Abstract
The microbial communities in our intestine have the potential to influence many aspects of our physiology via the production of metabolites, by fermenting dietary and host-derived substrates, and via interactions of microbial cells with host tissues. The possible impact of the gut microbiota upon obesity has been the subject of much intriguing research, but still remains to be clarified. While microbes contribute to the recovery of energy from the diet by fermenting otherwise non-digestible carbohydrates, it is not established that obese humans gain a greater proportion of their energy by this route than people of normal weight. In general, there is increasing evidence that the species composition of the gut microbiota in humans can be altered by dietary intake in the short- and long-term, and microbiota composition changes in individuals following weight-loss diets, or after bariatric surgery. Furthermore, experiments using rodents indicate that certain gut bacterial species may influence fat metabolism, energy intake and energy expenditure. This suggests that dietary interventions designed to manipulate the microbiota (e.g. with probiotics or prebiotics) might provide approaches for controlling adiposity, weight and metabolic health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
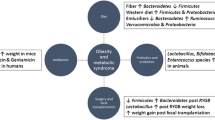
Interest in the role of the resident gut microbiota in human health and disease has increased rapidly over the past 10 years, including strong interest in the potential for our gut microbiota to influence weight gain and adiposity. Much of this interest was triggered by the hypothesis that the composition of the bacterial communities in our gut can influence ‘energy harvest’ from the diet [1] and by evidence for microbial influences on fat deposition [2]. As research has progressed, the interpretation of experimental data has often had to be refined, and many more interactions have emerged that suggest possible impacts of the microbial community on host physiology, energy intake and expenditure, as discussed in recent reviews [3–7] (Fig. 5.1). We will attempt here to give a brief overview of this rapidly developing field of research.
The ability to analyse gut microbial communities by non-cultural approaches, especially high-throughput sequencing, has led to a great deal of new information on the diversity and composition of the human colonic microbiota. Phylogenetic approaches based mainly on amplified 16S rRNA genes reveal that the two dominant bacterial phyla detected in faecal samples from healthy individuals are the Gram-negative Bacteroidetes and the Gram-positive Firmicutes, with Proteobacteria, Actinobacteria and Verrucomicrobia also represented. Although there is considerable inter-individual variation at the species level, 50–60 dominant species are present in most individuals [8–10]. It is not yet clear whether inter-individual variation in microbiota composition is continuous or semi-discrete, and evidence suggestive of different ‘enterotypes’ [11] within the human population is currently under debate. Communities dominated either by Prevotella spp. or Bacteroides spp. (both belonging to the Bacteroidetes phylum) have been reported from several large studies, but evidence for a third, Firmicutes-dominated, enterotype originally proposed by Arumugam et al. [11] appears less consistent [12, 13]. It now appears that diet may be a major factor driving such variation [12].
Impact of Diet on the Gut Microbiota
The main energy sources available to bacteria in the large intestine are non-digestible carbohydrates (plant cell wall polysaccharides and resistant starch) and host products, especially mucin. There is clear evidence that gut microbiota composition changes with dietary intake. This can be seen in short-term dietary intervention studies, where the representation of certain groups in the faecal microbiota is reported to increase within a few days of switching to diets high in particular non-digestible carbohydrates such as resistant starch [9, 14]. There is also clear evidence from numerous studies for microbiota changes in response to prebiotics [15–18]. These short-term shifts are however strongly influenced by inter-individual variation, and individual variation apparently remains the main factor determining the overall composition of the microbial community despite the consistent response of specific ‘diet-responsive’ bacterial groups [9]. Broadly-based shifts in the gut microbial community have nevertheless been reported in groups of subjects who differ in habitual, long-term dietary intake. In particular, Wu et al. [12] reported higher proportions of Bacteroidetes in subjects consuming diets high in protein, and higher proportions of Prevotella in those consuming more fibre. A similar shift was seen in a group of African children compared with Italian children whose diets differed in fibre and protein intake [19].
