Abstract
Weight loss in older adults has been a controversial topic for more than a decade. An obesity paradox has been previously described and the issue of weight status on health outcomes remains a highly debated topic. However, there is little doubt that physical activity (PA) has a myriad of benefits in older adults, especially in obese individuals who are inactive and have a poor cardiometabolic profile. In this review, we offer a critical view to clarify misunderstandings regarding the obesity paradox, particularly as it relates to obese older adults. We also review the evidence on PA and lifestyle interventions for the improvement of cardiorespiratory fitness, which can prevent disease and provide benefits to obese older adults, independent of weight changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health [1]. Current estimates classify more than 1.46 billion individuals globally as overweight, of whom, half a billion are obese. In the USA, obesity is associated with more than 112,000 deaths per year and an estimated annual medical cost close to 150 billion US dollars [2]. In 2008, the Obesity Society produced a white paper that reviewed the evidence and implications of classifying obesity as a disease [3•]. More recently, obesity has been recognized as such by the American Association of Clinical Endocrinologist [4] and the American Medical Association [5].
Obesity affects all age groups with current concerns focusing primarily on children, adolescents, and younger adults. Notwithstanding, the prevalence of obesity and obesity-related conditions continues to increase in older adults [2]. In older adults, all four geriatric domains are affected by obesity: medical (type 2 diabetes [T2D], cardiovascular disease [CVD], hypertension, stroke, breast cancer, colon cancer, gallbladder disease, osteoarthritis, and others), functional (impaired mobility and disability [6]), social (stigmatization, isolation, and others [7]), and psychological (depression [7] and dementia [8]).
There is evidence to support that moderate, intentional weight loss (IWL) has a beneficial effect on comorbidities, functional status, and quality of life, provided that regular physical activity (PA) is implemented [9]. Nevertheless, there is controversy regarding the impact of body weight and IWL in this age group, hence the need for further research to understand the most appropriate strategies and prescriptions for older adults [10]. This review aims to provide a critical discussion of the obesity paradox, PA, and IWL for the prevention of disease in obese older adults.
Obesity and Diseases in Older Adults
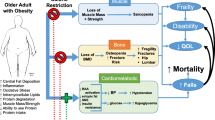
Obesity has been attributed mostly to an imbalance between caloric intake and caloric expenditure [11], and has been linked to a self-perpetuating vicious cycle of poor health and function, poorer quality of life, and accelerated morbidity and mortality (Fig. 1). Obesity is associated with a greater number of deaths worldwide than underweight, affecting the economically disadvantaged at an equal or greater rate than those in developed nations [1]. As we expand our knowledge on the biochemical, hormonal, and pathophysiological pathways involved in the development and persistence of obesity, we become aware of additional abnormalities that makes the process of weight gain so difficult and challenging to reverse [12]. However, the discussion regarding obesity as a disease and its specific pathophysiological pathways are beyond the scope of this review.
The results from inertia and intervention for obesity in older adults: Negative cycle (a): Without intervention, poor fitness, and fatness are followed by negative outcomes, disease, disability, and ultimately death. Positive cycle (b): Improved cardiorespiratory fitness even with only modest weight loss, prevents disease and may prolong survival. While major events (i.e., hospitalization, disease, etc.) may hinder the white arrow and interrupt the cycle, the benefits often continue as long as regular exercise and healthy diet are maintained
Obesity and Type 2 Diabetes (T2D)
Obesity has been described by DeFronzo et al. as the single most important factor responsible for the epidemic increase in T2D over the last 20 years. Weight gain and physical inactivity lead to insulin resistance, placing an increased insulin secretory demand on beta cells [13]. Older adults are at high risk for T2D due to the combined effects of increasing insulin resistance and impaired pancreatic islet function with aging, as reported by Kirkman et al. [14]. They summarize the pathophysiology and epidemiology of T2D in older adults, including the relevant demographic implications in the development of the disease and its complications. It is noteworthy that the prevalence of T2D seen in this age group results from the combination of newly diagnosed with long-standing cases, with varying clinical characteristics and implications for individualized interventions.
Obesity, Cardiovascular Disease (CVD), and Mortality
Obesity can produce hemodynamic alterations that predispose older adults to changes in cardiac morphology and ventricular function [15]. Obesity is an independent risk factor for the development and progression of hypertension and CVD [16]. Excess body fat, particularly visceral obesity, is associated with metabolic abnormalities, which can induce a variety of structural adaptations and alterations in cardiovascular (CV) structure and function [17]. The adverse effects of obesity on the development, severity, and progression of coronary heart disease (CHD) are also well known [18–21].
