Abstract
Exposure to ionizing radiation is associated with an increased risk of cancer. With the growing use of diagnostic imaging studies, there is concern for increasing the risk of radiation associated malignancy of the gastrointestinal tract. The purpose of this review is to summarize the existing literature for risk of gastrointestinal malignancy after ionizing radiation exposure from diagnostic imaging studies. Estimates of organ specific effective doses of radiation vary widely based on the method of measurement and patient factors. Most of the current data are based on calculations of organ effective doses from anthropomorphic phantoms and estimated cancer risk based on radiation exposure from environmental sources. Radiation associated cancer risk is dependent on both the cumulative radiation dose and the radiosensitivity of the particular organ. The majority of radiation exposure and risk associated with gastrointestinal malignancy comes from CT scans, especially of the abdomen/pelvis. Of the abdominal organs, the colon carries the highest lifetime attributable risk of radiation associated malignancy. The attributable risk of malignancy for an individual diagnostic imaging study is low, but measurable, and therefore imaging studies without radiation such as MRI and ultrasound should be considered, especially in patients who require repeated imaging studies. There is a shortage of epidemiological data and an absence of prospective data with adequate follow-up to describe accurate risk estimates of gastrointestinal cancers after diagnostic imaging. More studies are needed to better determine the risks of malignancy from diagnostic imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to ionizing radiation has been shown to have adverse effects on health. Radiation exposure has been associated with an increased risk of cancers, including lung, colon, breast, thyroid, and bladder [1, 2]. Leukemia was one of the first cancers related to radiation exposure, occurring within 5 years of exposure, whereas solid cancers have been observed within 10 years of radiation exposure [3, 4]. Most data on radiation associated malignancy are from Japanese atomic bomb survivors, and in the setting of occupational exposure [5, 6].
Radiation therapy for treatment of malignant and benign conditions has also been associated with increased cancer risk and occurrence of secondary malignancies. In the past, tinea capitis and enlargement of the thymus or tonsillar glands were treated with radiation therapy. Follow up of patients who have received these radiation treatments has shown an increased risk of cancer in the thyroid, breasts, bone marrow, brain, and skin [7–9]. Radiation therapy for prostate cancer after radical prostatectomy is associated with a second primary cancer, reported in up to 1747 cases per 100,000 during a 15-year follow-up period; the most frequent locations for a second primary cancer were bladder, rectum, and rectosigmoid junction [10]. A 3.4-fold increased risk relative to the general population of secondary stomach cancer has been reported in long-term survivors of testicular cancer and Hodgkin lymphoma who underwent radiation therapy [11]. Of patients who received radiation therapy to the ovaries or pituitary gland to treat hormonal infertility and amenorrhea, an overall 10% higher cancer mortality was noted, including an increased mortality from colon cancer with a standardized mortality ratio (SMR) of 1.9 (95% CI 1.1–3.1) [12].
The use of diagnostic imaging has grown tremendously over the last few decades. Nearly 70 million CT scans were performed in 2007 [13] and patients who require one CT are more likely to be exposed to multiple CT scans in the future [14]. Low doses of radiation exposure through diagnostic imaging have also been implicated in increased cancer risk. Breast, thyroid, and lung cancer have been associated with CT examinations of the chest [15, 16]. The relative risk of mortality from breast cancer is 1.36 (95%CI 1.11–1.67) in women who had received more than 100 mSv of radiation from fluoroscopic imaging during their treatment for tuberculosis [17]. An increased mortality from breast cancer was also observed in retrospective cohort studies of women with scoliosis who had undergone multiple radiographs, SMR 1.69 (95% CI, 1.3–2.1) [18]. Based on model estimates of radiation exposure, Smith-Bindman et al. calculated that among 40 year-old patients, 1 in 4360 women and 1 in 7350 men will develop radiation-induced cancer from a head CT, and 1 in 470 women, 1 in 620 men from an abdomen/pelvis CT [19]. Studies estimate that 0.7–2% of all cancers and 1% of total cancer mortality may be attributed to the radiation exposure from CT imaging [14, 20]. Radiation from CT examinations is estimated to cause 500 deaths annually in children less than 15 years old [21]. Using the BEIR VII lifetime risk model (described below), one patient in every 1000 exposed to 10 mSv from a CT scan of the abdomen will develop a radiation-induced malignancy in his or her lifetime [22•].