In obese subjects, gut microbiota changes have also been shown to result from weight-loss diets. In obese male volunteers there was a significant decrease in the faecal populations of the Roseburia + E. rectale group of Firmicutes bacteria and in bifidobacteria within 4 weeks following a shift to weight-loss diets with high protein and decreased carbohydrate contents [20, 21]. The cross-over design showed clearly that this change was driven by the diet rather than by weight loss, since it was partially reversed when there was a shift to a second weight-loss diet containing a higher content of total carbohydrate [22]. In an earlier study, Ley et al. [23] reported an increase in % Bacteroidetes in obese subjects following 52-week weight-loss regimes. The initial % Bacteroidetes in these subjects was lower than in most other studies, as discussed below.
Evidence for Changes in Microbiota Composition in Obese Humans
An early report, based on sequencing of amplified 16S rRNA genes, indicated a much higher ratio of Firmicutes to Bacteroidetes in faecal samples from 12 obese humans than in two lean controls [23]. This appeared consistent with the higher Firmicutes:Bacteroidetes ratio seen in genetically obese mice compared with lean mice, leading to the proposal that the ratio of these two phyla within the gut microbiota might be a causative factor in obesity [1]. Subsequent studies using FISH microscopy however either did not detect a change in % Bacteroidetes with BMI [22] or indicated a slightly increased % Bacteroidetes [24] in obese subjects. Numerous studies have now been performed using quantitative PCR, high-throughput sequencing of 16S rRNA genes or metagenome sequencing to analyse the faecal microbiota in obese subjects, leading to the conclusion that there is no consistent difference between lean, normal weight and obese subjects at the bacterial phylum level [4, 9, 25–27]. Some differences are however apparent at the genus and species levels [28] including between MZ twins who were discordant for BMI [29]. Given the impact of diet on microbiota composition at this level, discussed above, it seems likely that such differences will at least partially reflect differences on dietary intake between obese and normal-weight individuals. Yokota et al. [30] recently suggested that increased secretion of bile acids may contribute to alterations of the microbiota on high fat diets due to the antimicrobial activity of secondary bile acids. They demonstrated microbiota changes in line with those seen in several studies on high fat diets (i.e. an increase in Firmicutes at the expense of Bacteroidetes) after feeding rats increasing levels of cholic acid. There is also increasing evidence that type 2 diabetics show altered microbiota profiles when compared with healthy subjects, with a decreased representation of certain groups of Firmicutes and of bifidobacteria [31–34]. The increased incidence of metabolic syndrome and type 2 diabetes in obese subjects is therefore an important confounding factor when interpreting microbiota changes in the obese.
Recent work using metagenomic sequencing has shown that microbiota profiles in obese subjects can be distinguished as being of low (LGC), or high (HGC) gene count, reflecting high and low species diversity [28]. The LGC type tends to be dominated by the Gram-negative Bacteroides and may correspond to one of the ‘enterotypes’ proposed by Arumugam et al. [11]. Obese or overweight subjects, showing the LGC profile had significantly higher insulin resistance and fasting triglyceride levels, indicative of metabolic syndrome, compared with HGC individuals [28]. Moreover, obese LGC individuals showed more rapid past weight gain on average than obese HGC individuals. In a companion study, a 12-week intervention on weight-loss diets increased the gene count in the LGC group, while improving symptoms associated with metabolic syndrome in both groups [35]. The simplest interpretation of these findings is that gut microbiota composition in these subjects is largely driven by their dietary intake, although, conversely, consequences of changes in host physiology could also influence microbiota composition. A diet that is low in fibre and high in digestible carbohydrates, especially simple sugars, might account for the LGC profile (found in both obese and lean individuals) while at the same time promoting the development of metabolic syndrome.
In human studies it is usually not possible to distinguish between microbiota changes that are consequences of changes in diet and/or host physiology from any that might be contributing factors in obesity, adiposity and inflammation. However there is intriguing evidence, mainly from animal studies to suggest that individual bacteria could have more significant roles in influencing host nutrition, physiology and behaviour. A number of studies have shown that transfer of gut microbiota from obese humans, compared with non-obese donors, to germ-free mice results in increased weight gain and adiposity in the colonised mice. Most recently, this result has been reported for obese human twin pairs that were discordant for BMI with the microbiota from the obese twin promoting adiposity and weight gain when transferred into germ-free mice to a greater extent than the microbiota from the relatively lean twin [36]. Diet is likely to have driven the separation in the microbiota composition between the members of each twin pair, but the transfer experiments suggest that this altered composition is also contributing to adiposity and weight gain. Such effects require mechanistic explanations and some of the possibilities are considered below.