The Obesity Paradox and Cardiorespiratory Fitness (CRF)
The obesity paradox is understood as a decreased mortality and complications reportedly seen among older patients with obesity, whereas leaner counterparts had higher incidence of major in-hospital complications, including cardiac death. Grabowski and Ellis were among the first to report that higher BMI levels did not predict mortality in older adults [22]. Later, Gruberg et al. observed that, in patients who underwent percutaneous CV intervention, those with “very lean” and normal weight were at higher risk for in-hospital complications, cardiac death, and 1-year mortality [23]. Then, Hainer and Alhoon-Hainerová [24] support the case for an obesity paradox in CHD, heart failure, T2D, hypertension, peripheral artery disease, stroke, thromboembolism, and kidney and pulmonary diseases. In contrast, other investigations have not revealed evidence supporting the obesity paradox. Carnethon et al. [25] conducted a pooled analysis of five longitudinal cohort studies for a total of 2,625 participants with incident T2D. They found that overweight and obese individuals had higher mortality rates compared to those with normal weight, even after adjusting for demographic and CV risk factors [26]. More recently, Tobias et al. [27] studied more than 10,000 patients from the Nurses’ Health Study and the Health Professionals Follow-up Study, and found no evidence of an obesity paradox.
These contrasting findings regarding the obesity paradox may be, as described by Flegal et al. [28••], a consequence from studies using varying BMI and reference categories, making it difficult to compare and synthesize results together. Flegal et al. used sensitivity analyses to address these issues and concluded that, relative to normal weight, obesity was associated with significantly higher all-cause mortality, and overweight was associated with significantly lower all-cause mortality.
Adding further complexity to this issue is the impact of cardiorespiratory fitness (CRF), as several investigators have not observed the obesity paradox in populations with higher levels of CRF [29]. Lavie et al. [30, 31] outline the relative impact of fitness versus fatness on health outcomes and considers CRF to be the key contributing factor.
McAuley and Beavers [32] offer a review of five observational cohort studies that included adjusted multivariate analyses for combined fitness-adiposity categories and addressed the modifying influence of CRF on the obesity paradox in patients with CV diseases. They analyzed results according to CRF levels and found that among people with higher levels of CRF, risk of all-cause mortality is lowest for those with overweight rather than obesity, an association that was not seen among those with low CRF levels. Similarly, a recent meta-analysis conducted by Barry et al. [33] assessed the relationships between BMI and CRF with all-cause mortality. Individuals with poor fitness were at twice the risk of death regardless of BMI, while fit and overweight and obese individuals had similar mortality risk as their normal-weight counterparts. Remarkably, CRF is overlooked as a potential modifier of the inverse association between obesity and mortality in patients with known or suspected CVD, independent of BMI, waist circumference (WC), or percent body fat.
Alternatively, Banack and Kaufman [34] present evidence suggesting that the obesity paradox may be a measure of statistical bias. Preston and Stokes [35] address the potential confounding bias of smoking. Finally, Zheng et al. [36] address the heterogeneity in BMI trajectories among older populations, and that longitudinal increase in BMI past age 51 was more predictive of mortality risk than static BMI status.
In conclusion, the true nature of the obesity paradox may involve multiple factors: methodological problems in older studies focusing on BMI as the sole index for obesity, the inability of BMI to discriminate fat tissue from healthy muscle mass, and the independent association between fat distribution (i.e., central obesity) and CVD. Most importantly, we are concerned that misunderstanding the obesity paradox among medical providers, in the midst of a growing obesity epidemic, may lead to clinical inertia toward obesity.
Metabolically Healthy Obese: “Fitness and Fatness”
The concept of a metabolically healthy obese individual is controversial. The most common criteria used is by Wildman et al. [37], where metabolically healthy obese individuals are defined as those who have no more than one of the following: blood pressure >130/85 mmHg or use of antihypertensive drugs, triglycerides >1.7 mmol/L or use of lipid-lowering drugs, fasting glucose >5.6 mmol/L or use of medications for diabetes, HOMA-IR above the 90th percentile among all participants, and CRP above the 90th percentile among all. Some investigators, such as Hamer et al. [38], have found that metabolically healthy obese individuals are not at increased risk of CVD and all-cause CVD. However, others such as Hinnouho et al. [39] describe that both metabolically healthy and unhealthy obese phenotypes carry an elevated risk of mortality. Kramer et al. [40•] conducted a systematic review on studies that evaluated all-cause mortality or CV events according to BMI and metabolic status. They found that compared with metabolically healthy, normal-weight individuals, obese persons are at increased risk for adverse long-term outcomes, even in the absence of metabolic abnormalities, suggesting that there is not a healthy level of increased weight.
Sarcopenia, Dynapenia, and Functional Status
Sarcopenic obesity is characterized by muscle loss among older, obese persons or obese persons with severe disease burden or injury, and the decreased muscle mass or strength increases the risk for adverse health outcomes [41]. The comorbid disease burdens associated with obesity contributes to functional decline, but recent findings further implicate the obesity-associated inflammatory milieu, sarcopenia, and impairment of muscle function and strength in the pathogenesis of disability outcomes [42]. Kalyani et al. describe the pathophysiologic events that lead to the development of sarcopenic obesity [43•]. They conclude that while there is no cure for age- or disease-related muscle loss, regular exercise does help maintain muscle mass and strength, even though it does not completely halt the biological processes that ultimately lead to sarcopenia.