Although there is substantial literature reporting overall cancer risk related to environmental exposure, the risk of gastrointestinal malignancies associated with diagnostic imaging studies is unclear. The aim of this review is to summarize the existing literature on the risk of gastrointestinal cancers associated with radiation exposure from diagnostic imaging studies.
Methods of Quantifying Radiation Exposure and Malignancy Risk
Two models exist to account for radiation associated malignancy risk [23]. The dose response model is based on the assumption that cancer risk is related linearly to radiation exposure dosage and that no threshold limit exists for cancer risk. The threshold response model is dependent on a radiation threshold limit that must be reached before a risk of cancer exists. The second model is based on data from atomic bomb survivors, the linear no-threshold (LNT) risk model. The LNT model is based on dose response where even very small doses of radiation are associated with a small risk of cancer. The LNT risk model has been criticized as oversimplifying cancer risk at low radiation dosages and some groups do not recommend the LNT model to be applied at doses <50 mSv [23]. However, cancer risk was demonstrated at dosages as low as at 35 mSv based on atomic bomb survivor data [5, 24].
The actual dose of radiation an organ receives cannot be measured easily. Anthropomorphic phantoms are used to calculate the organ effective dose from a single imaging study. The phantom enables internally placed dosimeters to measure the absorbed organ doses using high sensitivity detectors. All of the studies reviewed used phantoms to estimate organ effective doses. However, actual organ radiation exposure may vary from the anthropomorphic phantom. Studies have shown discrepancies between organ doses measured from cohorts of real patients and measured from phantoms [19]. Phantom models are also limited because actual organ effective dose is dependent on patient specific factors such as size, body weight, and fat distribution.
Using the organ specific effective dose, the lifetime attributable risk (LAR) of cancer can be calculated using the data available in the Biological Effects of Ionizing Radiation (BEIR) VII report published by the National Research Council BEIR Committee [22•]. The LAR implies excess cancer risk above and beyond the baseline cancer risk. Some studies also report the relative risk (RR) using the LAR and the lifetime risk (LR), which is the baseline risk of cancer in unexposed populations. In the BEIR VII report, the risk of malignancy was extrapolated largely from epidemiological studies involving Japanese atomic bomb survivors, populations living near nuclear power plants during accidental release of radiation, and health care workers with occupational radiation exposure. There are limitations of the BEIR VII risk estimates. The BEIR VII report may underestimate cancer risk as the Japanese atomic bomb studies were based on gamma rays as opposed to x-rays. The BEIR VII report does not calculate LAR at radiation dosages less than 100 mSv; however many of the studies reviewed extrapolated the BEIR VII report to radiation doses less than 100 mSv. In another estimate of the cancer risk, the United Nations Scientific Committee on the Effects of Atomic Radiation Report (UNSCEAR) is a collection of epidemiological studies of radiation-induced malignancy, mostly from the atomic bomb, occupational exposure, and radiation therapy [25].
Gastric Cancer Risk
Stomach Effective Dose and Estimated Gastric Cancer Risk
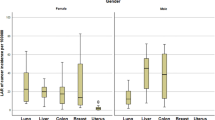
The amount of radiation the stomach is exposed to is dependent on the imaging study. Studies were identified estimating effective doses of radiation to the stomach from small bowel follow (SBFT) fluoroscopy and CT scans of the chest, abdomen, and pelvis (Table 1). There were no studies reporting stomach-specific effective doses of radiation associated with upper GI series. The total effective dose of radiation from upper GI series has been reported to range from 3 to 9.3 mSv per study, dependent on the number of radiographs taken per study [52]. All of the studies reviewed calculated stomach-effective doses using anthropomorphic phantoms or computer modeling software (ImPACT) (Table 2). Only one study estimated the effective dose of radiation to the stomach from SBFT, 0.8 mSv [26]. CT, on the other hand, exposes the stomach to a range of 3.6–68 mSv of radiation based on the type of CT. CT scan of the abdomen/pelvis exposes the stomach to 18–24 mSv of radiation [26, 27]. A single CT scan of the abdomen/pelvis in 18 year-olds was associated with a stomach cancer LAR of 0.008% [27]. The effective dose to the stomach from CT colonography (virtual colonoscopy) is similar to standard CT examinations (14.8 mSv) [30]. Although the amount of energy imparted per CT is larger in adults, children receive higher organ effective doses of radiation and have a higher risk of future malignancy. For example, cone beam CT scans, which are 3-dimensional CT scans used prior to the initiation of radiation therapy to visualize the target tumor in 3D imaging, had an effective dose of 68 mSv and a LAR of gastric cancer of 0.051% when studied in 5 year old children [29].