Potential for Microbiota Composition to Influence Energy Recovery from the Diet
The gut microbial community in the large intestine contributes to the overall ‘energy harvest’ from the diet by fermenting components that remain undigested by host enzymes in the small intestine. Short-chain fatty acids produced by microbial fermentation are efficiently transported across the gut wall and used as energy sources, with butyrate being preferentially utilised by the gut epithelium. The over-riding factor that determines how much energy is delivered via microbial fermentation is the non-digestible carbohydrate (fibre) content of the diet, together with gut transit, which is of course influenced by fibre content [5, 37]. More rapid whole gut transit may lead to a greater fraction of dietary intake failing to be digested in the upper GI tract, thus increasing the substrate available for fermentation in the large intestine [38]. On the other hand, rapid transit also tends to decrease the extent of fibre degradation and the efficiency of SCFA absorption [37]. Faecal SCFA concentrations are reported to be higher in obese subjects [24, 39, 40] which seems most likely to reflect higher dietary intake.
The potential factor that has attracted most speculation is the species composition of the gut microbiota [25, 41]. It is therefore worth considering in more detail the mechanisms by which changes in microbiota composition might affect energy recovery from the diet by considering the following questions.
Does Microbiota Composition Influence the Rate and Extent of Substrate Fermentation in the Colon?
If certain ‘keystone’ species were required to initiate degradation of recalcitrant substrates, then their absence from the microbiota could have a major impact on the release of energy from dietary residue. An example of this phenomenon comes from the finding that, among 14 obese human volunteers, ingested RS3 starch remained largely unfermented only in two individuals who lacked ruminococci in their faeces [9]. Relatives of R. bromii appear to be particularly potent degraders of this type of starch by comparison with other amylolytic species [42]. It is currently unclear how common such deficiencies in ‘keystone species’ are within the general population, but their consequence would be to reduce energy gain.
Does Microbiota Composition Influence the Stoichiometry of Fermentation in the Gut?
In vitro experiments show clearly that perturbation of the microbial community composition, e.g. resulting from a pH change, can result in major shifts in the ratios of the major fermentation products [43]. While acetate, propionate and butyrate all supply energy to the host, they are utilised by different tissues and have different physiological consequences, as discussed further below. Deficiencies in butyrate-producing bacteria (which belong to the Firmicutes within the human colonic community) have now been reported in several disease states, including type 2 diabetes [34], and overall decreases in these bacteria are known to result in decreased butyrate production [20, 43].
Hydrogen utilisation plays a central role in anaerobic metabolism, and the consequences of variation in hydrogen utilising microbes have been the subject of much speculation. Methanogenic archaea occur in high numbers in approximately half of the population; some reports indicate that they are increased in obese subjects [40, 44] while others suggest the contrary [24]. It is possible that in the absence of methanogenesis more carbon would be diverted into SCFA and therefore to the host (Table 5.1); indeed this is one of the goals of attempting to inhibit methanogenesis in the rumen [45]. In the absence of inhibition, however, the equivalent amount of carbon may simply be released as CO2 when methanogen populations are low. Another important group of hydrogen utilisers are acetogens, which have the ability to convert H2 and CO2 (or formate) to acetate. This introduces an additional non-dietary source of acetate carbon [46] but the contribution and degree of variability of this route for acetate synthesis in the human colon has not been fully established.
Another effect of hydrogen utilisation is to increase the growth efficiency of hydrogen-producing, substrate-degrading species [47]. As this is predicted to occur with both methanogens and acetogens it would be assumed to apply to any ‘normal’ gut microbial community, although there is some intriguing evidence that cellulolytic ruminococci may be dependent on the presence of methanogens within the microbiota [48]. If degradation of some dietary carbohydrates was increased in the presence of methanogens, this might tend to increase ‘energy harvest’ (Table 5.1).
