Abstract
The obesity epidemic has reached old age in most industrialized countries, but trials elucidating the benefits and risks of weight reduction in older adults above 70 years of age with obesity remain scarce. While some findings demonstrate a reduced risk of mortality and other negative health outcomes in older individuals with overweight and mild obesity (i.e. body mass index (BMI) < 35 kg/m2), other recent research indicates that voluntary weight loss can positively affect diverse health outcomes in older individuals with overweight and obesity (BMI > 27 kg/m2), especially when combined with exercise. However, in this age group weight reduction is usually associated with a reduction of muscle mass and bone mineral density. Since uncertainty persists as to which level overweight or obesity might be tolerable (or even beneficial) for older persons, current recommendations are to consider weight reducing diets only for older persons that are obese (BMI ≥ 30 kg/m2) and have weight-related health problems. Precise treatment modalities (e.g. appropriate level of caloric restriction and indicated dietary composition, such as specific dietary patterns or optimal protein content) as well as the most effective and safest way of adding exercise are still under research. Moreover, the long-term effects of weight-reducing interventions in older individuals remain to be clarified, and dietary concepts that work for older adults who are unable or unwilling to exercise are required. In conclusion, further research is needed to elucidate which interventions are effective in reducing obesity-related health risks in older adults without causing relevant harm in this vulnerable population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Obesity epidemic is reaching old age
According to the World Health Organization (WHO), obesity has been defined as an excessive accumulation of body fat mass (FM) [1]. Obesity is most often operationalized using a body mass index (BMI) ≥ 30 kg/m2 as crude population measure. However, as a consequence of age-related changes in body composition and height, the diagnostic accuracy of BMI is fairly poor in older people [2]. Available alternative diagnostic measures can be based on either the proportion of FM (≥ 35% in women (w) and ≥ 25% in men (m)) or a waist circumference (WC) of ≥ 88 cm (w) and ≥ 102 cm (m). In recent years, obesity prevalence in older adults has increased [3,4,5], with data of the NHANES study estimating the proportion of persons with a BMI ≥ 30 kg/m2 among adults aged ≥ 60 years in the U.S. to be 41% [4].
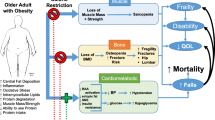
As in younger persons, obesity in older age may compromise health and well-being, but also everyday functioning [2, 6, 7]. It has been associated with a higher risk of adverse health outcomes, such as accelerated functional decline and loss of independence [8], pain [9], frailty [10], falls [11], cardiovascular disease [12] and higher mortality rates [13]. However, the traditional understanding that relates overweight (BMI ≥ 25 - < 30 kg/m2) and obesity to deleterious outcomes has been challenged for this age group, as recent findings demonstrated no increased or even a reduced risk of mortality and other negative health outcomes in older individuals with overweight and mild obesity (i.e. BMI < 35 kg/m2) compared to those with “normal” body weight (BW), in particular in case of illness [13,14,15,16]. This phenomenon is one aspect of the so-called ‘obesity paradox’ [17, 18]. Potential mechanism explaining these observations include the presence of better metabolic reserves that facilitate physiological resilience, especially when high levels of lean body mass (LM) are present together with the excess FM, or that disease-associated weight loss (WL) may be present in a relevant proportion of slim older persons [2, 7, 18]. While the protective effect of mild obesity is still debated, it has become widely accepted for overweight in the older population. However, additional research in this field is surely needed [6, 14, 19, 20]. In general, obesity also affects negatively the psychological well-being [21] and (health-related) quality of life (QoL) of older adults [22, 23]. For example, similar to younger age groups, about 40% of older persons with obesity feel dissatisfied with their weight status [24].
2 Potential benefits and harms of weight loss in older persons
It has been shown that intended weight reduction through caloric restriction (CR) can contribute to improvements in diverse health outcomes in older adults with obesity [6, 25, 26]. A recent systematic review included six trials published between 2005 and 2015 that were conducted in older persons with obesity (mean age ≥ 65 years, BMI ≥ 30.0 kg/m2 or WC ≥ 88 cm (w) / ≥ 102 cm (m)) [27]. The authors concluded that interventions to reduce obesity were able to improve physical functioning and QoL of participants, especially when CR was combined with exercise, which was tested in five of the trials [27]. With regard to metabolic outcomes, Normandin and co-workers [28] in a secondary analysis of a randomized controlled trial (RCT) [29] recently could document a reduction of the prevalence of the metabolic syndrome and an improvement in related biomarkers in older persons with overweight and obesity that participated in an intervention combining moderate CR (−600 kcal/d) with resistance training (RT) versus RT alone (see Tables 1 and 2 for details on trials described). Similarly, Nicklas and colleagues [30] revealed improvements in blood glucose, insulin and HOMA-IR as well as in peak respiratory capacity in older individuals with obesity receiving moderate CR (−600 kcal/d) or very moderate CR (−250 kcal/d) in combination with aerobic training (AT) versus AT alone. However, when interpreting these results it needs to be considered that - with few exceptions [31] - these studies mainly focussed on relatively young seniors. These are not representative of the older age group as a whole, which is threatened by functional deterioration at a much higher degree. People at more advanced age (> 75 years) as well as those with frailty and multimorbidity are underrepresented in available RCTs [32].
As a downside, WL - whether intentional or not - always goes at the expense of LM and bone mineral density (BMD), and may therefore exacerbate the age-related loss of muscle and bone mass [2, 6, 33,34,35,36,37]. Correspondingly, concerns have been raised whether weight-reducing diets in older persons with overweight and obesity may increase the risk of adverse health outcomes such as sarcopenia, fractures and functional decline [6, 37,38,39,40]. Furthermore, evidence suggests that the frequently seen weight regain after weight loss presumably facilitates unfavourable changes in body composition (i.e. increases in FM, reduced LM) [34, 41, 42]. In this context, the relatively new entity of sarcopenic obesity may be of special relevance [40, 43,44,45]. Sarcopenic obesity has been characterized as the confluence of two epidemics – that of obesity with that of an aging society leading to increasing rates of the age-related loss of muscle mass and function (sarcopenia) [45]. Both factors seem to share common aetiological pathways, such as the acceleration of body composition changes (fat mass gain and muscle loss). These are promoted by hormonal changes (mainly declining oestrogen and testosterone levels) in the female menopause as well as in the male aging process, and by an increasing insulin resistance associated with inflammatory processes [45]. Moreover, the muscle mass loss commonly observed with aging leads to a decreased resting metabolic rate, which in turn furthers the gain of body fat [45]. On the other hand, the infiltration of fat into the muscle contributes to the development of sarcopenia [45]. Therefore, both clinical entities synergistically increase the risk of negative health outcomes and earlier onset of disability [40, 42,43,44,45]. Older adults with sarcopenic obesity are thus a group at particular risk for functional disability [43,44,45,46].