Standard chest CT scans do not result in significant differences in the effective dose of radiation to the stomach when performed in addition to a CT abdomen/pelvis. Studies of CT scans of the abdomen/pelvis and full body CT scans have similar effective doses of radiation to the stomach [28]. Using the BEIR VII lifetime attributable risk model, cancer risk of annual chest CT scans starting at age 2 until survival age of 36 and 50 years for surveillance in cystic fibrosis (CF) patients was approximated using the cumulative radiation dose. After 34 annual chest CT scans, stomach cancer LAR was 0.0002% in males and 0.0003% in females [35]. When a total of 48 lifetime chest CT scans are performed, stomach cancer LAR was 0.0017% in males 0.0021% in females [35].
Although standard chest CT scans result in a minimal increase in effective dose of radiation to the stomach compared to CT scans of the abdomen/pelvis, several specific CT studies of the torso impart significantly higher effective doses of radiation to the stomach. CT angiography (CTA) has been studied as a substitute to conventional coronary angiography to assess coronary artery anatomy, but radiation doses are higher than for conventional angiography [31]. The stomach effective dose for ECG-gated coronary CT angiography was reported from 11.1 to 14.4 mSv [16, 32, 33]. For ECG-gated CTA studying pulmonary vein anatomy, the effective dose was 3.6 mSv, whereas CTA protocol to evaluate pulmonary emboli was 36.2 mSv [16].
As seen with abdominal CT scans, pediatric patients are exposed to higher effective doses of radiation of the stomach compared to adults using chest CT scans. Anthropomorphic phantoms of 5 year-old children demonstrated effective doses of 24.3 mSv from ECG-gated CTA [34]. Infant males were exposed to a 24.3 mSv from CT angiography, which corresponded to a LAR of 0.066% in males, 0.09% in females. 5 year-old boys and girls had a LAR of 0.057% and 0.076%, respectively, from one coronary CT angiography study, whereas 10 year-old boys had a LAR of 0.048%, girls 0.064% [34].
Epidemiologic Studies: Gastric Cancer
Although epidemiological studies following patients exposed to diagnostic imaging studies and their development of stomach cancer are lacking, estimations can be made based on the data existing for stomach cancer development among people exposed to radiation from other realms, such as atomic bomb exposure and radiation therapy.
Three studies reported the risk of gastric cancer from environmental radiation exposure. The Life Span Study, which followed atomic bomb survivors long-term to determine their incidence and mortality from cancer, found that stomach cancer risk of exposure-induced death (REID) was 0.1% per 1,000 mSv [36]. In a separate calculation, the UNSCEAR report estimated a relative risk of stomach cancer to be 2.1 in males and 2 in females per 1000 mSv [25]. The relative risk of stomach cancer in nuclear plant workers was 1.1 per 1000 mSv (95% CI, 0.01–3.4) or REID of 0.15% per 1000 mSv [38].
Four studies reported on the development of gastric cancer after exposure to radiation treatments. The relative risk of secondary stomach cancer after radiation treatment for cervical cancer was 1.54 or a REID of 0.18% per 1000 mSv [37]. The REID was 0.33% per 1000 mSv in patients with metropathia hemorrhagica treated with radiation therapy, [39]. Ankylosing spondylitis patients treated with radium had a relative risk of 1.56 of developing stomach cancer [40]. In patients with peptic ulcer disease treated with radiation, the relative risk of developing stomach cancer was 2.77 (95% CI, 1.6–4.8) [41].
