al. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902–14. https://doi.org/10.1016/j.celrep.2015.08.033.
Pals ST, de Gorter DJ, Spaargaren M. Lymphoma dissemination: the other face of lymphocyte homing. Blood. 2007;110(9):3102–11. https://doi.org/10.1182/blood-2007-05-075176.
Lemonnier F, Mak TW. Angioimmunoblastic T-cell lymphoma: more than a disease of T follicular helper cells. J Pathol. 2017;242(4):387–90. https://doi.org/10.1002/path.4920.
Schwartz FH, Cai Q, Fellmann E, Hartmann S, Mäyränpää MI, Karjalainen-Lindsberg ML, et al. TET2 mutations in B cells of patients affected by angioimmunoblastic T-cell lymphoma. J Pathol. 2017;242(2):129–33. https://doi.org/10.1002/path.4898.
•• Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014;123(19):3007–15. original observations of how gene expression profiles in T-cell lymphomas impact on microenvironmental changes and prognosis.
Werner MT, Zhao C, Zhang Q, Wasik MA. Nucleophosmin-anaplastic lymphoma kinase: the ultimate oncogene and therapeutic target. Blood. 2017;129(7):823–31. https://doi.org/10.1182/blood-2016-05-717793.
Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767–71. https://doi.org/10.1182/blood-2009-11-251926.
Rubio-Gonzalez B, Zain J, Rosen ST, Querfeld C. Clinical manifestations and pathogenesis of cutaneous lymphomas: current status and future directions. Br J Haematol. 2017;176(1):16–36. https://doi.org/10.1111/bjh.14402.
• Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–73. original observations of how infection with Epstein Barr virus upregulates the expression of PDL1.
Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9(1):109. https://doi.org/10.1186/s13045-016-0341-7.
Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877–90. https://doi.org/10.1007/s00262-017-1987-x.
Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, et al. Ratio of M2 macrophage expression is closely associated with poor prognosis for angioimmunoblastic T-cell lymphoma (AITL). Pathol Int. 2010;60(4):278–83. https://doi.org/10.1111/j.1440-1827.2010.02514.x.
Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–90. https://doi.org/10.3390/cancers6031670.
Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD, Weisenburger DD, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915–23.
Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13(1):25–40. https://doi.org/10.1038/nrclinonc.2015.187.
Wu X, Schulte BC, Zhou Y, Haribhai D, Mackinnon AC, Plaza JA, et al. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. J Invest Dermatol. 2014;134(11):2814–22. https://doi.org/10.1038/jid.2014.206.
Choi J, Goh G, Walradt T, Hong BS, Bunick CG, Chen K, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47(9):1011–9. https://doi.org/10.1038/ng.3356.
Chen J, Zhang Y, Petrus MN, Xiao W, Nicolae A, Raffeld M, et al. Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc Natl Acad Sci U S A. 2017;114(15):3975–80. https://doi.org/10.1073/pnas.1700682114.
Delfau-Larue MH, de Leval L, Joly B, Plonquet A, Challine D, Parrens M, et al. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica. 2012;97(10):1594–602. https://doi.org/10.3324/haematol.2011.061507.
Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood. 2014;124(14):2184–9. https://doi.org/10.1182/blood-2014-05-578286.
Toumishey E, Prasad A, Dueck G, Chua N, Finch D, Johnston J, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer. 2015;121(5):716–23. https://doi.org/10.1002/cncr.29103.
Querfeld C, Rosen ST, Guitart J, Duvic M, Kim YH, Dusza SW, et al. Results of an open-label multicenter phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sézary syndrome. Blood. 2014;123(8):1159–66. https://doi.org/10.1182/blood-2013-09-525915.
Bruns I, Fox F, Reinecke P, Kobbe G, Kronenwett R, Jung G, et al. Complete remission in a patient with relapsed angioimmunoblastic T-cell lymphoma following treatment with bevacizumab. Leukemia. 2005;19(11):1993–5. https://doi.org/10.1038/sj.leu.2403936.
Aguiar Bujanda D. Complete response of relapsed angioimmunoblastic T-cell lymphoma following therapy with bevacizumab. Ann Oncol. 2008;19(2):396–7. https://doi.org/10.1093/annonc/mdm579.
Ganjoo K, Hong F, Horning SJ, Gascoyne RD, Natkunam Y, Swinnen LJ, et al. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404). Leuk Lymphoma. 2014;55(4):768–72. https://doi.org/10.3109/10428194.2013.816700.
Ogura M, Ishida T, Hatake K, Taniwaki M, Ando K, Tobinai K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–63. https://doi.org/10.1200/JCO.2013.52.0924.
Duvic M, Pinter-Brown LC, Foss FM, Sokol L, Jorgensen JL, Challagundla P, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883–9. https://doi.org/10.1182/blood-2014-09-600924.
Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110(44):17945–50. https://doi.org/10.1073/pnas.1316796110.
Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–704. https://doi.org/10.1200/JCO.2015.65.9789.
Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2017.
Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Öllinger R, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552(7683):121–5. https://doi.org/10.1038/nature24649.
• Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129(17):2437–42. original observations of the high efficacy of PD1 blockade in NK-cell lymphoma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on T-Cell and Other Lymphoproliferative Malignancies
Rights and permissions
About this article
Cite this article
Tse, E., Kwong, YL. Immunologic Milieu of Mature T-Cell and NK-Cell Lymphomas—Implications for Therapy. Curr Hematol Malig Rep 13, 37–43 (2018). https://doi.org/10.1007/s11899-018-0437-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-018-0437-y