Abstract
In this review article, we first present a brief overview of the vascular and neural components of diabetic retinopathy. Next, the multifocal electroretinogram (mfERG) technique, which can map neuroretinal function noninvasively, is described. Findings in diabetic retinal disease using the mfERG are reviewed. We then describe the progress that has been made to predict the onset and progression of diabetic retinopathy and edema in specific retinal locations, using quantitative models based on the mfERG. Finally, we consider the implications for the future of these predictive models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes is the leading cause of new blindness in people of working age in the United States [1, 2]. Every year, over 24,000 new cases of blindness occur in the U.S. due to diabetic retinopathy [3]. Between 2005 and 2008, 4.2 million diabetes patients 40 years or more of age had diabetic retinopathy in the U.S., and 4.4 % of the patients with diabetes had advanced diabetic retinopathy that could produce severe loss of vision [4].
Preventing abnormally elevated blood glucose significantly reduces the risk for development and progression of ocular and visual complications of type 1 and type 2 diabetes, as has been shown by epidemiological studies [5–7]. Control of blood glucose concentration is, however, often difficult to achieve and/or maintain for many patients. Worse, sight-threatening diabetic retinopathy can occur even among those patients who manage their diabetes well [5].
Traditionally, diabetic retinopathy has been considered to be, and has been defined as, a pathology of the microvasculature within the inner retina layers [8]. The earliest form of diabetic retinopathy is nonproliferative diabetic retinopathy (NPDR). NPDR has also been referred to as background or simple diabetic retinopathy. In NPDR, retinal blood vessels experience weakening of their vascular walls and membranes, are often abnormally dilated, have outpouching/distension, have leakage and bleeding, and release fluid into the retina. There may also be retinal ischemia.
A more advanced form, proliferative diabetic retinopathy (PDR), increases significantly a patient’s risk for vision loss. In PDR, in addition to the pathological changes associated with NPDR, there is neovascularization, the formation of abnormal new blood vessels that have weak vascular walls that are prone to leakage. Neovascularization can lead to vitreal hemorrhages, retinal traction, and/or retinal detachments. The primary cause of severe vision loss in type 1 diabetes is PDR [9, 10].
Patients with types 1 and 2 diabetes are also at risk for the development of diabetic retinal edema, resulting from the breakdown of the blood–retinal barrier and the leakage of blood plasma components primarily into the middle retinal layers. Diabetic retinal edema can occur locally (cystoid edema) and/or diffusely within the retina. Clinically significant macular edema and diabetic retinopathy are the primary causes for the irreversible visual consequences of diabetes [11, 12].
Neural Components of Diabetic Retinopathy
In addition to the microvascular changes that constitute the clinical signs of retinopathy, it has been known for decades that there are also neural changes produced by diabetes. Many of these neural changes occur early in the disease process, before visible signs of vasculopathy are present [13]. Indeed, as we shall discuss later in this review, certain changes in neuroretinal function are highly predictive of the appearance of the visible vascular changes that are the traditional signs of diabetic retinopathy.
The view that diabetes directly affects the neurosensory retina, independently of the clinically observed vascular changes, has been proposed for more than 50 years [14]. Nearly 30 years ago, Bresnick suggested that diabetic retinopathy should be redefined as a dual-component disease, a neurosensory disorder resulting from metabolic and systemic insults to the retina and the clinically observed vascular changes [15]. A number of sensitive electrophysiological measurements of neural function within the human retina and psychophysical (behavioral) measurements of visual function have shown that there are early abnormalities that occur before the clinical signs of diabetic retinopathy [16–18]. Consistent with these reports, animal models of diabetes have shown that there are increased inflammatory factors within the retina, structural changes of the retinal glia, and retinal ganglion cell apoptosis prior to the overt vascular changes associated with clinical retinopathy. It should be emphasized, however, that good animal models of human diabetic retinopathy, ones that closely resemble the disease in humans, have not yet been made available for study [19].
At this time, it is unclear whether neural changes in the retina precede subtle microvascular changes or follow microvascular changes or whether neural and microvascular changes occur in parallel. Advances in methodologies and technologies to detect, more sensitively, the neural and vascular neural complications of diabetes in the retina could very well provide evidence for all three possibilities in the future. However, there is a growing amount of evidence that early neural and vascular changes produced by diabetes involve complicated feed-forward interactions that serve to exacerbate both types of changes, leading to the clinical signs of retinopathy [17, 18]. An important point is that early biomarkers of neuroretinal dysfunction, especially those associated with an increased risk for the development of the clinical signs of diabetic retinopathy, would provide an opportunity for intervention. As will be discussed later, the multifocal electroretinogram (mfERG) provides such a biomarker that is predictive of the clinical signs of diabetic retinopathy.