Only one study reported on cancer risk in patients exposed to diagnostic radiation over time [42•]. The death rate from cancer was 8% higher than the general population in 5573 women who had undergone multiple X-rays examinations for scoliosis and other spinal disorders [42•]. Average cumulative effective dose was 109 mSv in the breast, 41 mSv in the lung and 10 mSv in the bone marrow. No association with stomach cancer mortality was found, SMR 0.35 (95% CI, 0.04–1.25); however, the stomach effective dose was not reported.
Liver Cancer Risk
Liver Effective Dose
Since the liver is a large organ in the abdominal compartment, it is at risk for radiation exposure with any diagnostic imaging. SBFT fluoroscopy exposes the liver to an effective dose of 6.4 mSv [26]. The liver effective dose amongst CT scans was dependent on the type of study. CT abdomen/pelvis studies expose the liver to 16–33 mSv [26, 27]. Addition of a CT chest to a CT abdomen/pelvis was reported to provide a similar liver effective dose as a CT abdominal/pelvis alone, 18 mSv [27]. The LAR of liver cancer is 0.005% from a single CT examination of the abdomen/pelvis and increases slightly to 0.006% when the CT imaging is of the chest/abdomen/pelvis [27]. In the study previously described calculating cancer risk of annual surveillance chest CT scans in cystic fibrosis, 34 annual CT examinations of the chest was associated with a LAR for liver cancer of 0.0003%, and a LAR or 0.003.8% in those who received 48 annual chest CT scans.
As described with stomach effective doses, special CT scans of the chest may results in higher liver effective doses of radiation. CT angiography for pulmonary emboli emits 57.8 mSv of radiation to the liver [16]. ECG-gated CTA evaluating pulmonary vein anatomy has an effective dose of 15 mSv to the liver; whereas, ECG-gated CTA for coronary arteries has a liver effective dose of 19.2–53.4 mSv [6, 32, 33] in the adult liver and 20.8 mSv to the pediatric liver [34]. A single cone beam CT has a liver cancer LAR of 0.023% with a RR of 1.049. For children exposed to ECG-gated coronary CT angiography, the LAR is influenced by the age at time of exposure. The highest risk of cancer was in infants with a LAR of 0.071% in infant males and 0.031% in infant females. In 5 year-old subjects, the LAR among males was 0.058% and females 0.026%. In 10 year-old children the LAR of liver cancer was 0.05% in males and 0.022% in females [34].
Epidemiologic Studies: Liver Cancer
Only two studies reported the development of liver cancer related to environmental radiation exposure. The incidence of liver cancer in atomic bomb survivors in the Life Span Study showed an excess relative risk of 0.48 per 1000 mSv [36]. The UNSCEAR report, estimated the RR of liver cancer is 2.8 per 1000 mSv in males and 0.9 per 1000 mSv in females [25].
Interestingly, liver cancer was not associated with either therapeutic radiation (to the cervix) or in serial abdominal x-rays. Among cervical cancer patients treated with radiation therapy, the excess relative risk of liver cancer was −0.06, as the expected number of liver cancer cases exceeded the actual number of cases [43]. In the study described previously in women who had undergone multiple X-rays examinations for scoliosis and other spinal disorders, mortality from liver cancer was less than expected in the general population, SMR 0.17 (95% CI, 0.00–0.94); however the liver effective dose was not reported.