Does Microbiota Composition Affect the Uptake of SCFA by the Gut Epithelium?
As already noted, gut transit is thought to have an important influence of SCFA uptake. Conversely, SCFA are themselves known to affect gut motility and transit via interactions with receptors that influence gut hormones, although these effects may differ in different regions of the intestine. This creates complex feedback loops whereby microbiota composition may influence absorption of fermentation products by the host. There is also intriguing evidence that methane may slow gut transit [49]; methanogens appear to be associated with slow gut transit [50] but whether this is because of their slow growth rate or their impact on gut motility is not known.
Potential for Microbiota to Influence Energy Expenditure and Adiposity
The intestinal microbiota influences host physiology beyond their direct actions in the gut (Fig. 5.2). Several possible routes of cross-talk exist between the microbes and host tissues, encompassing metabolic, immunological, endocrine and neural pathways [3, 51, 52], and the exact mechanisms of interaction are currently under extensive research. Another factor that has to be taken into consideration is the role host genetics play in determining the response to obesogenic diets as well as the composition of the microbiota. High heritability of gene-by-diet interactions has recently been demonstrated in a genome-wide association study in different mouse strains on a high fat/high sugar diet [53]. A parallel investigation of the gut microbiota revealed significant phylum-level shifts in response to diet across different genetic backgrounds, however, effects of the genetic background on the composition and plasticity of the microbiota were also evident. Only one of the genetic loci found to be associated with body fat, which include three amylase genes, was found to be associated with significant changes in microbiota composition, namely an enrichment of Enterobacteriaceae within the phylum Proteobacteria. In addition, three specific microbiota phylotypes showed a modest correlation with obesity traits. Intriguingly, Akkermansia displayed a negative correlation with body fat percentage despite the fact that this genus showed the strongest overall enrichment on the high fat/high sugar diet [53].
Some studies have found that energy balance could be profoundly influenced in animal models by the introduction of a single bacterial species. Administration of a purified probiotic strain of Lactobacillus reuteri led to the prevention of weight gain without significantly affecting the existing microbiota or calorie consumption in mice on a Western diet. The underlying effect appeared to be a modulation of the immune system towards a more anti-inflammatory tone, and the phenotype was transferable to naïve hosts via purified CD4+ T cells from animals consuming the probiotic [54].
Separately, it has been reported that Akkermansia muciniphila (which comprises 3–5 % of the colonic microbiota in healthy adults) abundance correlates inversely with body weight. This bacterium has a specialist role and derives its carbon and energy from the mucus layer lining the intestinal tract. In contrast to Parks et al. [53] who found an increase of Akkermansia on a high fat/high sugar diet (see above), Everard et al. [55] demonstrated that populations of this organism are diminished on high fat diets, which resulted in a reduction in the thickness of the mucus layer. Moreover, re-introduction of A. muciniphila by gavage to mice fed a high fat diet reduced body weight and improved body composition without changes in food intake. It also restored the mucus layer, decreased circulating lipopolysaccharide (LPS) levels and increased glucose tolerance compared to control animals gavaged with either PBS or killed cells [55]. Both studies found that the reduction in weight gain and body fat was achieved without a significant reduction in food intake, indicating that energy balance regulation was influenced via other factors, such as locomotor activity and heat production.
A direct link between obesity, glucose metabolism and low-grade inflammation has previously been demonstrated by subcutaneous administration of LPS, which led to insulin resistance and fat mass development in mice [56]. An intact gut barrier function is crucial in preventing LPS from crossing from the gut into the systemic circulation (increased plasma LPS levels have been termed metabolic endotoxemia), and the gut microbiota may influence gut permeability via actions on the mucus layer or regulatory effects on epithelial cells (e.g. tight junction protein expression) [57]. Bacterial signalling appears to involve the endocannabinoid system, endogenous bioactive lipids that regulate barrier function, as well as the enteroendocrine peptide glucagon-like peptide-2 [55].