In addition to the health risks of sarcopenic obesity, the nutritional status of older people with obesity may be compromised despite the excess of FM, for example due to inadequate intake of micronutrients or protein [7, 47]. WL-interventions can (further) negatively affect nutritional status, e.g. when strict dietary regimens are prescribed or when dietary counselling is insufficient and CR leads to unbalanced diets [48]. It is therefore crucial to consider these risks when evaluating the benefits of intentional WL through CR for older individuals with obesity. In frail and multimorbid older persons, and especially in those with low muscle mass, a very prudent approach to WL has thus to be chosen. Effects should not be extrapolated from results of studies conducted in younger persons. Thus, specific concepts are needed to combat obesity in older adults without endangering their muscular and bone health as well as their nutritional status. Health care professionals should be aware that “generic” WL-programs need to be adapted for this specific target group.
3 New ESPEN guideline on “Nutrition in Geriatrics”
The recently updated guideline on “Nutrition in Geriatrics” of the European Society for Clinical Nutrition and Metabolism (ESPEN) now provides specific recommendations for obesity treatment in older adults [32]. These recommendations are based on evidence from RCTs and systematic reviews published until 2016 that focussed on older adults beyond the age of 65 years. Taking into consideration the potential “obesity paradox” and the risks of WL for older persons [6], the first recommendation in the ESPEN guideline is that in older individuals with overweight (BMI 25 - < 30 kg/m2), weight-reducing diets shall be avoided (recommendation 54) [32]. However, it is recommended to consider WL-interventions in older persons with obesity (BMI ≥ 30 kg/m2) who suffer from weight-related health problems (recommendation 55) [32]. It is stated that especially in frail and multimorbid persons which are more vulnerable to stressors, the careful individual weighing of benefits and risks of WL is required, taking into account individual functional resources, metabolic risk, comorbidities and the patients’ perspective and priorities. In addition, the effects of such a WL program on a patient’s QoL have to be discussed [32]. If a decision is taken against weight reduction, the guidelines` advice is to focus on weight stability and the avoidance of further weight gain [32].
With regard to the exact modalities for the dietary treatment of obesity in older adults, the ESPEN guideline recommends that energy restriction shall be only moderate, thereby aiming at slow WL in order to preserve muscle mass (recommendation 56) [32]. Moderate CR in the guideline has been defined in line with previous recommendations [25, 26, 49] as around 500 kcal/d below the estimated energy needs, while maintaining a minimum intake of 1000–1200 kcal/d. This strategy aims at a WL of 0.25–1 kg/week and a reduction of around 5–10% of initial BW after a period of at least 6 months. At the same time, a balanced diet as generally recommended for older adults should be assured, including a protein intake of at least 1 g/kg BW/d and an appropriate intake of micronutrients [32, 50, 51]. This will usually need regular nutritional counselling to help the older person maintaining sufficient nutrient intake (especially of protein) from foods during the energy restriction period in order to avoid malnutrition. In some cases it may be necessary to aid the achievement of these nutritional goals with nutritional supplements (e.g. providing extra protein or micronutrients), but this aspect has not been discussed in the guideline [32].
In accordance with previous recommendations [25, 26, 49], diets with very low caloric intake (VLCDs, i.e. <1000 kcal/day) in the guideline are strongly discouraged for the older population due to the risk of developing malnutrition, promoting muscle loss and accelerated functional decline [32]. Since trials actually testing such VLCDs in persons 65 years and older were not identified at the time of the guideline production, these recommendations are, however, “good practice points” (i.e. expert opinion), based on considerations on the potential dangers of extreme WL for older adults’ muscle mass and physical function, as described above.
4 Very low calorie diets (VLCDs, dietary intake <1000 kcal/d) for older persons
Since the finalization of the ESPEN guideline [32], new studies on the dietary treatment of obesity in older adults have been published. In a recent systematic review, Haywood and Sumithran [52] included RCTs published until January 2018 examining the effects of any type of WL-intervention tested in persons aged 60 years and beyond with obesity (BMI ≥ 30 kg/m2). They identified two new small trials [53,54,55] actually testing VLCDs with an energy intake around 800–1000 kcal/d through meal replacements (Optifast program) over a relatively short period of time (3–6 months) in older adults without functional impairment or frailty at baseline (age range 65–85 years). One of them was partly industry-funded by Nestlé [53]. The VLCD concepts applied in these studies were tested in combination with exercise and compared to moderate CR (see Tables 1 and 2 for details). In both trials WL was greatest in the VLCD group, with comparable differences in the reduction of FM and WC. Haywood and colleagues in their trial also analysed changes in biomarkers of cardiovascular risk and found that improvements were more pronounced in the VLCD group [55]. However, the loss of LM and BMD was also greater in the VLCD group [54]. In the second trial of Ard et al., no significant differences in the amount of LM loss between both CR concepts were reported, but the authors failed to report the absolute magnitude of the LM loss [53]. In one of the studies, physical function improved in all three groups without significant differences [54], while in the other the intervention had no effects on the tested functional parameters [53]. Haywood et al. reported adverse events to be infrequent and non-severe (mainly constipation and dizziness), but they tended to be more frequent in the VLCD group [54]. Ard et al. did not describe adverse events in detail, but only as having been “musculoskeletal in nature and generally exacerbations of previous chronic conditions” [53].