Colon Cancer Risk
Colonic Effective Dose
Since the incidence of colon cancer is the highest among cancers of the gastrointestinal tract, radiation exposure increasing the risk of malignancy must be scrutinized. SBFT fluoroscopy exposes the colon to 1.6 mSv of radiation [26]. The colonic radiation dose from CT scans varies depending on the type of imaging study. CT scans of the abdomen/pelvis expose the colon to 17–21.8 mSv [26, 27]. As with stomach and liver effective doses, addition of a standard chest CT to a CT abdomen/pelvis results in only minimal changes in radiation effective dose to the colon [27]. The LAR from colon cancer is higher than that of stomach and liver cancer for the same amount of radiation absorbed. The LAR of colon cancer was reported at 0.03% from a single CT examination of the abdomen/pelvis [27]. The effective radiation dose to the colon from a CT colonography was similar to that of a standard CT abdomen pelvis, 13.2 mSv per study [30]. However, serial CT colonography for colon cancer screening for national screening and surveillance may result in high cumulative effective doses of radiation, the results of which have not yet been investigated. Although not used as frequently today as in past years, barium enemas were once commonly used as screening tests for colon cancer and inflammatory bowel disease. Studies reporting organ specific effective doses of radiation from barium enemas were lacking, but estimates of total effective doses of radiation report 10.7 mSv per barium enema [51]. The effect of serial barium enema examinations and cancer risk has not been reported.
Epidemiologic Studies: Colon Cancer
Two studies reported the risk of colon cancer associated with environmental radiation exposure with differing results. The Life Span Study reported a REID 0.51% per 1,000 mSv of radiation for colon cancer, higher than the REID of 0.1% for gastric cancer [36]. However, the UNSCEAR study, reported the RR of colon cancer was 1.1 per 1,000 mSv in males and 1.9 in females, which was less than gastric cancer (2.1 and 2 in males, females, respectively) [25].
Three studies reported on the association of colon cancer and therapeutic radiation exposure. In a study of benign gynecological disease treated with radiation therapy, the REID was 0.31% per 1000 mSv of radiation [48]. Studies of peptic ulcer treated with radiation therapy found an excess relative risk of 0.05 per 1000 mSv (95% CI 0.05–0.22) or REID of 0.04% per 1000 mSv [41]. Radiation therapy used to treat skin hemangiomas found an excess relative risk of colon cancer of 0.37 per 1000 mSv [49].
Only one study, described earlier, reported the association of colon cancer and serial X- rays for spinal disorders [42•]. The mortality risk from colorectal cancer was not significantly more than the general population with colon cancer SMR 0.99 (95% CI, 0.65–1.45), and rectal cancer SMR 0.66 (95% CI, 0.13–1.93) [42•], although the colon effective dose was not reported.
Esophagus
Esophagus Effective Dose
Due to its location in the chest, the esophagus is more susceptible to radiation from thoracic CT imaging studies compared to other gastrointestinal organs. In adults, the radiation dose reported from a coronary CT angiography varied between 23.6 and 71.9 mSv [16, 32–34]. ECG-gated pulmonary vein CT angiography and CT angiography for pulmonary emboli exposed the esophagus to 48.6 mSv and 48 mSv, respectively [16]. The effective dose of radiation to the esophagus from Cone beam CT in 5 year-old children was 13 mSV and 16.2 mSv from full body CT [28, 29]. The LAR of esophageal cancer from 34 annual chest CT scans was 0.004% in males and 0.002% per 100,000 in females. The LAR increased to 0.0066% in males and 0.002.5% in females with 48 annual CT scans [35].
Epidemiological Studies: Esophageal Cancer
Two studies reported on the association of esophageal cancer and environmental radiation exposure. The Life Span Study, showed an excess RR of esophageal cancer incidence of 0.41 in males and 0.84 in females per 1000 mSv of radiation exposure [36]. Based on the UNSCEAR report, the excess RR esophageal cancer is 0.2 per 1000 mSv of exposure in males, 0.1 in females [25].
Two studies reported on the association of esophageal cancer and therapeutic radiation exposure. Radiation therapy for cervical cancer was associated with a relative risk for esophageal cancer of 1.26 per 1000 mSv (95% CI, 1.1–1.3) [50]. However, the association between radiation treatment for ankylosing spondylitis and esophageal cancer was not statistically significant, excess RR 0.17 per 1000 mSv (95% CI, 0.09–0.25) [40].
