Abstract
Purpose of Review
We sought to examine the effects of the gut microbial makeup on weight gain and obesity. We wanted to find out what the current research on this topic was and what the effect of the gut microbiota on energy metabolism is, as well the effects of diet on the microbiome and what effect the microbiome has on metabolic syndrome.
Recent Findings
Obesity is thought to be due to greater calorie intake than expenditure. Recently, research has looked into the effects of the microbiome on obesity. Our gut flora is made up of trillions of microbes and there is evidence to suggest that even from the earliest stages of life, altering that flora can affect human’s ability to gain and lose weight, which can lead to obesity and ultimately other disease such as cardiovascular disease, diabetes mellitus, and liver disease.
Summary
Obesity can affect the human body in profound ways and lead to a multitude of comorbidities. We found that the obesity pandemic appears to parallel the increased use of antibiotics seen across the US. In addition, the use of antibiotics can alter the gut flora even from the earliest stages of life and the altered microbiome can alter our body habitus and energy metabolism through antibiotics, diet, and bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The human digestive system is made up of trillions of microbes, including bacteria, fungi, and viruses, which collectively are known as the microbiome. The gut microbiota is made up mainly of four phyla, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria [1••]. Recently, there has been a great deal of research into the relationship between the microbiome and metabolic diseases, namely obesity, diabetes mellitus, and cardiovascular disease.
Obesity has become a major health epidemic worldwide. According to the World Health Organization, worldwide obesity has more than doubled since 1980. As of 2014, nearly 40% of the world’s adult population, ages 18 and older, were overweight and 13% were obese [2]. Children unfortunately are not immune to this epidemic, as it is estimated that 41 million children under the age of 5 were either overweight or obese [2]. It was initially industrialized countries that dealt with obesity, but it has quickly become a global pandemic. For centuries, attempts have been made to try and pinpoint which factors contribute to obesity. Simply put, obesity is generally thought to be caused by more calorie intake than expenditure. This phenomenon is primarily driven by lifestyle changes including increased sedentary lifestyle and diets rich in calorie-dense foods. However, over the years, researchers have been delving into some of the underlying mechanisms causing obesity. One that has garnered much attention is the link between obesity and the gut microbiome. Obesity is associated with multiple comorbidities, increased mortality, and significant socio-economic burden. Thus, any research could prove extremely beneficial.
Initial attempts to use antibiotics for malnutrition were unsuccessful and were later widely used by humans and livestock as pro-growth stimulators. There appears to be a parallel link between obesity and another healthcare epidemic—antibiotic resistance. There appears to be a correlation between the states with the highest prevalence of obesity and the highest annual antibiotic prescriptions as demonstrated by Figs. 1 and 2 below. This raises the notion that the use of antibiotics possibly altering the gut microbiome might be an important driving factor in the obesity epidemic.
Antibiotic prescriptions per 1000 persons of all ages, 2010 [3]. From New England Journal of Medicine, Hicks et al., U.S. Outpatient Antibiotic Prescribing, 2010, 368(15), pp. 1461–1462, copyright 2013. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
Prevalence of self-reported obesity among US adults by state and territory, 2015 [4]. Reprinted by permission from the Centers for Disease Control and Prevention: https://www.cdc.gov/obesity/data/prevalence-maps.html, copyright 2017; reprinted with permission from Massachusetts Medical Society
Microbiome and Obesity
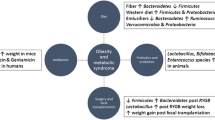
The interplay between the microbiome and obesity is a complex one, still undergoing much research, with many factors believed to be involved. For over the past decade, this issue has been one undergoing much investigation. In 2005, Ley et al. published their work entitled Obesity alters gut microbial ecology in which they looked at the effect of polysaccharide-rich diets on mice microbiota. They discussed the fact that humans benefit from the trillions of microorganisms inhabiting their digestive tract by extracting energy in the form of calories. Based on that, they hypothesized that individuals predisposed to obesity may have a microbiome that is more efficient in extracting and storing energy from the food that the host eats. They analyzed the bacterial rRNA from the microbiome of mice that were all fed the same diet and found that obese mice had a statistically significant reduction, nearly 50%, in Bacteroidetes and a higher percentage of Firmicutes [5]. Based on this finding, they concluded that not only does obesity affect the makeup of the gut microbiome, but conversely changing the diversity of the microbiome could also change the energy balance in obese individuals. Over time with low-fat diet, the ratio gradually and steadily decreased and at 1 year, the ratio closely resembled that of a lean individual.