To date, no definite conclusions can be drawn from these two small studies, but it appears that in older adults moderate CR induces less WL but also less LM and BMD loss than VLCDs (when combined with exercise). Moreover, the higher WL achieved with VLCDs did not translate into any improvements of physical function compared to moderate CR. And even if there was apparently no relevant risk of serious adverse events with VLCDs, minor adverse events like digestive complaints, hypoglycaemia and hypotension were described, which may be already a concern in the vulnerable geriatric population even when not dieting. Haywood et al. from their results concluded that there is a need for close observation and for the adaption of medication for hypertension and diabetes in older persons on a VLCD, as in their trial changes in blood pressure and glucose were rapid [54]. Taken together, we think that the results of these new trials do not justify any changes to the recommendation of the current ESPEN guideline indicating that only moderate CR is appropriate in WL-interventions for older persons [32].
5 Specific modifications of dietary composition to support weight loss in older adults
Different dietary compositions to support WL are being tested, with research currently having focussed mainly on protein intake [56]. Buckinx et al. [57] investigated the potential influence that different levels of protein intake may have on the effects of a 12-week high-intensity interval exercise training (HIIT) in 30 sedentary older (≥ 60 years) adults with obesity (see Tables 1 and 2 for study details). Participants were a posteriori divided into two groups (matched for age, sex and BMI) depending on the ingested amount of protein (< 20 g in one meal or ≥ 20 g in each meal). They concluded that a higher intake of protein at each meal was not associated with an improvement of muscle performance after HIIT in older persons [57]. A RCT by Beavers and colleagues [58,59,60] (partly industry-funded by Medifast) compared a 6-month WL-intervention using a high-protein meal replacement (Medifast 4&2&1 Plan, 1.2–1.5 g protein/kg BW/d and 1100–1300 kcal/d) to a weight-stable control arm in a sample of 96 fit persons with obesity aged 65 to 79 years. They could show that while total BW and FM declined in the high-protein diet group, LM and gait speed remained stable [58]. The high protein diet also improved the lumbar spine trabecular bone score, while total hip, femoral neck and lumbar spine BMD did not decline significantly during the WL-intervention compared to the control group [59]. Regarding cardiometabolic markers, fasting glucose, insulin, HOMA-IR, and triglycerides were significantly reduced in the high protein group, while no changes in the weight stable control group were observed except for a reduction in HDL cholesterol [60].
A narrative review by Al-Nimr [61] summarized available evidence of RCTs on modifications of dietary protein content in older persons (≥ 60 years) with obesity published between 2013 and 2018. Included trials (n = 13) were often small and had a high proportion of females. They concluded that an intake of 1.0–1.2 g or up to 1.4 g protein per kg BW/d may be needed for better functional outcomes and LM retention in older adults losing weight. An even distribution of protein intake over the meals (20-30 g per meal) may also reduce LM loss during WL-interventions [61]. On the other hand, in their systematic review, Haywood and Sumithran [52] concluded from the RCTs they had identified for this topic (no overlap with the 13 trials discussed by Al-Nimr [61]), that – provided that energy intakes were equal - increasing the protein content above 0.9 g/kg BW/d [62,63,64] or changing the protein source [65] did not result in any differences in WL or LM loss.
Moreover, there is no consensus about whether to use the actual BW or rather a “healthy” BW (referring to BMI 18.5–24 kg/m2 for adults < 71 years and 22–27 kg/m2 for persons ≥ 71 years) as reference to calculate protein requirements [66]. In individuals with obesity using the actual BW could lead to an overestimation of protein needs, as the BW consists to a large part of excess FM presumably not needing additional protein. Consequently, as body composition phenotypes differ between individuals, the question has also been raised whether LM rather than BW should be considered when estimating protein needs [67]. At the moment, it seems sensible to recommend at least 1.0 g protein/kg of actual BW/d for older adults during a weight loss program. It remains still unclear whether they would benefit from protein intake levels above this value.
Apart from that, for older persons it might be challenging to achieve this minimum requirement with the usual diet, and even more so with an energy-restricted one. Several of the studies mentioned above have therefore employed complete/partial meal replacements or protein/amino acid supplements to assure adequate nutrient intake of their participants. Then again, other concepts tested have been able to achieve a high protein intake from protein-rich foods such as meat and dairy products alone [52, 56,57,58,59,60,61]. In clinical practice, regular nutritional counselling is surely needed to help dieting older persons to achieve sufficient intake of nutrients from foods and thus to avoid malnutrition, and possibly this should be complemented by nutritional supplements. More well-powered and well-conducted RCTs are needed to better elucidate these aspects, as to date no certain recommendations for the optimal protein content of WL diets for older persons with obesity can be derived from available evidence.
Regarding the assessment of specific dietary patterns such as “low carb” or ketogenic diets for weight loss in older adults, at the time of this review (November 2019) we found no recent studies specifically including older and frailer adults. However, there are some preliminary results after one year of intervention available from the 6-year PREDIMED-Plus trial investigating the effects of an energy-restricted Mediterranean diet combined with physical activity (PA) promotion and behavioural support (−600 kcal/day, WL aim: 7–10% of BW after 6 months) [68]. This approach was compared to an unrestricted-calorie Mediterranean dietary pattern in 626 young-old (55–75 years) persons with metabolic syndrome and overweight or obesity (BMI 27 - < 40 kg/m2) [68]. The results suggest that adding CR and PA to a Mediterranean dietary pattern has additional beneficial effects on BW and several metabolic risk factors [68]. In the control group, following a Meditarranean diet alone has not shown significant effects on BW [68], thereby corroborating the recently published corrected results of the preceding much larger (n = 7447) PREDIMED trial (testing the unrestricted-calorie Mediterranean dietary pattern without CR in the same age group), where only marginal effects on BW and WC could be demonstrated [69]. Certainly, the final results of the PREDIMED-Plus trial, which are expected in five years, will help to better understand the potential of this dietary pattern (with or without CR) for promoting health in obese young-old adults. Nevertheless, as for most other dietary obesity treatments, this concept still remains to be specifically tested in an older, frail and multimorbid population.