The Multifocal Electroretinogram (mfERG)
The mfERG technique was introduced by Sutter and Tran in 1992 [20] and initially was used to map retinal function in patients a few years later [21, 22]. The mfERG method extracts retinal responses generated by as many as hundreds of individual small retinal locations from a single recording acquired using a thread, foil, or contact lens electrode. Commonly, 32–103 mfERG stimulus patches are used. The separate retinal locations are tested concurrently (in parallel). Detailed description of this technology is beyond the scope of this review, but such details can be found in multiple previous reports [22–25]. However, a key to the technique is that the temporal sequences used to “pseudorandomly” modulate (usually, black–white) the individual stimulus patches are not correlated. Therefore, cross-correlating each stimulus patch’s temporal pattern with the complex retinal signal extracts the local response generated within each of the corresponding individual retinal patches. In many implementations/instruments, this decomposition generates a series of response “kernels,” which represent different properties of the retinal responses at each location (e.g., flash response and adaptation) [23].
The length of the recording required to achieve sufficient quality (signal-to-noise ratio) of the locally generated responses varies with the size and the number of retinal patches that are stimulated, as well as stimulus conditions. For a given stimulus condition, shorter recordings are required for fewer and larger retinal patches, and longer recordings are needed for more and smaller patches to achieve the same response quality. Highly reproducible results can be obtained with 103 stimulus locations covering the central 45° of the retina using a 7.5-min recording using a contact lens electrode [26].
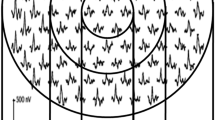
Figure 1 depicts the “standard” 103-element mfERG stimulus array that we primarily use, the array of corresponding local mfERG responses, and a local mfERG response. The stimulus array is scaled with retinal eccentricity to approximately equalize the response sizes (and the signal-to-noise ratio) at each retinal location. The “X” at the center of the array is a fixation aid upon which the patient steadily gazes, and the circle centered at about 15° on the left-hand side represents the location of the optic nerve head of a left eye’s retina. The flash luminance we use is 200 cd/m2. Using dilated pupils, the mfERG is primarily initiated by the activity of cone photoreceptors. The mfERG response array shows the local response waveforms typical of a healthy eye that are recorded in 7.5 min. The single local mfERG response shows the commonly reported “first-order” kernel (mean local flash response) waveform in detail and two common measurements. The first measurement is implicit time (IT), the time from a focal flash to the positive peak (P1) that follows the earlier negative trough. The second measurement is amplitude (Amp), the voltage difference between the P1 peak and the preceding trough.
Testing the diabetic retina using small, localized stimuli has advantages over large or full-field stimuli. It is reasonable to expect that the diabetes-induced changes in neuroretinal function will be nonuniform across the retina, as is the case in most retinal diseases. The anatomical changes evident in early NPDR occur usually in isolated, small patches where vascular abnormalities (microaneurysms and dot hemorrhages), infarcts (cotton wool spots), exudates, and/or edema are present. Therefore, abnormal neuroretinal function might also occur within relatively small patches of the retina. Visual stimuli that activate large retinal areas, relative to small, functionally abnormal areas, are less sensitive to dysfunction than small stimuli because the retinal responses are dominated by the larger, relatively healthy retinal areas.
Another advantage of the mfERG is that it allows for mapping of retinal function and identification of the retinal location(s) and spatial extent of dysfunction. This could be very important because, if localized neuroretinal dysfunction was known to be associated with subsequent diabetic retinopathy and/or edema development close to the fovea, where the best visual acuity occurs, it would be of greater clinical importance than if it occurred in the retinal periphery. Finally, various components of the mfERG that can be enhanced using various stimulus conditions have been associated with specific functions and different retinal layers [23, 25, 27–34]. Thus, it is possible to examine these response components and their putative cellular mechanisms at different locations across the posterior pole of the retina.