It appears that disruption of the microbiome at the very early stages of life can predispose one to obesity. In a 2013 paper, Trasande et al. considered the effects of exposure of antibiotics during the first 2 years of life on weight and body mass index (BMI) by age 7. Their study was based on over 11,000 children and it showed that antibiotic exposure in infants less than 6 months old was associated with increased body mass in children 10–38 months; however, antibiotic exposure at 6–14 months or 15–23 months was not consistently associated with an increased BMI [6]. In a 2015 study, Cox et al. used mice to delve into whether disruption of the microbiome by exposing subjects to low-dose antibiotics during the maturation period could affect one’s metabolism and make them more susceptible to becoming obese. Their study showed that mice that received antibiotics, namely penicillin, chlortetracycline, or vancomycin resulted in more weight gain as compared to the control mice [7]. Their work was based on the thought that early life is a very important point for metabolic development and that changes made to the gut microbiome could lead to an increase in weight and thus obesity later in life. Administration of antibiotics leads to the loss of protective bacteria. With high-fat diets, the mice became obese. Further, when the microbiota was transferred to germ-free mice, they also became obese. In addition to antibiotics, they also discussed how the method of delivery can affect the microbiota of infants, which is based on several studies. In a study by Dominguez-Bello et al., they found that infants who were vaginally delivered acquired a microbiota like that of their mother’s vaginal microbiota, mostly containing Lactobacillus, Prevotella, or Sneathia species, whereas those born via cesarean section had microbiota from the skin surface, which mostly included Staphylococcus, Corynebacterium, Propionibacterium species [8]. Women who received a C-section also received a dose of cephalosporin prior to delivery, whereas women who underwent vaginal delivery received no antibiotics. From there, several studies aimed to look at the association of C-section and obesity. Blustein et al. looked at over 10,000 births, about 9% of which were via C-section. They found that although children born via C-section had a lower birth weight, by age 11 those same children were almost twice as likely to be overweight or obese [9]. The microbiome must be given much consideration, even at the very early stages of life.
Energy Metabolism and Gut Microbiota
What do gut microbiota do about energy metabolism and energy homeostasis? They are involved in carbohydrate metabolism and salvage. Energy metabolism is the major determinant of body habitus. With the ingestion of dietary fiber, we get bacterial fermentation and degradation from the fiber, resulting in oligosaccharides and monosaccharides. These monosaccharides derived from fermentation are thought to be excess energy as humans do not have the metabolic pathway to digest complex fibers Fig. 3. The oligosaccharides and monosaccharides are then fermented to short-chain fatty acids (SCFAs). The three major SCFAs derived by gut bacteria are acetate, propionate, and butyrate. The former two can be used primary substrates for hepatic lipogenesis and gluconeogenesis and the latter provides energy for colonic epithelial cells [10••]. SCFAs are involved in regulation of gene expression by binding to the G-protein-coupled receptors GPR41 and GPR43 with several potential outcomes. One of which is the increased expression of GLP-1 which in turn leads to increased insulin secretion [10••]. This increases nutrient absorption which might have adipogenic effects but the overall net effect is antidiabetic. On the other hand, GLP-1’s effect on enteroendocrine L-cells and adipose tissue (leptin) with increased satiety eventually leads to anti-adipogenic affects with reduction in energy intake as seen in Fig. 4.
Effects of colonic fermentation of dietary fibers [11]. Reprinted by permission from Macmillan Publishers Ltd.: Nature (https://www.nature.com/nature/journal/v489/n7415/full/nature11552.html), copyright 2012
Simplified scheme showing the roles of SCFA in host metabolism [12]. Reprinted by permission from Cambridge University Press: Proceedings of the Nutrition Society (https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/gut-microbiota-and-energy-balance-role-in-obesity/7D68A28E4810FE35ED5EB6A5C812C203/core-reader), copyright 2014
Further examining the relationship between energy metabolism and the microbiome, a study was done that considered the energy balance and the possibility that microbiota can be a cause of obesity by increasing energy extraction. Based on experimentation in mice, it was concluded that obesity-related microbiomes had an increased capacity to harvest energy from the diet and the primary cause being a leptin deficiency [13]. Leptin is a hormone produced by adipose cell to inhibit hunger. Mice with mutations in the leptin genes saw increased food consumption and the microbiomes of obese mice were transmitted to germ-free recipients who developed increase in total body fat, again showing the effects of the microbiome as a factor that can contribute to the development of obesity [13].
Bariatric surgery can possibly even alter the gut microbiota composition. There have been associations with Roux-en-Y gastric bypass (RYGB) surgery and its effects on the gut microbial composition, some of which is seen even before the post-surgical weight loss begins. In a 2013 study, Liou et al. sought to investigate these effects. They transferred the cecal contents from RYGB donors into lean, germ-free mice. The recipient mice exhibited significant weight loss whereas mice inoculated from the cecal contents of those who had undergone “sham” surgery and the germ-free control showed no significant weight change [14]. It appears that even alterations of the microbiome via bariatric surgery influence obesity. The exact mechanism of this is unclear. The authors of this study thought that it could also relate back to SCFAs as previously discussed above [14].