6 Combining dietary treatment with exercise and physical activity
A sedentary or inactive lifestyle throughout lifetime facilitates the development of obesity [70] and exercise is of major relevance in all persons with obesity [71, 72]. Exercise - especially at higher intensities - contributes to a negative energy balance and offers a multitude of health benefits, among others a reduced risk for developing obesity-related comorbidity by enhancing cardiovascular and musculoskeletal fitness [71, 73]. As has been described above, WL through CR in older people comes at the cost of reduced LM and BMD. It may therefore accelerate the “natural” deterioration of these parameters during the aging process. Based on the assessment of 12 RCTs that all compared the effects of a dietary WL-intervention alone to the same diet combined with exercise in older persons, the recent ESPEN guideline recommends that in older persons with obesity, dietary WL-interventions should be combined with structured, supervised physical exercise whenever possible [32]. This should be supported by an increase in everyday PA [32]. In 10 of these 12 trials, a weight-reducing diet alone resulted in WL, but also in the loss of LM. While only modest WL was found with exercise alone, as is often seen [27, 56, 71], the WL effect was more pronounced with CR alone and it was highest when both intervention types were combined [32]. The latter also resulted in increased preservation of LM. Moreover, in the combined diet and exercise groups greater improvements in strength and physical performance measures were observed than in the diet only groups [32]. In these trials, exercise training was conducted between 2 and 5 times per week and a single session lasted between 45 and 90 min. Most studies administered a multicomponent training including a combination of AT, RT and flexibility components [32]. New meta-analytic evidence supports the notion that RT in older persons may fully prevent muscle loss induced by CR, while changes in BW and FM were not different between groups in the analysed samples [74]. A recent RCT confirmed the above mentioned findings and emphasizes the enhanced efficacy of combined RT and AT [75]. Besides enhanced muscle mass, other well-known adaptations to exercise (especially to RT and power training, but also to challenging balance exercises and AT) in older people - including those with sarcopenic obesity - include improvements in strength, physical performance and functioning, reduced fall and fracture risk and a decelerated decline of BMD [76,77,78,79,80,81]. Of note, exercise participation can achieve these health benefits even in the absence of WL [71] and it appears that the protection against serious health consequences of obesity is related to the fitness level achieved rather than to the amount of PA [82]. In addition, there is strong evidence for the efficacy of exercise and PA in maintaining BW after WL [71, 83]. Taking this into consideration, it comes as no surprise that the majority of interventions included in the recent systematic review of Haywood and Sumithran [52] used combinations of nutritional interventions (mostly CR) with exercise as well as PA (29 of 36 trials). They found only five trials that compared different “pure” nutritional interventions without any additional exercise/PA component [62, 65, 84,85,86]. This highlights the apparent general acknowledgement in the field that in order to achieve optimal results, i.e. relevant WL without substantial LM loss, for older adults CR should be combined with appropriate exercise/PA whenever possible.
Several other recently published RCT results deserve to be mentioned, as they extend existing knowledge [33, 87,88,89,90,91,92,93]. Their details are displayed in Tables 1 and 2. Some of these have confirmed earlier findings that WL promotes decreases in BMD, but also indicate that adding RT components may attenuate these negative side effects, predominantly at the hip [33, 35, 87, 88, 92]. This may indicate that the mechanical loading through gravitation and muscle contractions in interventions administered to people with obesity is higher in RT than in rather joint-protecting low-impact AT programs and thus results in a better preservation of bone structures [94]. Beavers and colleagues emphasized the need of RT, as they found that the decrease of bone mass may persist after the WL has stopped or even when weight is regained [33]. Based on a secondary analysis of data from a RCT by Villareal et al. [31], Colleluori et al. suggest that exercise promotes an increase in BMD by inhibiting bone resorption and bone turnover, while CR increases these processes [87]. Finally, in a small-scale RCT in older individuals with obesity comparing moderate CR alone to moderate CR in combination with a weight vest worn several hours over the day, no group differences were found for several measures of body composition and WL, while BMD loss at the hip was nearly three times higher in the diet only group [88, 89]. However, none of these studies has provided data on fracture risk. Surprisingly, wearing weight vests for on average 6.7 h/day did not lead to significant group differences in LM or functional performance, with the exception of lower extremity power [89]. It has been demonstrated that for physical functioning in older persons, power is more important than strength [95, 96]. Independent of the vest, both groups improved in gait speed, confirming earlier findings that WL alone may lead to improved physical functioning [56, 89]. Another article demonstrated differential task-specific effects of different exercise regimens on different functional self-efficacy domains in older individuals with obesity, supporting the idea of combining both AT and RT with CR [90]. Rejeski and colleagues found that a WL-intervention with only very moderate CR (−330 kcal/d) in combination with either RT or AT was able to reduce CRP levels in older people with obesity, and that reductions were highest in combination with RT [91]. Finally, Hugenschmidt and colleagues could demonstrate that adding CR to a 20-week AT did not negatively affect cognitive measures of several domains (e.g. executive functions) [93].
7 Long-term effects of weight-loss interventions
As described above, dietary interventions to treat obesity in older people have repeatedly been shown to effectively reduce BW and improve health and functional outcomes, especially when combining CR and physical exercise [27]. However, outcomes were usually measured only at the end of the intervention phase and the knowledge about long-term weight maintenance and persistent health effects of such interventions in older adults is still scarce [97,98,99,100]. Three recently published studies add further knowledge to this topic [33, 34, 36, 101]. In an 18-month RCT with 12-month follow-up (FU) a very moderate CR (−330 kcal/day, WL aim: 7–10% of BW) was compared to the same diet combined with AT or with RT, respectively, in older people with obesity (n = 187, aged 67 ± 5 years, BMI 35 ± 4 kg/m2) [33]. At month 30, a lower BW compared to baseline was maintained in all three groups despite some weight regain [33]. Total hip BMD decreased by about 2% during the intervention phase and was stable at the lower level afterwards [33]. An additional analysis of the same study with a subset of 77 participants revealed a tendency towards a partial restoration of hip BMD loss after 12 months FU in those who had regained weight compared to weight maintainers [36].