A study by Chung et al. [44] compared cardiovascular risk factors among 2,943 older adults, according to the presence of sarcopenia and obesity (sarcopenic obese, sarcopenic nonobese, nonsarcopenic obese, and nonsarcopenic nonobese groups) and found that those with sarcopenic obesity had a higher likelihood of having insulin resistance, metabolic syndrome, and CVD risk factors. Of note, they defined sarcopenia as an appendicular skeletal muscle mass divided by weight (%) of <1 SD below the sex-specific mean for young adults, which may differ from other definitions previously used.
The definition of sarcopenia remains under discussion between international societies, with some arguing for the inclusion of loss of muscle strength (dynapenia) while others focus only on decreased muscle mass (sarcopenia alone). Dynapenia is only partially explained by sarcopenia, and these two conditions should be considered independent of one another [45]. Regardless of the definition, a decrease in muscle strength and increase in body fatness both greatly influence functional status in old age. Thus, dynapenic obesity is a concept that needs to be studied further to establish potential modifiable determinants for preventing functional decline in older persons [46]. Although there is not a general scientific agreement on how to define sarcopenia, there is a growing body of evidence that both sarcopenic obesity and the quality of muscle mass impact cardiometabolic health, development of disease, and mortality.
Weight Loss and Lifestyle Interventions for Disease Prevention in Obese Older Adults
The increasing prevalence of obesity around the world, including that observed in older adults, requires immediate attention. With a growing number of morbidities being linked to obesity, there is an urgent need for action. In their 2013 fact sheet, the American Heart Association suggests that Americans are obese because they “overeat and are sedentary” [11]. Intensive lifestyle interventions (ILI) have been shown to be effective in producing improvements in CRF and CV risk factors, with [47] or without IWL [48]. Successful strategies for sustained IWL typically include the following: (1) consuming fewer calories, (2) increasing PA levels, and (3) modifying unhealthy behaviors [49].
Diabetes Prevention
For individuals with prediabetes, lifestyle modification is the cornerstone of T2D prevention, with evidence of a 40–70 % reduction in relative risk [50] as found in major studies such as the Finnish Diabetes Prevention Study [51] and the Diabetes Prevention Program (DPP) [52]. In the DPP, the group receiving ILI resulted in a loss of body weight that was disproportionately higher in older participants, an effect which may be partly explained by the reversibility in age-related insulin resistance [14]. Among those aged 65 or older, 60 % met the 7 % weight loss goal at end of core curriculum (2 years), whereas only 43 % of those aged under 45 years did. Similarly, at the final visit (mean 3.2 years), 63 % of those aged 65 or older met the weight loss goal, compared with only 27 % of those under 45 years of age. In multivariate analyses, being older was the strongest predictor of achieving prescribed weight loss and ILI activity goals [53]. This influence of age in the DPP was addressed by Crandall et al. [54], who concluded that lifestyle modification was exceptionally effective in preventing diabetes in older individuals; a finding largely explained by greater weight loss and physical activity. The DPP also showed that the effects of an ILI can have a lasting impact on total energy intake up to 9 years later. Additionally, levels of PA during the long-term follow-up remained significantly associated with the sustainment of positive dietary habits. Initial success in achieving IWL, reducing fat and energy intake, and attaining PA goals appear to predict long-term ability to maintain these changes [55]. Delahanty et al. found in a group of middle age participants that the following baseline patient characteristics were independent and durable predictors of successful IWL: age when first overweight, fewer previous self-implemented IWL attempts, greater exercise self-efficacy, fewer fat-related dietary behaviors, greater dietary restraint, and more time spent in sedentary behaviors. Additionally, improvements in low-fat diet self-efficacy and dietary restraint skills were independent predictors of long-term weight outcomes [56]. A population-based cohort study in Southern Germany investigated the impact of baseline BMI and BMI change, as well as baseline WC and changes in WC, on reversion from prediabetes to normal glucose tolerance (NGT) and maintenance of long-term NGT [57]. It was concluded that for older adults, IWL strongly increased the chances of reversal from prediabetes to NGT irrespective of initial BMI, and that long-term persistence of NGT was influenced by both initial BMI and changes in BMI.
Cardiometabolic Outcomes
Low levels of CRF have been associated with an increase in CVD events in men with T2D [58]. Recently, the Look AHEAD study compared the effect of an ILI to support and education in overweight and obese older adults with T2D [59]. Although the results did not indicate that IWL and increased PA were associated with reduction in mortality, CVD, and its complications, individuals in the ILI had lower levels of prescription drug use, improvements in CVD risk factors, decreased levels of sleep apnea and depression, and a higher quality of life [60].
It has been stated that IWL should not be the primary therapeutic goal for obese patients with heart failure. Mild to moderate IWL with the aim of improving quality of life and alleviating other medical conditions may be more reasonable in severely obese patients [61]. Supporting this opinion, Atkins et al. [62] reported the results of a prospective cohort study that enrolled more than 4,000 older adults, age 60 to 79 who were followed for 11.4 years. The investigators found that sarcopenia and central adiposity were significantly associated with greater CV and all-cause mortality and that men with sarcopenic obesity had the highest risk of all-cause, but not CV mortality.