Risk of Other Gastrointestinal Malignancies
Pancreas
The effective dose of radiation to the pancreas is dependent on the type and location of the diagnostic study. SBFT fluoroscopy exposes the pancreas to 4.7 mSv of radiation compared to 31.4 mSv from a CT abdomen/pelvis [26]. There were no reported estimates of pancreatic cancer related to abdominopelvic CT scans, but the LAR of pancreatic cancer from 48 annually consecutive CT scans of the chest is 0.001% in males and 0.002% in females [35]. In children, coronary CTA results in 6.9 mSv and a cone beam CT of 56 mSv to the pancreas [29, 34]. Peptic ulcer disease treated with radiation therapy has been associated with pancreatic cancer with a relative risk of 1.87 (95% CI, 1.0–3.4) [41].
Small Bowel
Data are scarce regarding the association of small bowel cancer and diagnostic imaging. The small bowel receives 7.1 mSv of radiation from a SBFT fluoroscopy, which is higher than any other gastrointestinal organ from SBFT. In contrast, CT of the abdomen/pelvis results in a small bowel effective dose (16.3 mSv) similar to other gastrointestinal organs [26]. CT coronary angiography exposes the small bowel to 16 mSv of radiation in adult males and 9 mSv in females [32]. There are no estimates of the attributable risk of small bowel cancers to radiation exposure.
Chronic Disease and Repeated Radiation Exposure
Cumulative effect of the scans must be taken into consideration in patients with chronic disease such as inflammatory bowel disease (IBD). Crohn’s disease (CD) patients received an average of 1.8 SBFT examinations and 2.3 CT scans in their lifetime [26]. In a population based study of IBD patients, the radiation exposure in IBD patients was similar to the average annual background environmental radiation exposure. However, the upper quartile of patients with IBD received 2–11 times the dose of background environmental radiation exposure [53]. In a separate study, Desmond et al. [44•] retrospectively studied 354 Crohn’s disease patients to quantify the cumulative effective dose received by patients through diagnostic imaging studies during a 15 year period. High cumulative effective dose was defined as >75 mSv, which has been associated with increased cancer mortality by 7.3% [45]. The mean cumulative effective dose among these patients was 36.1 mSv with 15.5% of patients receiving more than 75 mSv. An average of 12.4 studies were performed per patient in the 15 years of follow-up. Risk factors associated with a high cumulative effective dose were: age <17 years old at time of diagnosis, hazard ratio 2.1 (95% CI, 1.1–4.1, P = 0.02), upper GI tract disease, odds ratio (OR) 2.4 (95% CI, 1.2–4.9, P = 0.02), penetrating or stricturing disease, OR 2.0 (95% CI, 1.0–3.9, P < 0.0001), oral steroid requirement, OR 3.8 (95% CI, 1.1–12.7, P < 0.01), IV steroid requirement, OR 3.7 (95% CI, 2.0–6.6, P < 0.0001), infliximab requirement, OR 2.3 (95% CI, 1.2–4.4, P = 0.01), or more than one surgery, OR 2.7 (95% CI, 1.4–5.4, P < 0.001). Crohn’s disease patients who required more than one surgery received the highest mean cumulative effective dose of all groups with mean cumulative effective dose 66.6 mSv (95% CI, 52.9–80.3 mSv). The majority of the cumulative effective dose was accounted for by CT imaging. Patients with Crohn’s disease require multiple and frequent imaging to establish diagnosis, monitor the extent of disease and response to therapy, and detect complications from Crohn’s disease. The possible risk of malignancy from radiation exposure must be considered especially in Crohn’s patients who already have an increased risk of tumors in the gastrointestinal tract, liver or biliary system [46], and small bowel lymphomas [47].
Conclusions
As the use of diagnostic imaging studies increases, the risk of malignancy must be considered and further studied. The majority of radiation exposure and risk associated with gastrointestinal malignancy comes from CT scans, especially of the abdomen/pelvis. Chest CT scans expose only a small amount of radiation and malignancy risk to the stomach and liver, although the risk may be greater in the colon and esophagus. The colon is more susceptible to cancer risk from radiation exposure compared to the other gastrointestinal organs. Children in particular have a higher cancer risk associated with radiation exposure compared to adults. The overall risk of cancer from a single diagnostic imaging study is low, but measurable. However, patients with chronic conditions such as inflammatory bowel disease are at risk of high cumulative radiation exposure and potentially higher cancer risks.