Inflammation may be mediated by several bacterial products such as lipopeptides, LPS and flagellins that act as ligands for toll-like receptors (TLRs) 2, 4 and 5, whilst other TLRs detect nucleic acid motifs. In most cell types detection of these bacterial ligands evokes a potent inflammatory response inducing myeloid-differentiation factor 88 (MyD88) and NF-kappa B which results in a broad array of pro-inflammatory chemokines and cytokines. By contrast, recognition of these bacterial moieties by intestinal epithelial cells has been reported to lead to enhancement of barrier function, and epithelial repair rather than overt inflammatory responses.
LPS is continuously released in the intestinal tract as a consequence of bacterial cell lysis and serum LPS was shown to be 76 % higher in type 2 diabetics compared to the control cohort and consumption of a high fat meal resulted in a 50 % higher endotoxin level [58]. High fat diets can increase absorption of LPS present in the cell walls of Gram-negative bacteria either by incorporation into chylomicrons or by increasing intestinal permeability [56]. LPS is a potent inflammatory mediator that signals in a TLR4-dependent manner and infusion of LPS can increase weight gain, adiposity, insulin resistance and liver triglycerides. Separately deletion of TLR5 in mice, which senses bacterial flagellin, results in an alteration in the composition of the gut microbiota and also to features of metabolic syndrome including insulin resistance, increased adiposity and blood pressure and increased cholesterol levels [59].
An important role of inflammation in the development of obesity is in line with the notion that germ-free animals are resistant to diet-induced obesity [60]. However, Fleissner et al. [61] demonstrated that this effect is dependent on the specific dietary ingredients of high fat diets and that germ-free mice are not generally protected against obesity by comparing different types of high-fat diet with equal macronutrient content. Furthermore, it has recently been shown that, in contrast to germ-free mice, germ-free rats did not exhibit decreased adiposity compared to their conventional counterparts, and alterations in host lipid metabolism differed between rats and mice [62]. Therefore, differences in host metabolism as well as morphological and physiological alterations of germ-free animals compared to conventional animals require careful consideration in the assessment of microbiota-mediated effects on adipogenesis.
Recently Upadhyay et al. [63] have linked effects of the microbiota in diet-induced obesity in mice to gut immunity by investigating mice deficient in lymphotoxin, which is involved in normal mucosal defence against pathogens. Lymphotoxin-deficient mice were resistant to diet-induced obesity and also showed changes in gut microbiota composition, particularly an increase in segmented filamentous bacteria. Germ-free animals receiving the microbiota from lymphotoxin-deficient animals remained lean, whereas cohousing of animals with lymphotoxin negative and positive genetic background lead to weight gain in the negative background, indicating transferability of the host phenotype via the microbiota. The authors postulate that changes in gut mucosal host immunity in response to diet influence the microbiota, which in turn affects systemic host physiology.
Microbes may also signal to the host via short-chain fatty acids, the major metabolic end products of fermentation by bacteria in the colon, via G-protein-coupled receptors GPR41 (or free fatty acid receptor (FFAR)3 and GPR43 (FFAR2). The dominant acids usually detected are acetate, propionate and butyrate, all of which activate FFAR2 and 3 with different potency [64]. The receptors are expressed in various tissues, including the gut, a range of immune cells and adipose tissue, but their prime site of action remains under study and there is some conflicting evidence in the literature with regard to their function [64]. Nevertheless, FFAR3 has recently been linked to activation of sympathetic neurons via SCFA, whereas ketone bodies acted as antagonists [65]. Thus SCFA may influence energy expenditure by affecting heart rate and thermogenesis via this route. FFAR2 knockout mice, on the other hand, were shown to develop obesity on a normal diet, whereas overexpression of FFAR2 in adipose tissue promoted a lean phenotype even on a high fat diet [66]. These effects were abolished when the animals were raised under germ-free conditions, indicating an involvement of the gut microbiota. On a molecular basis, the activation of FFAR2 suppressed insulin signalling specifically in white adipose tissue, with a consequent inhibition of fat accumulation, while promoting energy expenditure in other tissues [66].