Effects of Diet on the Microbiome
Without a doubt, diet is one of the single biggest factors contributing to obesity. But can ones’ diet affect the microbiome? Diet can alter the composition of the microbiota starting at an early age. Whether an infant is given breast milk or is formula-fed could affect its microbiome. This is believed to be due to the effect of oligosaccharides which are fermented by bacteria. Human milk is rich in oligosaccharides and infants who were breast-fed were found to have an increased proportion of Bifidobacteria [15]. According to this paper, a microbiome rich in Bifidobacteria is associated with improved health [15]. In adults, high-fat diets tend to correlate with obesity. High-fat diets are associated with decreased microbiota richness, as opposed to high-fruit and high-vegetable diets, as well as low-calorie diets, which lead to microbiota richness. As shown in Fig. 5 below, our dietary consistency can contribute to our microbiome, whether it is protein, fat, or carbohydrate [16]. One study found that those who took in a high-protein/low-carbohydrate diet had a decrease in Roseburia and Eubacterium rectale in their microbiome and a decrease in butyrate in the feces [17]. As previously discussed, butyrate and other SCFAs can influence obesity. With regard to a high-fat diet, one study showed that pigs fed high-fat diets showed a higher number of Bacteroides and Enterobacteriaceae than those seen in low-fat diets [18]. Similar results can be seen with diets rich in carbohydrates. One study showed that an increase in carbohydrates from whole date-fruit extracts resulted in an increase in Bacteroides [19]. Of course, Bacteroides is not the only species affected by the dietary change, but given its abundance in our microbiome, it is one that has garnered much attention. While we already know that high-calorie diets can lead to obesity, we are now learning more from the studies above and several others that these diets can affect our microbiome.
Impact of diet on gut microbiome and human health [16]. Reprinted by permission from BioMed Central: Journal of Translational Medicine (https://translationalmedicine.biomedcentral.com/articles/10.1186/s12967-017-1175-y), copyright 2017
Microbiome and Metabolic Syndrome
In addition to obesity, metabolic syndrome is related to a host of other chronic condition, including type II diabetes mellitus, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD). With the rise seen in obesity over the years, there has also been a linear increase in diabetes mellitus, predominately type II. In a study by Cani et al. in which they altered the gut microbiome of mice by administering antibiotics to determine the effects on inflammation and its causation of obesity and diabetes, they found that mice that were high-fat fed and treated with antibiotics had a reduced amount of lipopolysaccharide (LPS), an important component of the cell wall in gram-negative bacteria, resulting in a reduced metabolic endotoxemia [20]. Subcutaneous infusion of LPS was shown to cause weight gain and insulin resistance. Those mice lacking in metabolic endotoxins had decreased weight gain and glucose intolerance [20]. In a later study, the intestinal microbiome was transplanted from lean donors to recipients with metabolic syndrome. Six weeks post-transplantation, recipients were found to have an improvement in peripheral insulin sensitivity [21]. The thought behind the cause of this finding is that role of butyrate derived from microbial metabolism in the gut. Butyrate has been shown to decrease body weight and fasting glucose. The effects were profound, with a reduction of 10% in weight and 30% in fasting glucose in obese mice given a 5-week treatment of butyrate [22]. As previously mentioned, this again points to the effects of SCFAs on obesity.
Cardiovascular disease is still the leading cause of death worldwide. Obesity and diabetes are among the risk factors for developing cardiovascular disease. There has been a link seen with the microbiome and its effect on cardiovascular disease through its main precursor—atherosclerosis. It is thought that there is a link between atherosclerosis and trimethylamine-N-oxide (TMAO), which is a gut flora metabolite that comes from the oxidation of trimethylamine (TMA). Our gut bacteria produce TMA from our dietary amines which then get converted into TMAO, which can play a role in atherosclerosis by inhibiting reverse cholesterol transport [23]. One dietary amine, choline, has been studied to see its effects on cardiovascular disease. In patients undergoing elective cardiac catheterization, elevated levels of plasma choline were related to increased risk of cardiovascular disease only in patients who also had concomitantly elevated levels of TMAO [24]. Though obesity in and of itself is a risk factor for the development of cardiovascular disease, our gut flora may also play a contributing role.
The third metabolic disease looked at involved the liver. The liver is one of the organs most affected by obesity, giving way to NAFLD. In a 2012 study, the inflammatory effects on microbiota leading to the progression of NAFLD and with that weight gain and obesity were considered. It was shown that toll-like receptors and NOD-like receptors were both involved in facilitating inflammatory processes in the gut that resulted in the abnormal accumulation of bacteria, leading to NAFLD and obesity [25]. NAFLD can unfortunately progress to non-alcoholic steatohepatitis, which can then lead to cirrhosis and the unfortunate consequences that come with it, which are not limited to portal hypertension, hepatic encephalopathy, and even increased mortality.