In a pilot study, a weighted, random sample of older people with obesity (n = 42, mean age ≥ 65 y, mean BMI ≥ 30 kg/m2) who had previously participated in one of five RCTs that lasted between 5 and 18 months [29, 30, 102,103,104] - all with a randomization to CR (between −250 and − 1000 kcal/d) in combination with exercise (AT, RT or both) or to exercise alone - was reinvestigated after 3.5 ± 1 years to evaluate long-term changes on body composition and physical function after WL-interventions [34]. In both groups, BW at FU was still slightly lower than at baseline. Even though the CR plus exercise group had greater WL, FM and LM loss during the intervention period than the exercise alone group, results of both groups did not substantially differ at FU after 3.5 ± 1 years [34]. Unfavourable changes in body composition occurred in both groups at FU, with a more pronounced regain in FM in the combined CR and exercise group and a greater loss of LM in the exercise alone group [34]. Time to complete a 400 m walk at FU had returned to baseline levels in both groups, while in the exercise alone group improved SPPB results were still visible [34].
Moreover, in 2018 data on long-term effects on physical function of the Look AHEAD lifestyle intervention were published in a stratified analysis for participants aged 60 years and older with type 2 diabetes (BMI ≈ 36 ± 6 kg/m2) [101]. The study lasted approximately 11.4 years from baseline to the stop of the intervention, and FU was about 1.6 years. Participants received either an intensive lifestyle intervention with a special focus on WL (−10% of BW) and PA, or diabetes support plus education [101]. Long-term benefits were found for certain parameters of physical performance like gait speed and SPPB in the intervention group compared to the educational group, while for others (handgrip strength, impairment in lower extremity function) no differences were found [101].
The above mentioned results partially refer to secondary analyses of prior RCTs that are based on reduced samples and pooling of intervention groups. As for other aspects, the evidence on long-term effects of dietary weight-loss interventions in older adults with obesity is still limited and inconclusive. Further sufficiently powered RCTs with appropriate FU considering the development of body composition, health and functional measures as well as QoL after the end of WL-interventions are needed.
8 Conclusions and need for further research
Summarizing the available evidence, there is still a considerable lack of studies elucidating the risks and benefits of weight reduction in older adults with obesity above 70 years of age, especially if they have functional limitations and health impairments. With few exceptions, most participants of published trials were less than 75 years old and they were not physically impaired. Therefore, their results may not be generalizable to the older population, and especially not to more frail individuals. To date, there remains uncertainty which level of excess fat mass is tolerable (or even beneficial) for older persons, and at what point the harmful consequences of obesity require a loss of weight. The advantages of the combination of moderate caloric restriction and exercise have become more and more evident, although larger and sufficiently powered trials are still needed. However, the long-term effects of such interventions as well as the most effective modalities regarding dietary composition (for example specific dietary patterns and optimal protein content), the level of caloric restriction, the types and amount of exercise and the best methods to implement such interventions in order to facilitate adherence in this specific population are currently less than clear. Moreover, practical strategies for dietary weight loss sparing lean and bone mass as well as bone structure are highly needed for older persons with obesity-related health problems that are not able (and/or sometimes also not willing) to exercise. Evidence on the possible benefits and harms of weight reduction in the most vulnerable groups of older individuals, e.g. persons with obesity in nursing homes or hospitals, is also lacking. Further research is urgently required here, as in these settings obesity increasingly contributes to dependence, it complicates care procedures and it impacts quality of life of those affected and of their care-givers [47, 105]. More work needs to be done in order to elucidate which interventions are effective for combating obesity-related health risks in older adults without causing more harm than good.
References
World Health Organization (WHO). Health topics: Obesity. https://www.who.int/topics/obesity/en/.
Batsis JA, Zagaria AB. Addressing obesity in aging patients. Med Clin North Am. 2018;102(1):65–85. https://doi.org/10.1016/j.mcna.2017.08.007.
Fakhouri THI, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States. NCHS. Data Brief. 2007-2010;2012(106):1–8.
Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–5. https://doi.org/10.1001/jama.2018.3060.
Peralta M, Ramos M, Lipert A, Martins J, Marques A. Prevalence and trends of overweight and obesity in older adults from 10 European countries from 2005 to 2013. Scand J Public Health. 2018;46(5):522–9. https://doi.org/10.1177/1403494818764810.
DiMilia PR, Mittman AC, Batsis JA. Benefit-to-risk balance of weight loss interventions in older adults with obesity. Curr Diab Rep. 2019;19(11):114. https://doi.org/10.1007/s11892-019-1249-8.
Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr. 2017;36(4):917–38. https://doi.org/10.1016/j.clnu.2016.11.007.
Bell JA, Sabia S, Singh-Manoux A, Hamer M, Kivimaki M. Healthy obesity and risk of accelerated functional decline and disability. Int J Obes. 2017;41(6):866–72. https://doi.org/10.1038/ijo.2017.51.
Taylor R Jr, Pergolizzi JV, Raffa RB, Nalamachu S, Balestrieri PJ. Pain and obesity in the older adult. Curr Pharm Des. 2014;20(38):6037–41. https://doi.org/10.2174/1381612820666140316131431.
Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One. 2017;12(6):e0178383. https://doi.org/10.1371/journal.pone.0178383.
Mitchell RJ, Lord SR, Harvey LA, Close JC. Obesity and falls in older people: mediating effects of disease, sedentary behavior, mood, pain and medication use. Arch Gerontol Geriatr. 2015;60(1):52–8. https://doi.org/10.1016/j.archger.2014.09.006.
Colpani V, Baena CP, Jaspers L, van Dijk GM, Farajzadegan Z, Dhana K, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(9):831–45. https://doi.org/10.1007/s10654-018-0374-z.
Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. https://doi.org/10.1016/s0140-6736(16)30175-1.
Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of endocrinology comprehensive clinical practice guidelines for medical Care of Patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. https://doi.org/10.4158/EP161365.GL.
Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99(4):875–90. https://doi.org/10.3945/ajcn.113.068122.