Sarcopenic Obesity and Functional Decline in Older Adults
Obesity is a known risk factor for mobility limitations in older adults, a population for which exercise interventions can be feasibly and effectively implemented. Identification of older adults at risk for mobility limitations can be accomplished through routine screening in the ambulatory setting. Addressing functional deficits and environmental barriers with exercise and mobility devices can lead to improved function, safety, and quality of life [63]. Lifestyle interventions incorporating diet and regular exercise appear to be the optimal treatment for sarcopenic obesity [64]. However, there is no cure for age- or disease-related muscle loss. Exercise helps maintain muscle mass and strength, but probably does not affect the biological process that ultimately leads to sarcopenia. Consequently, there is a need for innovative interventions for preserving muscle mass in older adults. The Lifestyle Interventions and Independence for Elders (LIFE) study recruited 1,635 sedentary people aged 70 to 89 at risk for disability. The intervention consisted of a structured, moderate-intensity PA program, which was compared to a health education program. The researchers found a reduction in major mobility disability by more than 2.6 years with the intervention, suggesting a high level of benefit to mobility from structured programs while targeting vulnerable older adults [65].
Other Outcomes
Health care providers seeking to translate lifestyle interventions into clinical practice settings can use evidence-based practices to guide their decisions on who, how, and when to treat patients. Patients with T2D and obesity-related conditions benefit from lifestyle modification programs targeting osteoarthritis and knee pain, physical mobility, fatty liver, urinary incontinence, sexual dysfunction, sleep apnea, subclinical inflammation, retinopathy, and nephropathy [66]. In the DPP, overweight and obese adults at high risk for T2D showed small improvement in most physical and vitality components of the health-related quality of life (HRQoL), through IWL and increased PA [67].
Considerations for Effective Implementation
A systematic review by Ali et al. [68] addressed studies that translated the DPP lifestyle intervention in various community settings. Despite limitations in the quality of the included studies, they noted clinically significant levels of IWL in adults at high risk of T2D. Among participants of an adapted DPP, Harwell et al. [69] found that the highest percentage of achievers (weight loss goal of 7 %) were older adults, especially those who frequently monitored their dietary intake and markedly increased their PA levels. Ali et al. also suggested that lay health educators (LHE) can be trained as lifestyle professionals to help individuals achieve lifestyle intervention goals at a reduced cost without sacrificing the effectiveness of the program. West et al. [70] conducted a study to determine whether an adaptation of the DPP lifestyle program delivered by lay health educators and conducted in senior centers is effective in promoting weight loss among older adults. They successfully trained 20 LHE, who assisted in the recruitment of participants in senior centers and delivered a well-received lifestyle intervention. The LHE model provides an effective mean to deliver health promotion programs and may be particularly relevant for rural areas with limited access to health providers. The use of LHE could also be part of a patient-centric approach in urban locations, such as assisted living facilities, where it may be feasible to recruit and engage large number of older adults for lifestyle interventions [71].
Implementation Experience at Miami VA Healthcare System
Observations from our team suggest that the implementation of evidence-based, exercise programs are feasible in clinical settings. Enhance Fitness (EF) [72] is an evidence-based, supervised group exercise program. We were awarded a grant by the Health Aging Regional Collaborative [73] to implement EF in the Miami VA Healthcare System. We recruited overweight and obese older Veterans from the MOVE! Program [74]. MOVE! is a comprehensive weight management program, but lacked an exercise component. In our older, mostly male Veterans subjects, we found significant improvements in weight, WC, as well as functional and metabolic parameters similar to those seen in larger trials with greater resources and conducted in more highly controlled settings. Interestingly, a proportion of our participants did not lose weight but decreased their WC; although we did not assess body composition, we hypothesized that with exercise, they improved their muscle mass and decreased their fat mass, maintaining the weight and still showing improvements in medical, functional, and psychosocial outcomes. We also found improvement in HRQoL and depression scores, and decreased self-reported utilization of medications for T2D and hypertension. Participant testimonials describe improvements in their symptoms of post-traumatic stress disorder and improved socialization. The socialization benefits and the “spirit of the corps,” as commented by our participants, offer additional interesting avenues for future research in overweight/obese older adults.
Limitations for Weight Loss and Exercise in Older Adults
Mobility impairment is common in older adults. The most common risk factors are older age, strength or balance impairment, and chronic diseases such as T2D and osteoarthritis [63]. Among other studies, the Look AHEAD [75] found that older adults with diabetes are capable of losing weight and experience substantial mobility benefits and reduction in knee pain from doing so. The ILI group had a relative risk reduction of 48 % in loss of mobility as compared with the support group. Both weight loss and improved fitness were significant mediators of this effect. Although it has been shown that older adults can be very successful and motivated to adhere to IWL/PA programs, the programs must be tailored for many of the age-related barrier they face, such as comorbidities, loss of muscle mass, and frailty [76, 77]. The conditions or diseases associated with obesity are also barriers for potential interventions for its management and further disease prevention.