In Summary
Obesity can have an array of effects on the human body, some of which can be devastating. As discussed, obesity in and of itself is a major risk factor for many diseases which can cause early death. Based on the available data, it appears that the gut microbiota is linked to obesity; however, the magnitude of this association is not entirely clear. Here is what we do know:
-
1.
The obesity pandemic parallels increased antibiotic use and resistance.
-
2.
Altered gut microbiota has the potential to alter body habitus and energy metabolism through antibiotics, diet, and bariatric surgery.
-
3.
Diet and sedentary lifestyle remain the primary drivers of obesity.
-
4.
Obesity involves complex interplay between multiple other factors that require more study like host genetics, environment, and gut permeability.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. 2013;36(1):103–14. This paper provides a very good overall breakdown of the gut microbiome and how it can impact the development of obesity. It also discusses the microbiome’s ability to affect metabolic syndrome and other obesity-related diseases.
World Health Organization. (2017). Obesity and overweight. [Online] Available at: http://www.who.int/mediacentre/factsheets/fs311/en/ [Accessed 23 July 2017].
Hicks L, Taylor T, Hunkler R. Antibiotic prescriptions per 1000 persons of all ages according to state, 2010. [Online image] In U.S. Outpatient Antibiotic Prescribing, 2010. N Engl J Med. 2013;368(15):1461–2. Permission granted via email message 9/11/2017
Centers for Disease Control and Prevention. Adult Obesity Prevalence Maps, (2017). [Online Image] Available at: https://www.cdc.gov/obesity/data/prevalence-maps.html [Retrieved 13 July 2017]. Permission granted via email message 9/8/2017
Ley R, Backhed F, Turnbaugh P, Lozupone C, Knight R, Gordon J. Obesity alters gut microbial ecology. Proc Natl Acad Sci. 2005;102(31):11070–5.
Trasande L, Blustein J, Liu M, Corwin E, Cox L, Blaser M. Infant antibiotic exposures and early-life body mass. Int J Obes. 2012;37(1):16–23.
Cox L, Blaser M. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11(3):182–90.
Dominguez-Bello M, Costello E, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107(26):11971–5.
Blustein J, Attina T, Liu M, Ryan A, Cox L, Blaser M, et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes. 2013;37(7):900–6.
•• Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. Provides a good description of how the foods we eat get broken down and can affect our energy harvest. Also discusses how our diet can alter human gut microbiota.
Tremaroli V, Bäckhed F. Effects of colonic fermentation of dietary fibres. [Online image] In Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):244. Permission granted via email message 9/7/2017
Blaut M. Simplified scheme showing the roles of SCFA in host metabolism. [Online image] In gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2014;74(03):230. Permission granted via email message 9/7/2017
Turnbaugh P, Ley R, Mahowald M, Magrini V, Mardis E, Gordon J. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Liou A, Paziuk M, Luevano J, Machineni S, Turnbaugh P, Kaplan L. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41.
Albenberg L, Wu G. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146(6):1564–72.
Singh R, et al. “Impact of diet on the gut microbiome and human health.” [Online image] In influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(73):3. Permission granted online 9/7/2017
Russell W, Gratz S, Duncan S, Holtrop G, Ince J, Scobbie L, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93(5):1062–72.
Heinritz S, Weiss E, Eklund M, Aumiller T, Heyer C, Messner S, et al. Impact of a high-fat or high-fiber diet on intestinal microbiota and metabolic markers in a pig model. Nutrients. 2016;8(5):317.
Eid N, Enani S, Walton G, Corona G, Costabile A, Gibson G, et al. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J Nutr Sci. 2014;3(46):1–9.
Cani P, Bibiloni R, Knauf C, Waget A, Neyrinck A, Delzenne N, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81.
Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte R, Bartelsman J, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.
Gao Z, Yin J, Zhang J, Ward R, Martin R, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17.
Wang Z, Klipfell E, Bennett B, Koeth R, Levison B, DuGar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63.
Tang W, Wang Z, Levison B, Koeth R, Britt E, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84.
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal W, Strowig T, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7984):179–85.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nutrition and Obesity
Rights and permissions
About this article
Cite this article
Omer, E., Atassi, H. The Microbiome That Shapes Us: Can It Cause Obesity?. Curr Gastroenterol Rep 19, 59 (2017). https://doi.org/10.1007/s11894-017-0600-y
Published:
DOI: https://doi.org/10.1007/s11894-017-0600-y