Many of the studies reviewed calculated cancer risk based on the organ-specific effective doses from diagnostic imaging studies using phantoms and computer models. There is paucity of epidemiologic studies with adequate follow up after diagnostic imaging to assess gastrointestinal malignancy risk. Radiation-induced solid malignancies have been shown to emerge one or two decades after the time of exposure, so future studies will require long-term monitoring for neoplasm in these patients [22•]. Directions of future studies should include long term follow up of patients exposed to radiation from diagnostic imaging and development of radiation reduction technologies such as ultrasound, magnetic resonance, capsule endoscopy, and other novel techniques.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162(4):377–89.
Li CI, Nishi N, McDougall JA, Semmens EO, Sugiyama H, Soda M, et al. Relationship between radiation exposure and risk of second primary cancers among atomic bomb survivors. Cancer Res. 2010;70(18):7187–98.
Folley JH, Borges W, Yamawaki T. Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med. 1952;13(3):311–21.
Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009;29(2A):A43–59.
Cardis E, Vrijheid M, Blettner M, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ. 2005;331(7508):77.
Muirhead CR, Goodill AA, Haylock RG, et al. Occupational radiation exposure and mortality: second analysis of the National Registry for Radiation Workers. J Radiol Prot. 1999;19(1):3–26.
Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36 Suppl 2:121–5.
Ron E, Modan B, Boice Jr JD, Alfandary E, Stovall M, Chetrit A, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033–9.
Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–32.
Abdel-Wahab M, Reis IM, Wu J, Duncan R. Second primary cancer risk of radiation therapy after radical prostatectomy for prostate cancer: an analysis of SEER data. Urology. 2009;74(4):866–71.
Van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75(5):1420–9.
Ron E, Boice Jr JD, Hamburger S, Stovall M. Mortality following radiation treatment for infertility of hormonal origin or amenorrhoea. Int J Epidemiol. 1994;23(6):1165–73.
Amis Jr ES, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4(5):272–84.
Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, et al. Cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251(1):175–84.
Berrington de Gonzales A, Darby A. Risk of cancer from diagnostic x-rays: estimates for the UK and 14 other countries. Lancet. 2004;363(9406):345–51.
Hurwitz LM, Reiman RE, Yoshizumi TT, Goodman PC, Toncheva G, Nguyen G, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007;245(3):742–50.
Miller AB, Howe GR, Sherman GJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Engl J Med. 1989;321(19):1285–9.
Doody MM, Lonstein JE, Stovall M, Hacker DG, Luckyanov N, Land CE. Breast cancer mortality after diagnostic radiography: findings from the U.S. Scoliosis Cohort Study. Spine (Phila Pa 1976). 2000;25(16):2052–63.
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–86.
Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84.
Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176(2):289–96.
• National Research Council BEIR Committee. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press, 2006. The BEIR VII report complied many of the existing epidemiological data on the risk of malignancy from ionizing radiation and developed a model to calculate the risk of cancer based on radiation dosages.
Health Physics Society. Radiation risk in perspective: Position statement of the Health Physics Society. Adopted January 1996, revised August 2004. http://hps.org/documents/radiationrisk.pdf. Accessed June 14, 2007.
Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100(24):13761–6.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation). Sources and Effects of Ionizing Radiation. UNSCEAR Report to the General Assembly, Volume II: Effects. New York: United Nations, 2000b.
Jaffe TA, Gaca AM, Delaney S, Yoshizumi TT, Toncheva G, Nguyen G, et al. Radiation doses from small-bowel follow-through and abdominopelvic MDCT in Crohn’s disease. AJR Am J Roentgenol. 2007;189(5):1015–22.
Tarin TV, Sonn G, Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J Urol. 2009;181(2):627–32. discussion 632–3.
Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232(3):735–8.
Kim S, Yoshizumi TT, Frush DP, Toncheva G, Yin FF. Radiation dose from cone beam CT in a pediatric phantom: risk estimation of cancer incidence. AJR Am J Roentgenol. 2010;194(1):186–90.