Other effects of SCFA, such as increased expression and production of hormones involved in appetite regulation (glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in the gut; leptin in adipose tissue) may also be mediated by FFAR2 and 3. An involvement of FFAR2 in GLP-1 release from colonic L cells has been demonstrated in cell cultures as well as in vivo [67]. It remains to be established, however, how transferrable results from animal models are to humans, as it has been shown that there are differences between hosts with regard to the potency and selectivity of different SCFA on the receptors, as well as the receptor interaction with downstream effectors [64]. Regardless of the underlying mechanisms of action, there is also evidence for an anti-obesogenic effect for both propionate and butyrate when given orally [68, 69], and modulation of host energy balance through dietary stimulation of microbial SCFA production is an attractive concept to help tackle obesity. Prebiotic supplementation has been shown to be effective in reducing inflammation in animal models and increasing satiety in humans [7], but the complexity of the microbiota as well as the multitude of possible molecular routes for interaction with the host require further investigation before specific members of the microbiota or certain microbiota profiles can unequivocally be assigned a role in preventing or promoting obesity.
Impact of Antibiotics
Antimicrobials potentially alter microbiota composition [70] and epidemiological studies in humans have shown that antibiotic treatment during the first 6 months of life [71] may have an effect as this is a time when the host adipocytes are developing [72]. In young mice sub-therapeutic levels of antibiotics were recently found to change gut microbiota composition and increase fat mass [73]. Additionally this study identified an increase in SCFA in the large intestine, suggested to reflect increased fermentation. On the other hand, Cani et al. [74] reported that treatment of obese and diabetic mice with antibiotics (ampicillin and neomycin) for a period of 4 weeks led to a reduction of metabolic endotoxemia, body weight and body fat. The impact of antibiotics is likely to depend critically on dosage, on the particular antibiotic/s used, and on events both in the small and large intestine [75] making generic interpretations difficult and probably unwise.
Conclusions
There is increasing evidence from studies with small animal models that the microbiota of the gut can influence adiposity and weight gain. Explanations for these effects appear to lie with the impact of microbial activities and metabolic output upon host physiology. Although the microbial fermentation of non-digestible dietary residue contributes energy to the host, the hypothesis that the gut microbiota of obese and lean individuals differ in the efficiency with which they retrieve energy from dietary residue (‘energy harvest’) remains unproven. On the other hand, small animal experiments indicate that adiposity and weight gain can be promoted by the transfer of ‘obesogenic’ microbiota into germ-free animals, with some evidence that individual species can play a role. Potential mechanisms include influences on food intake and satiety, energy expenditure and the control of pathways that influence inflammation pathways, glucose homeostasis and adipogenesis. It appears that these microbial factors can have an influence on human obesity, but their exact contribution has still to be fully assessed.
References
Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23.
Blaut M, Klaus S. Intestinal microbiota and obesity. In: Joost H-G, editors. Appetite control. Handbook of experimental pharmacology vol 209. Heidelberg: Springer; 2012. DOI: 10.1007/978-3-642-24716-3_11.
Tagliabue A, Elli M. The role of gut microbiota in human obesity: recent findings and future perspectives. Nutr Metab Cardiovasc Dis. 2013;23:160–8.
Flint HJ. Obesity and the gut microbiota. J Clin Gastroenterol. 2011;45:S128–132.
Tremaroli V, Kovatcheva-Datchary P, Backhed F. A role for the gut microbiota in energy harvesting? Gut. 2010;59:1589–90.
Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83.
Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–84.
Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30.
Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473: 174–80.
Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8.
Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84.
Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE. 2010;5:e15046.
Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–89.
Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24:710–4.
Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of non-digestible carbohydrates to stimulate faecal bifidobacteria in healthy humans: a double blind, randomized, placebo-controlled, parallel-group, dose response relation study. Am J Clin Nutr. 2004;80:1658–64.
Ramirez-Farias C, Slezak K, Fuller Z, et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–50.
De Filippo C, Cavalieri D, Di Paolo M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107: 14691–6.