In addition, it has been considered that implementing lifestyle interventions for all individuals at high risk for diabetes is not economically feasible, stressing the importance of implementing preventive measures prior to disease progression. Moreover, 40–50 % of patients with prediabetes still progress to T2D despite IWL [13], illustrating the difficulty in maintaining lifestyle interventions long-term. Kirkman et al. [14] described that diabetes prevention through lifestyle intervention be pursued in relatively healthy older adults. A review by Blüher et al. addressed which individuals should be targeted for T2D prevention [78]. They mentioned the need to identify and address key issues in high-risk populations, but older adults were not mentioned. Similarly, the DPP did not enroll significant numbers of individuals aged >70 years or those with functional or cognitive impairment [14], exemplifying the lack of attention given to these subpopulations and a bias toward a perceived lack of benefit.
Health Economic Consequences and the Obese Older Adult
Basu et al. [79] used data from the National Health and Nutrition Examination Survey (1999–2010) to construct and validate a micro-simulation model of obesity in the US population for the years 2010 to 2020. Among age groups, obesity prevalence increased most substantially in the oldest group (60+ years), from 41 % of the population to 44 %. With obesity levels in the USA associated with more than 112,000 deaths per year and an estimated annual medical cost close to 150 billion dollars [2], it is obvious that there is a major need to support preventive lifestyle interventions that can lower health care costs while offering a broad spectrum of additional health benefits that targeted pharmacotherapy may not be able to provide. Given the continued rise in obesity and its associations with multi-morbidities, impaired mobility and disability, depression, dementia, and others, we can only expect costs to increase unless population-level action is taken immediately.
Avenell et al. [80] conducted a systematic review of the long-term effects and economic consequences of treatments for obesity and the implications for health improvement. Assuming that high-risk individuals who changed their behavior maintained this change over a 2-year period of time, the cost of combined diet and exercise interventions are comparable to that of other treatments, such as pharmacotherapy. In the DPP, an economic analysis conducted by Herman et al. [81] concluded that, over 10 years, the ILI and metformin groups showed long-term cost savings compared to treatment as usual. Krukowski et al. [82] reported that a successful, behavioral lifestyle weight loss intervention delivered by LHEs could serve as a promising vehicle for the translation of evidence-based obesity treatment programs in underserved areas. A cost effectiveness analysis of their study [83] found that the total estimated cost to implement the ILI was $2,731 per senior center or $165 per participant. With an average weight loss of 3.7 kg after 4 months, the implementation of the program cost $45 per kilogram lost. This effective, low cost intervention certainly offers promise for the dissemination of cost-effective lifestyle modification interventions in community settings. Overall, these results suggest that ILIs represent good return on investment and highlights the fact that lifestyle changes are most cost-effective when the lifestyle modifications are sustained long-term.
Study Case
Mr. MNM is a 72-year-old male with obesity, defined by a body mass index (BMI) of 34.4 kg/m2. He is 69-in. tall and weighs 233 lb. He has prediabetes, moderate bilateral knee osteoarthritis, mild depression, and presents to the clinic asking how much weight he should lose.
How much IWL is appropriate? Note that if he lost 20 lb, he would still be considered obese.
Follow Up
Mr. MNM enrolls in a supervised exercise program for older adults. Not only does he improve his functional parameters, but also he makes new friends and reports feeling better about himself. Although he was not clinically depressed at the beginning of the exercise program, he states that he feels much better now, with more energy and purpose in life. He has been recommending that his friends engage in exercise as well. The “cherry of the pie,” as he describes, is that he lost 10 % of his body weight (23 lb) over the last year and had to buy new pants. Although his weight has decreased to 210 lb, his BMI (31.0 kg/m2) remains in the obese category. Whether he will benefit from “remaining obese” or “losing more weight” remains a matter of discussion. His physicians note that Mr. MNM, now age 73, has greatly improved his cardiometabolic profile, as well as his fitness level, physical functionality, mental health, and socialization.
Discussion and Questions
Mr. MNM lost weight but continues to be obese, albeit metabolically healthier.
-
1.
Would the patient benefit from further IWL?
-
2.
Are there any concerns from further IWL?
-
3.
Is there a role for pharmacologic interventions and bariatric surgery in this patient?
Agenda for the Future
Lifestyle interventions focusing on the relative contributions of IWL and exercise for specific subpopulations of obese older adults remain a critical research and clinical management and implementation topic. As mentioned by Kirkman et al. [14], the DPP did not enroll significant numbers age >70 years or those with functional or cognitive impairment. A recent systematic review of randomized controlled trials targeting IWL in obese older adults showed that only one of the ten included studies investigated long-term weight maintenance [77], reflecting the limited evidence available on maintenance of IWL and associated health implications in this age group. Furthermore, more research is needed to define in which circumstances dietary restrictions can be reasonably justified to induce IWL in specific subpopulations of older adults. For example, in the older (>80 years), frail individuals, the best course of action may be to abstain from recommending dietary restriction and IWL [9], placing the focus on tailored PA programs. Notably, it would be not feasible to treat all older adults with obesity and risk factors for diabetes, and future translational research and clinical programs may help to better channel available resources and efforts to target those who are the highest risk for diabetes and other obesity-related morbidities.