Woolley C, Thompson C, Hakendorf P, Horwood C. The effect of age upon the interrelationship of BMI and inpatient health outcomes. J Nutr Health Aging. 2019;23(6):558–63. https://doi.org/10.1007/s12603-019-1206-x.
Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25(4):643–59. https://doi.org/10.1016/j.cger.2009.07.005.
Wang S, Ren J. Obesity paradox in aging: from prevalence to pathophysiology. Prog Cardiovasc Dis. 2018;61(2):182–9. https://doi.org/10.1016/j.pcad.2018.07.011.
Bowman K, Delgado J, Henley WE, Masoli JA, Kos K, Brayne C, et al. Obesity in older people with and without conditions associated with weight loss: follow-up of 955,000 primary care patients. J Gerontol A Biol Sci Med Sci. 2017;72(2):203–9. https://doi.org/10.1093/gerona/glw147.
Gill LE, Bartels SJ, Batsis JA. Weight Management in Older Adults. Curr Obes Rep. 2015;4(3):379–88. https://doi.org/10.1007/s13679-015-0161-z.
Jackson SE, Beeken RJ, Wardle J. Obesity, perceived weight discrimination, and psychological well-being in older adults in England. Obesity (Silver Spring). 2015;23(5):1105–11. https://doi.org/10.1002/oby.21052.
Batsis JA, Zbehlik AJ, Pidgeon D, Bartels SJ. Dynapenic obesity and the effect on long-term physical function and quality of life: data from the osteoarthritis initiative. BMC Geriatr. 2015;15:118. https://doi.org/10.1186/s12877-015-0118-9.
Wang L, Crawford JD, Reppermund S, Trollor J, Campbell L, Baune BT, et al. Body mass index and waist circumference predict health-related quality of life, but not satisfaction with life, in the elderly. Qual Life Res. 2018;27(10):2653–65. https://doi.org/10.1007/s11136-018-1904-6.
Monteagudo C, Dijkstra SC, Visser M. Self- perception of body weight status in older Dutch adults. J Nutr Health Aging. 2015;19(6):612–8. https://doi.org/10.1007/s12603-015-0486-z.
Mathus-Vliegen EM, Obesity OMTFotEAftSo. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts 2012;5(3):460–483. doi:https://doi.org/10.1159/000341193.
Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO. Obesity Society Obes Res. 2005;13(11):1849–63. https://doi.org/10.1038/oby.2005.228.
Batsis JA, Gill LE, Masutani RK, Adachi-Mejia AM, Blunt HB, Bagley PJ, et al. Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc. 2017;65(2):257–68. https://doi.org/10.1111/jgs.14514.
Normandin E, Chmelo E, Lyles MF, Marsh AP, Nicklas BJ. Effect of resistance training and caloric restriction on the metabolic syndrome. Med Sci Sports Exerc. 2017;49(3):413–9. https://doi.org/10.1249/MSS.0000000000001122.
Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–9. https://doi.org/10.3945/ajcn.114.105270.
Nicklas BJ, Brinkley TE, Houston DK, Lyles MF, Hugenschmidt CE, Beavers KM, et al. Effects of caloric restriction on cardiorespiratory fitness, fatigue, and disability responses to aerobic exercise in older adults with obesity: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019;74(7):1084–90. https://doi.org/10.1093/gerona/gly159.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29. https://doi.org/10.1056/NEJMoa1008234.
Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. https://doi.org/10.1016/j.clnu.2018.05.024.
Beavers KM, Walkup MP, Weaver AA, Lenchik L, Kritchevsky SB, Nicklas BJ, et al. Effect of exercise modality during weight loss on bone health in older adults with obesity and cardiovascular disease or metabolic syndrome: a randomized controlled trial. J Bone Miner Res. 2018;33(12):2140–9. https://doi.org/10.1002/jbmr.3555.
Houston DK, Miller ME, Kitzman DW, Rejeski WJ, Messier SP, Lyles MF, et al. Long-term effects of randomization to a weight loss intervention in older adults: a pilot study. J Nutr Gerontol Geriatr. 2019;38(1):83–99. https://doi.org/10.1080/21551197.2019.1572570.
Jiang BC, Villareal DT. Weight loss-induced reduction of bone mineral density in older adults with obesity. J Nutr Gerontol Geriatr. 2019;38(1):100–14. https://doi.org/10.1080/21551197.2018.1564721.
Kammire DE, Walkup MP, Ambrosius WT, Lenchik L, Shapses SA, Nicklas BJ, et al. Effect of weight change following intentional weight loss on bone health in older adults with obesity. Obesity (Silver Spring). 2019;27(11):1839–45. https://doi.org/10.1002/oby.22604.
Papageorgiou M, Kerschan-Schindl K, Sathyapalan T, Pietschmann P. Is weight loss harmful for skeletal health in obese older adults? Gerontology. 2019;66:1–13. https://doi.org/10.1159/000500779.
Cetin DC, Nasr G. Obesity in the elderly: more complicated than you think. Cleve Clin J Med. 2014;81(1):51–61. https://doi.org/10.3949/ccjm.81a.12165.
Johnson KC, Bray GA, Cheskin LJ, Clark JM, Egan CM, Foreyt JP, et al. The effect of intentional weight loss on fracture risk in persons with diabetes: results from the look AHEAD randomized clinical trial. J Bone Miner Res. 2017;32(11):2278–87. https://doi.org/10.1002/jbmr.3214.
Parr EB, Coffey VG, Hawley JA. 'Sarcobesity': a metabolic conundrum. Maturitas. 2013;74(2):109–13. https://doi.org/10.1016/j.maturitas.2012.10.014.
Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, et al. Weight loss and regain and effects on body composition: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2010;65(1):78–83. https://doi.org/10.1093/gerona/glp042.
Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the health, aging and body composition study. Am J Clin Nutr. 2005;82(4):872–8. https://doi.org/10.1093/ajcn/82.4.872.
Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons--a narrative review. Clin Interv Aging. 2015;10:1267–82. https://doi.org/10.2147/CIA.S82454.
Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: Time to meet the challenge. Clin Nutr. 2018;37(6 Pt A):1787–93. https://doi.org/10.1016/j.clnu.2018.04.018.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37. https://doi.org/10.1038/s41574-018-0062-9.
Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. https://doi.org/10.1093/epirev/mxs006.
Porter Starr KN, McDonald SR, Weidner JA, Bales CW. Challenges in the Management of Geriatric Obesity in High Risk Populations. Nutrients. 2016;8(5). doi:https://doi.org/10.3390/nu8050262.
Zeanandin G, Molato O, Le Duff F, Guerin O, Hebuterne X, Schneider SM. Impact of restrictive diets on the risk of undernutrition in a free-living elderly population. Clin Nutr. 2012;31(1):69–73. https://doi.org/10.1016/j.clnu.2011.08.007.
Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–24. https://doi.org/10.1159/000442721.
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–59. https://doi.org/10.1016/j.jamda.2013.05.021.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014;33(6):929–36. https://doi.org/10.1016/j.clnu.2014.04.007.
Haywood C, Sumithran P. Treatment of obesity in older persons-a systematic review. Obes Rev. 2019;20(4):588–98. https://doi.org/10.1111/obr.12815.
Ard JD, Cook M, Rushing J, Frain A, Beavers K, Miller G, et al. Impact on weight and physical function of intensive medical weight loss in older adults with stage II and III obesity. Obesity (Silver Spring). 2016;24(9):1861–6. https://doi.org/10.1002/oby.21569.
Haywood CJ, Prendergast LA, Purcell K, Le Fevre L, Lim WK, Galea M, et al. Very low calorie diets for weight loss in obese older adults-a randomized trial. J Gerontol A Biol Sci Med Sci. 2017;73(1):59–65. https://doi.org/10.1093/gerona/glx012.
Haywood CJ, Prendergast LA, Lim R, Lappas M, Lim WK, Proietto J. Obesity in older adults: effect of degree of weight loss on cardiovascular markers and medications. Clin Obes. 2019;9(4):e12316. https://doi.org/10.1111/cob.12316.
Bales CW, Porter Starr KN. Obesity interventions for older adults: diet as a determinant of physical function. Adv Nutr. 2018;9(2):151–9. https://doi.org/10.1093/advances/nmx016.
Buckinx F, Gaudreau P, Marcangeli V, Boutros GEH, Dulac MC, Morais JA, et al. Muscle adaptation in response to a high-intensity interval training in obese older adults: effect of daily protein intake distribution. Aging Clin Exp Res. 2019;31(6):863–74. https://doi.org/10.1007/s40520-019-01149-y.
Beavers KM, Nesbit BA, Kiel JR, Sheedy JL, Arterburn LM, Collins AE, et al. Effect of an energy-restricted, nutritionally complete, higher protein meal plan on body composition and mobility in older adults with obesity: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2019;74(6):929–35. https://doi.org/10.1093/gerona/gly146.
Weaver AA, Houston DK, Shapses SA, Lyles MF, Henderson RM, Beavers DP, et al. Effect of a hypocaloric, nutritionally complete, higher-protein meal plan on bone density and quality in older adults with obesity: a randomized trial. Am J Clin Nutr. 2019;109(2):478–86. https://doi.org/10.1093/ajcn/nqy237.
Serra M, Beavers D, Henderson R, Kelleher J, Kiel J, Beavers K. Effects of a Hypocaloric, nutritionally complete, higher protein meal plan on regional body fat and Cardiometabolic biomarkers in older adults with obesity. Ann Nutr Metab. 2019;74(2):149–55. https://doi.org/10.1159/000497066.
Al-Nimr RI. Optimal protein intake during weight loss interventions in older adults with obesity. J Nutr Gerontol Geriatr. 2019;38(1):50–68. https://doi.org/10.1080/21551197.2018.1544533.
Backx EM, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, de Groot LC. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes. 2016;40(2):299–304. https://doi.org/10.1038/ijo.2015.182.
Verreijen AM, Engberink MF, Memelink RG, van der Plas SE, Visser M, Weijs PJ. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr J. 2017;16(1):10. https://doi.org/10.1186/s12937-017-0229-6.
Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279–86. https://doi.org/10.3945/ajcn.114.090290.
Beavers KM, Gordon MM, Easter L, Beavers DP, Hairston KG, Nicklas BJ, et al. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study. J Nutr Health Aging. 2015;19(1):87–95. https://doi.org/10.1007/s12603-015-0438-7.
Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113(6):809–15. https://doi.org/10.1016/j.jand.2013.01.014.
Geisler C, Prado CM, Muller MJ. Inadequacy of Body Weight-Based Recommendations for Individual Protein Intake-Lessons from Body Composition Analysis. Nutrients. 2016;9(1). doi:https://doi.org/10.3390/nu9010023.
Salas-Salvado J, Diaz-Lopez A, Ruiz-Canela M, Basora J, Fito M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42(5):777–88. https://doi.org/10.2337/dc18-0836.
Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Chiva-Blanch G, et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):e6–e17. https://doi.org/10.1016/s2213-8587(19)30074-9.
Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiol. 2012;2(2):1143–211. https://doi.org/10.1002/cphy.c110025.
Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. https://doi.org/10.1111/sms.12581.
Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet (London, England). 2016;387(10031):1947–56. https://doi.org/10.1016/s0140-6736(16)00271-3.
American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–30. https://doi.org/10.1249/MSS.0b013e3181a0c95c.
Sardeli AV, Komatsu TR, Mori MA, Gaspari AF, Chacon-Mikahil MPT. Resistance Training Prevents Muscle Loss Induced by Caloric Restriction in Obese Elderly Individuals: A Systematic Review and Meta-Analysis. Nutrients. 2018;10(4). doi:https://doi.org/10.3390/nu10040423.
Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943–55. https://doi.org/10.1056/NEJMoa1616338.
El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2013;347:f6234. https://doi.org/10.1136/bmj.f6234.