Duncan SH, Belenguer A, Holtrop G, et al. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–8.
Russell WR, Gratz S, Duncan SH, et al. High protein, reduced carbohydrate diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93:1062–72.
Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–4.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology – human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Schwiertz A, Tars D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2009;18:190–5.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core microbiome in obese and lean twins. Nature. 2009; 457:480–4.
Jumpertz R, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65.
Zupanic ML, Cantarel BL, Liu Z, et al. Analysis of the gut microbiota in the old order Amish and its relation to the metabolic syndrome. PLoS ONE. 2012;7:e43052.
Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–9.
Tims S, Derom C, Jonkers DM, et al. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–17.
Yokota A, Fukiya S, Ooka T, Ogura Y, Hayashi T, Ishizuka S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 2012;3:455–9.
Wu X, Ma C, Han L, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61:69–78.
Larsen N, Vogensen FK, van den Berg FWJ, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085.
Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–105.
Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbiota richness. Nature. 2013;500:585–90.
Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214.
Stephen AM, Wiggins HS, Cummings JH. Effect of changing transit time on colonic microbial metabolism in man. Gut. 1987;28:601–9.
Chapman RW, Sillery JK, Graham MM, et al. Absorption of starch by healthy ileostomates: effect of transit time and of carbohydrate load. Am J Clin Nutr. 1985;41:1244–9.
Teixeira TFS, Grzeskowiak L, Franceschini SCC, et al. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr. 2013;109:914–9.
Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57.
Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–42.
Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6: 1535–43.
Walker AW, Duncan SH, Leitch ECM, et al. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. 2005;71:3692–700.
Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2008;106:2365–70.
Wood TA, Wallace RJ, Rowe A, et al. Encapsulated fumaric acid as a feed ingredient to decrease methane emissions. Anim Feed Sci Technol. 2009;152:62–71.
Miller TL, Wolin MJ. Pathways of acetate, propionate and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62:1589–92.
Latham MJ, Wolin MJ. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl Environ Microbiol. 1977;34:297–301.
Robert C, Bernalier-Donadille A. The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol. 2003;46: 81–9.
Pimental M, Lin HC, Enayatt P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290: G1089–95.
El Oufir L, Flourié B, Bruley des Varannes S, et al. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut 1996; 38:870–7.
Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–12.
Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336: 1262–7.
Parks BW, Nam E, Org E, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141–52.
Poutahidis T, Kleinewietfeld M, Smillie C, et al. Microbial reprogramming inhibits Western diet-associated obesity. PLoS ONE. 2013;8:e68596.
Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130:202–12.
Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92.
Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328: 228–31.
Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–84.
Fleissner CK, Huebel N, Mostafa M, et al. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr. 2010;104:919–29.
Swartz TD, Sakar Y, Duca FA, Covasa M. Preserved adiposity in the Fischer 344 rat devoid of gut microbiota. FASEB J. 2013;27:1701–10.
Upadhyay V, Poroyko V, Kim T, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol. 2012;13:947–53.
Hudson BD, Murdoch H, Milligan G. Minireview: the effects of species ortholog and SNP variation on receptors for free fatty acids. Mol Endocrinol. 2013; 27:1177–87.
Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA. 2011;108:8030–5.
Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chair fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71.
Arora T, Sharma R, Frost G. Propionate. Anti-obesity and satiety enhancing factor? Appetite. 2011;56: 511–5.
Gao Z, Yin J, Zhang J, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17.
Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280.
Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond). 2013;37:16–23.
Greenwood MR, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. J Lipid Res. 1974;15:474–83.
Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6.
Cani P, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Flint HJ. Antibiotics and adiposity. Nature. 2012;488: 601–2.
Acknowledgments
The authors receive support from the Scottish Government Food, Land and People programme.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Flint, H.J., Duncan, S.H., Louis, P. (2014). Gut Microbiome and Obesity. In: Kushner, R., Bessesen, D. (eds) Treatment of the Obese Patient. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1203-2_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1203-2_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1202-5
Online ISBN: 978-1-4939-1203-2
eBook Packages: MedicineMedicine (R0)