There is substantial variation within diverse older populations for type and level of PA [84]. Statistics on older Americans illustrate that engagement in leisure-time PA (hobbies, sports, or exercise) is greater among younger (aged 60 to 69) than older (aged 70 years and older) adults, and higher among older men than women. Thus, while some retirees may remain or become more active, travel, and enjoy the support of family and friends, the trend among older adults is toward more spent in and around their home engaged in sedentary behavior. According to a report from the Centers for Disease Control and Prevention [85], 22.5 % of those aged 65 and older were never active and only 15.3 % were at a high level of overall PA. Wang et al. [86] comment that promoting active lifestyles in the Medicare population, especially overweight and obese groups, could potentially improve their well-being and save a substantial amount of health care expenditures. However, despite the large body of evidence supporting the efficacy of a variety of PA and behavior change programs to assist older persons to become active, the majority are yet to become optimally active. These sedentary obese older adults will be at greater risk for physical and mental health decline, worsening when their social support network decreases. This situation clearly represents an important public health challenge, i.e., how to increase the engagement of older persons in routine PA [87]. Future research should consider the influence of the neighborhood environment on health outcomes and include measures of the social and built environment [88].
Conclusions
Lifestyle management with increased PA and healthier diet is feasible and effective for disease prevention in obese older adults. A modest amount of IWL resulting from exercise and controlled caloric intake is likely to reflect a positive change, and delay or prevent the development of T2D. The prevention of CVD and mortality seem to be mostly related to improvements in CRP rather than weight alone, and the prevention of functional decline requires exercise interventions to preserve muscle mass and function. Unfortunately, these interventions are still not widely implemented nor regularly encouraged both by providers and the healthcare systems, albeit some private insurers started to cover memberships to gyms. As pointed out by Chrysant et al. [89], the obesity paradox may be a misnomer that conveys a confusing message to the general public (e.g., "obesity can be protective") and may negate national and international efforts working to improve obesity prevention and safe IWL. As described by Swift et al. [90], all clinicians must continue educating patients on lifestyle management with reasonable expectations, and emphasize that numerous health benefits occur from PA programs, even in the absence of IWL. Obesity and the vicious cycle that results when left untreated will have negative consequences on all four geriatric domains, but many remain unsure about implementing IWL and exercise programs in older adult populations. Further research is needed to provide clearer guidelines for the medical community in the treatment of obese older adults, and conflicting findings on obesity and sarcopenic obesity need to be synthesized and translated in clinically applicable interventions, specially for vulnerable individuals. Finally, more pragmatic studies are needed on how best to implement evidence-based programs focused on IWL and increasing PA, and how to enhance access to such lifestyle management programs in “real world” settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO 10 facts on obesity. World Health Organization 2013. http://www.who.int/features/factfiles/obesity/en/. Accessed 25 Apr 2014.
CDC Overweight and obesity facts. Centers for Disease Control and Prevention. 2014. http://www.cdc.gov/obesity/data/adult.html. Accessed 21 April 2014.
TOS Obesity Writing Group, Allison DB, Downey M, Atkinson RL, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the council of The Obesity Society. Obesity. 2008;16:1161–77. This article, ahead of its time, allows the reader not only to better understand the definition and implications of defining obesity and disease, but also to incorporate language and concepts that will be extremely useful when discussing with patients about the definition of obesity as a disease.
AACE Position Statement, Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American association of clinical endocrinologists’ position statement on obesity and obesity medicine. Endocr Pract. 2012;18:642–8.
AMA News Room. AMA Adopts new policies on second day of voting at annual meeting. Obesity as a disease. American Medical Association 2013. http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page. Accessed 21 Apr 2014
WHO Global strategy on diet, physical activity and health. Obesity and Overweight Facts. World Health Organization. 2003. http://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf. Accessed April 21, 2014.
TOS Obesity, bias and stigmatization. The Obesity Society 2014. http://www.obesity.org/resources-for/obesity-bias-and-stigmatization.htm. Accessed May 5, 2014.
Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity. 2013;21:E51–5.
Darmon P. Intentional weight loss in older adults: useful or wasting disease generating strategy. Curr Opin Clin Nutr Metab Care. 2013;16:284–9.
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS obesity guideline. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and The Obesity Society. Circulation 2013; 00:000–000.
AHA FACTS with a very heavy heart. Obesity and cardiovascular disease (CVD). American Heart Association 2013. http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_305059.pdf. Accessed 21 Apr 2014.
Reed JL, Chaput J-P, Tremblay A, Doucet E. The maintenance of energy balance is compromised after weight loss. Can J Diabetes. 2013;37:121–7.
DeFronzo RA, Abdul-Ghani MA. Preservation of β-cell function: the key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354–66.