Martinez-Amat A, Aibar-Almazan A, Fabrega-Cuadros R, Cruz-Diaz D, Jimenez-Garcia JD, Perez-Lopez FR, et al. Exercise alone or combined with dietary supplements for sarcopenic obesity in community-dwelling older people: a systematic review of randomized controlled trials. Maturitas. 2018;110:92–103. https://doi.org/10.1016/j.maturitas.2018.02.005.
Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:Cd012424. https://doi.org/10.1002/14651858.CD012424.pub2.
Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2010;42(5):902–14. https://doi.org/10.1249/MSS.0b013e3181c34465.
Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. Ageing Res Rev. 2013;12(1):329–38. https://doi.org/10.1016/j.arr.2012.10.001.
Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr). 2012;34(6):1493–515. https://doi.org/10.1007/s11357-011-9311-8.
Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev. 2010;11(3):202–21. https://doi.org/10.1111/j.1467-789X.2009.00653.x.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–7. https://doi.org/10.1016/j.pcad.2013.09.012.
McDonald SR, Porter Starr KN, Mauceri L, Orenduff M, Granville E, Ocampo C, et al. Meal-based enhancement of protein quality and quantity during weight loss in obese older adults with mobility limitations: rationale and design for the MEASUR-UP trial. Contemp Clin Trials. 2015;40:112–23. https://doi.org/10.1016/j.cct.2014.11.010.
Christensen P, Bliddal H, Riecke BF, Leeds AR, Astrup A, Christensen R. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clin Obes. 2011;1(1):31–40. https://doi.org/10.1111/j.1758-8111.2011.00006.x.
Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collab Res Group JAMA. 1998;279(11):839–46. https://doi.org/10.1001/jama.279.11.839.
Colleluori G, Napoli N, Phadnis U, Armamento-Villareal R, Villareal DT. Effect of weight loss, exercise, or both on Undercarboxylated Osteocalcin and insulin secretion in frail. Obese Older Adults Oxidative Med and Cell Longevity. 2017;2017:4807046–12. https://doi.org/10.1155/2017/4807046.
Kelleher JL, Beavers DP, Henderson RM, Yow D, Crotts C, Kiel J et al. Weighted Vest Use during Dietary Weight Loss on Bone Health in Older Adults with Obesity. Journal of osteoporosis and physical activity. 2017;5(4). doi:https://doi.org/10.4172/2329-9509.1000210.
Normandin E, Yow D, Crotts C, Kiel J, Beavers KM, Nicklas BJ. Feasibility of Weighted Vest Use during a Dietary Weight Loss Intervention and Effects on Body Composition and Physical Function in Older Adults. J Frailty Aging. 2018;7(3):198–203. https://doi.org/10.14283/jfa.2018.17.
Fanning J, Walkup MP, Ambrosius WT, Brawley LR, Ip EH, Marsh AP, et al. Change in health-related quality of life and social cognitive outcomes in obese, older adults in a randomized controlled weight loss trial: does physical activity behavior matter? J Behav Med. 2018;41(3):299–308. https://doi.org/10.1007/s10865-017-9903-6.
Rejeski WJ, Marsh AP, Fanning J, Ambrosius WT, Walkup MP, Nicklas BJ. Dietary weight loss, exercise, and inflammation in older adults with overweight or obesity and Cardiometabolic disease. Obesity (Silver Spring). 2019;27(11):1805–11. https://doi.org/10.1002/oby.22600.
Beavers KM, Beavers DP, Martin SB, Marsh AP, Lyles MF, Lenchik L, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci. 2017;72(11):1582–5. https://doi.org/10.1093/gerona/glx048.
Hugenschmidt CE, Leng X, Lyles M, Michael L, Dougherty A, Babcock P, et al. Cognitive effects of adding caloric restriction to aerobic exercise training in older adults with obesity. Obesity (Silver Spring). 2019;27(8):1266–74. https://doi.org/10.1002/oby.22525.
Kohrt WM, Barry DW, Schwartz RS. Muscle forces or gravity: what predominates mechanical loading on bone? Med Sci Sports Exerc. 2009;41(11):2050–5. https://doi.org/10.1249/MSS.0b013e3181a8c717.
Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58(8):728–33. https://doi.org/10.1093/gerona/58.8.m728.
Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. https://doi.org/10.1097/JES.0b013e31823b5f13.
Shea MK, Nicklas BJ, Houston DK, Miller ME, Davis CC, Kitzman DW, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr. 2011;94(3):839–46. https://doi.org/10.3945/ajcn.110.006379.
Shea MK, Houston DK, Nicklas BJ, Messier SP, Davis CC, Miller ME, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT study. J Gerontol A Biol Sci Med Sci. 2010;65(5):519–25. https://doi.org/10.1093/gerona/glp217.
Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging. 2013;17(1):3–7. https://doi.org/10.1007/s12603-012-0421-5.
Chmelo EA, Beavers DP, Lyles MF, Marsh AP, Nicklas BJ, Beavers KM. Legacy effects of short-term intentional weight loss on total body and thigh composition in overweight and obese older adults. Nutr Diabetes. 2016;6:e203. https://doi.org/10.1038/nutd.2016.8.
Houston DK, Neiberg RH, Miller ME, Hill JO, Jakicic JM, Johnson KC, et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: the look AHEAD study. J Gerontol A Biol Sci Med Sci. 2018;73(11):1552–9. https://doi.org/10.1093/gerona/glx204.
Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46. https://doi.org/10.1001/jama.2015.17346.
Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–73. https://doi.org/10.1001/jama.2013.277669.
Rejeski WJ, Brubaker PH, Goff DC Jr, Bearon LB, McClelland JW, Perri MG, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–6. https://doi.org/10.1001/archinternmed.2010.522.
Zanandrea V, Barreto de Souto P, Cesari M, Vellas B, Rolland Y. Obesity and nursing home: a review and an update. Clin Nutr. 2013;32(5):679–85. https://doi.org/10.1016/j.clnu.2013.05.008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goisser, S., Kiesswetter, E., Schoene, D. et al. Dietary weight-loss interventions for the management of obesity in older adults. Rev Endocr Metab Disord 21, 355–368 (2020). https://doi.org/10.1007/s11154-020-09577-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09577-2