Brenner D, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129(1):328–37.
Hunold P, Vogt FM, Schmermund A, Debatin JF, Kerkhoff G, Budde T, et al. Radiation exposure during cardiac CT: effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003;226:145–52.
Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298(3):317–23.
Huang B, Li J, Law MW, Zhang J, Shen Y, Khong PL. Radiation dose and cancer risk in retrospectively and prospectively ECG-gated coronary angiography using 64-slice multidetector CT. Br J Radiol. 2010;83(986):152–8.
Huang B, Law MW, Mak HK, Kwok SP, Khong PL. Pediatric 64-MDCT coronary angiography with ECG-modulated tube current: radiation dose and cancer risk. AJR Am J Roentgenol. 2009;193(2):539–44.
de González AB, Kim KP, Samet JM. Radiation-induced cancer risk from annual computed tomography for patients with cystic fibrosis. Am J Respir Crit Care Med. 2007;176(10):970–3.
Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, et al. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat Res. 1994;137(2 Suppl):S17–67.
Boice Jr JD, Engholm G, Kleinerman RA, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116(1):3–55.
Zhuntova GV. Role of occupational radiation in oncologic morbidity among “Mayak” production association workers. Med Tr Prom Ekol. 2009;8:20–5.
Darby SC, Reeves G, Key T, Doll R, Stovall M. Mortality in a cohort of women given X-ray therapy for metropathia haemorrhagica. Int J Cancer. 1994;56(6):793–801.
Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis. Int J Cancer. 1994;59(3):327–38.
Griem ML, Kleinerman RA, Boice Jr JD, Stovall M, Shefner D, Lubin JH. Cancer following radiotherapy for peptic ulcer. J Natl Cancer Inst. 1994;86(11):842–9.
• Ronckers CM, Land CE, Miller JS, Stovall M, Lonstein JE, Doody MM. Cancer mortality among women frequently exposed to radiographic examinations for spinal disorders. Radiat Res. 2010;174(1):83–90. This is one of the few epidemiological studies documenting the mortality risk with serial diagnostic X-ray examinations. Cancer mortality overall was higher than expected, although no increased risk was detected for gastrointestinal cancers.
Boice Jr JD, Day NE, Andersen A, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst. 1985;74(5):955–75.
• Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57(11):1524–9. This study identified predictors of high radiation exposure in patients with Crohn’s disease, including: upper GI tract disease, penetrating disease, requirement for intravenous steroid or infliximab, and history of multiple surgeries.
Cardis E, Vrijheid M, Blettner M, et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:3962416.
Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62.
Jess T, Loftus Jr EV, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted county, Minnesota. Gastroenterology. 2006;130:1039–46.
Inskip PD, Monson RR, Wagoner JK, Stovall M, Davis FG, Kleinerman RA, et al. Cancer mortality following radium treatment for uterine bleeding. Radiat Res. 1990;123(3):331–44.
Lundell M, Holm LE. Risk of solid tumors after irradiation in infancy. Acta Oncol. 1995;34(6):727–34.
Boice Jr JD, Day NE, Andersen A, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst. 1985;74(5):955–75.
Hirofuji Y, Aoyama T, Koyama S, Kawaura C, Fujii K. Evaluation of patient dose for barium enemas and CT colonography in Japan. Br J Radiol. 2009;82(975):219–27.
Geleijns J, Broerse JJ, Chandie Shaw MP, Schultz FW, Teeuwisse W, Van Unnik JG, et al. A comparison of patient dose for examinations of the upper gastrointestinal tract at 11 conventional and digital X-ray units in The Netherlands. Br J Radiol. 1998;71(847):745–53.
Peloquin JM, Pardi DS, Sandborn WJ, Fletcher JG, McCollough CH, Schueler BA, et al. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103(8):2015–22.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, M.L., Hou, J.K. Cancer Risk Related to Gastrointestinal Diagnostic Radiation Exposure. Curr Gastroenterol Rep 13, 449–457 (2011). https://doi.org/10.1007/s11894-011-0214-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-011-0214-8