Kirkman S, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–56.
Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400.
Chrostowska M, Szyndler A, Hoffmann M, Narkiewicz K. Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab. 2013;27:147–56.
Bastien M, Poirier P, Lemieux I, Després J-P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–81.
Kannel WB, Wilson PW, Nam BH, D’Agostino RB. Risk stratification of obesity as a coronary risk factor. Am J Cardiol. 2002;90:687–701.
McClain J, Hsu F, Brown E, et al. Pericardial adipose tissue and coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Obesity. 2013;21:1056–63.
Labounty TM, Gomez MJ, Achenbach S, et al. Eur Heart J Cardiovasc Imaging. 2013;14:456–63.
Wassel CL, Laughlin GA, Araneta MR, et al. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity. 2013;21:1704–12.
Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the longitudinal study of aging. JA GS. 2001;49:968–79.
Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–84.
Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36 Suppl 2:S276–81.
Carnethon MR, De Chavez PJD, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–90.
Florez H, Castillo-Florez S. Beyond the obesity paradox in diabetes. Fitness, fatness and mortality. Editorial. JAMA. 2012;308:619–20.
Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44.
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories. A systematic review and meta-analysis. JAMA. 2013;309:71–82. This meta-analysis confirms the expected association between obesity and increased risk for all-cause mortality. The data was stronger in older adults, which is the focus of this review.
De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease—the obesity paradox. Prog Cardiovasc Dis. 2014;56:401–8.
Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN. Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox. J Am Coll Cardiol. 2014;63:1345–54.
Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. J Am Coll Cardiol HF. 2013;1:93–102.
McAuley PA, Beavers KM. Contribution of cardiorespiratory fitness to the obesity paradox. Prog Cardiovasc Dis. 2014;56:434–40.
Barry VW, Baruth M, Beets MW, Durstine JL, Liu J. Blair SN. Fitness vs fatness on all-cause mortality: a meta-analysis. Prog Cardiovasc Dis. 2014;56:382–90.
Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102.
Preston SH, Stokes A. Obesity paradox. Conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiol. 2014;25:454–61.
Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol. 2013;178:1591–9.
Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering. Prevalence and correlates of 2 phenotypes among the US populations (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–24.
Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab. 2012;97:2482–8.
Hinnouho G-M, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality. Does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–300.
Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69. This systematic review offers a great insight to the suggested concept of healthy obesity, with results consistent with the prevalent need to address and manage obesity.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky S, Ferrucci L. Sarcopenic obesity—definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700.
Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51.
Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014; Mar 6 [Epub ahead of print]. This review offers a great insight to the pathophysiology of sarcopenic obesity and the metabolic and endocrinologic issues of sarcopenia, age-related or disease-related.
Chung J-Y, Kang H-T, Lee D-K, Lee H-R, Lee Y-J. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013;56:270–8.
Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67A:28–40.
Visser M. Symposium 2: exercise and protein nutrition. Obesity, sarcopenia and their functional consequences in old age. Proc Nutr Soc. 2011;70:114–8.
Espeland MA, Rejeski WJ, West DS, et al. Intensive weight loss intervention in older individuals: results from the action for health in diabetes type 2 diabetes mellitus trial. J Am Geriatr Soc. 2013;61:912–22.
Gaesser GA, Angadi SS, Sawyer BJ. Exercise and diet, independent of weight loss, improve cardiometabolic risk profile in overweight and obese individuals. Phys Sports Med. 2011;39:87–97.
AHA. Treating obesity as a disease. American Heart Association. 2014. https://www.heart.org/HEARTORG/GettingHealthy/WeightManagement/Obesity/Treating-Obesity-as-a-Disease_UCM_459557_Article.jsp. Accessed April 21, 2014.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–90.
Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50.
Knowler WC, Barrett-Connor E, Flowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle interventions or metformin. N Engl J Med. 2002;346:393–403.
Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34.
Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol: Med Sci. 2006;61A:1075–81.
Davis N, Ma Y, Delahanty LM, et al. Predictors of sustained reduction in energy and fat intake in the Diabetes Prevention Program Outcomes Study intensive lifestyle intervention. J Acad Nutr Diet. 2013;113:1455–64.
Delahanty LM, Peyrot M, Shrader PJ, et al. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP). Diabetes Care. 2013;36:34–40.
Kowall B, Rathmann W, Heier M, et al. Impact of weight and weight change on normalization of prediabetes and on persistence of normal glucose tolerance in an older population: the KORA S4/F4 study. Int J Obes. 2012;36:826–33.
Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165:2114–20.
Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD study. Int J Obes. 2009;33:305–16.
Korytkowski MT. Lessons from the look action for health in diabetes study. Indian J Endocrinol Metab. 2013;17 suppl 3:S650–3.
Clark AL, Fonarow GC, Horwich TB. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2014;56:409–14.
Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–60.
Brown CJ, Flood KL. Mobility limitation in the older patient. A clinical review. JAMA. 2013;310:1168–77.
Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20:412–9.
Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults. The LIFE study randomized clinical trial. JAMA. 2014;311:2387–96.
Delahanty LM. The Look AHEAD study: implications for clinical practice go beyond the headlines. J Acad Nutr Diet. 2014;114:537–42.
Florez H, Pan Q, Ackerman RT, et al. Impact of lifestyle intervention and metformin on health-related quality of life: the Diabetes Prevention Program randomized trial. J Gen Intern Med. 2012;27:1594–601.
Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012;31:67–75.
Harwell TS, Vanderwood KK, Hall TO, Butcher MK, Helgerson SD. The Montana Cardiovascular Disease and Diabetes Prevention Workgroup. Factors associated with achieving a weight loss goal among participants in an adapted Diabetes Prevention Program. Prim Care Diabetes. 2011;5:125–9.
West DS, Bursac Z, Cornell CE, et al. Lay health educators translate a weight-loss intervention in senior centers: a randomized controlled trial. Am J Prev Med. 2011;41:385–91.
Johnson JA, McIlroy WE, Papaioannou A, Thabane L, Giangregorio L. Feasibility study of walking for exercise in individuals living in assisted living settings. J Geriatr Phys Ther. 2013;36:175–81.
National Council on Aging. Center for aging. Enhance fitness. http://www.ncoa.org/improve-health/center-for-healthy-aging/enhance-fi.html. Accessed April 25, 2014.
Healthy Aging Regional Collaborative. http://www.healthyagingsf.org/. Accessed April 25, 2014.
MOVE! Weight Management Program. http://www.move.va.gov/. Accessed April 25, 2014.
Rejeski WJ, Ip EH, Bertoni AG, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. NEJM. 2012;366:1209–17.
Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–20.
Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol. 2013;48:1054–61.
Blüher S, Markert J, Hergert S, et al. Who should we target for diabetes prevention and diabetes risk reduction? Curr Diabetes Rep. 2012;12:147–56.
Basu S, Seligman H, Winkleby M. A metabolic-epidemiological microsimulation model to estimate the changes in energy intake and physical activity necessary to meet the Health People 2020 obesity objective. Am J Public Health. 2014;104:1209–16.
Avenell A, Broom J, Brown TJ, et al. A systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:1–182.
Herman WH, Edelstein SL, Ratner R, et al. Effectiveness and cost-effectiveness of diabetes prevention among adherent participants. Am J Manag Care. 2013;19:194–202.
Krukowski RA, Lensing S, Love S, et al. Training of lay health educators to implement an evidence-based behavioral weight loss intervention in rural senior centers. The Gerontologist. 2012;53:162–71.
Krukowski RA, Pope RA, Love S, et al. Examination of costs for a lay health educator-delivered translation of the Diabetes Prevention Program in senior centers. Prev Med. 2013;57:400–2.
Prohaska T, Belansky E, Belza B, et al. Physical activity, public health, and aging: critical issues and research priorities. J Gerontol: Soc Sci. 2006;61:S267–73.
Barnes PM, Schoenborn CA. Physical activity among adults: United States, 2000: advance data from vital and health statistics. CDC Natl Cent Health Stat. 2003;333:15.
Wang F, McDonald T, Reffitt B, Edington DW. BMI, physical activity, and health care utilization/costs among medicare retirees. Obes Res. 2005;13:1450–7.
Biedenweg K, Meischke H, Bohl A, et al. Understanding older adults’ motivators and barriers to participating in organized programs supporting exercise behaviors. J Prim Prev. 2014;35:1–11.
Michael YL, Nagel CL, Gold R, Hillier TA. Does change in the neighborhood environment prevent obesity in older women? Soc Sci Med. 2014;102:129–37.
Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7:85–94.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–7.
Acknowledgments
We would like to thank Dr. Maria van Zuilen for her review of this manuscript. The work contributed by Dr. Mark Stoutenberg was supported by Grant Number 1KL2TR000461 from the Miami Clinical and Translational Science Institute, the National Center for Advancing Translational Sciences, and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the NIH.
We would like to thank the Health Foundation of South Florida and its Healthy Aging Regional Collaborative, which supported the grant that allowed us to implement the Enhance Fitness exercise program at Miami VAHS.
We would also like to thank the Department of Human and Health Services, which supported our grant NIH grant 1R18AE000049-01, “Peer-lead and Telehealth Activated Care (PACT) in Diabetes Prevention and Management.”
We would like to thank the support of the Geriatric Research, Education and Clinical Center at Miami VAHS.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Willy Marcos Valencia, Mark Stoutenberg, and Hermes Florez declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lifestyle Management to Reduce Diabetes/Cardiovascular Risk
Rights and permissions
About this article
Cite this article
Valencia, W.M., Stoutenberg, M. & Florez, H. Weight Loss and Physical Activity for Disease Prevention in Obese Older Adults: an Important Role for Lifestyle Management. Curr Diab Rep 14, 539 (2014). https://doi.org/10.1007/s11892-014-0539-4
Published:
DOI: https://doi.org/10.1007/s11892-014-0539-4