The mfERG in Diabetes, Diabetic Retinopathy, and Edema
The earliest mfERG study to focus on patients with diabetes was conducted by Palmowski and colleagues and was published in 1997 [35]. This small study comprised 8 patients without and 8 with diabetic retinopathy and 19 nondiabetic healthy control subjects. The mfERGs were analyzed after averaging the 103 local waveforms across the 50° stimulus array into a single waveform and also within concentric rings around the fovea of the retina. The first-order response kernels, which represent the mean flash responses, and second-order kernels (which represent changes in the mean flash responses produced by preceding flashes) were examined. In diabetic patients without retinopathy, only the second-order kernel Amp was significantly reduced. In the patients with retinopathy, the ITs of the first-order and second-order kernels were significantly longer, and their Amps were reduced. They concluded that the results were evidence that the inner retina was primarily affected in “subclinical” diabetic retinopathy. It should be noted, however, that this study’s small sample sizes and the fact that local responses were not individually measured might have limited the ability to detect abnormalities of retinal function sensitively in diabetic patients without retinopathy.
The first study to analyze local (individual) mfERG responses in diabetes was that of Fortune et al. in 1999 [36]. This study examined local response characteristics in 8 diabetic patients with retinopathy and 8 patients without retinopathy, as compared with 16 nondiabetic healthy control subjects. A template-scaling method for measuring local first-order mfERG IT and Amp, developed by Hood and Li, was used [37]. Fortune and colleagues found that, in eyes with NPDR and/or edema, retinal locations with lesions had longer ITs than did locations without lesions and that IT increased with the graded severity of the retinal lesion. Many retinal locations without lesions also had significantly longer ITs, in both eyes with and without retinopathy, indicating dysfunction in the absence of clinical diabetic retinopathy. In contrast, local mfERG Amp was not related consistently to retinopathic lesions and was not affected in eyes without retinopathy [36].
The main findings of these initial two studies have been replicated quite consistently [38–40], although most studies did not measure individual mfERG responses [35, 41–47]. Studies with larger sample sizes than those first two studies have reported more significant neuroretinal function changes in diabetes patients without retinopathy [38–41, 48, 49]. The mfERG IT is significantly delayed in 14 %–21 % of the retinal locations tested in adults with diabetes before retinopathy onset [38, 40].
Consistently, patients with diabetic retinopathy have demonstrated worse neuroretinal function than those without retinopathy. Of the different nonproliferative changes associated with diabetic retinopathy, edema appears to be associated with the longest mfERG IT (worst dysfunction) [50]. Patients with clinically significant diabetic macular edema and diabetic retinopathy have delayed mfERG ITs and reduced mfERG Amps [51]. The presence of hard exudates (associated with diabetic macular edema) was shown to be associated with increased (worse) mfERG IT [52]. Focal laser treatment of edema decreases retinal swelling and appears to increase mfERG Amps [53]. However, some mfERG studies have not reported improved function [54]. Some studies reporting significant abnormalities of neuroretinal function in diabetes patients with and without retinopathy have used mfERG techniques differing from the “standard” mfERG technique described earlier. These techniques include the slow flash technique [39, 55], isolation of high-frequency mfERG oscillatory potentials [32, 55], and a “global flash mfERG technique” [45].
Retinal dysfunction appears to occur early in the disease, prior to development of clinical retinopathy. It has been documented with the mfERG in adolescents with type 1 diabetes [56, 57•, 58], adolescents with type 2 diabetes [57•, 59], and adults with recently diagnosed type 2 diabetes [60] without retinopathy. Recently, it was reported that mfERG IT is linearly correlated with HbA1c in adolescent type 1 patients without retinopathy, suggesting that long-term blood glucose control in this disease determines the degree of neuroretinal dysfunction before diabetic retinopathy is diagnosed [61•]. Interestingly, in both type 1 and type 2 adult patients without clinical signs of retinopathy, local mfERG IT and AMP abnormalities are not spatially associated with local retinal thickness changes measured by optical coherence tomography [62•].
Is the mfERG a Predictor of Diabetic Retinopathy?
In the initial predictive study, 11 adult diabetic patients with mild and moderate NPDR and 11 adult diabetic patients without retinopathy were examined [63]. They were tested at baseline and 1 year later, using the 103-element mfERG stimulus described earlier. Fundus photos were also taken at each examination. Local mfERG ITs were measured using a template-stretching method [37] and converted to Z-scores. In the eyes that developed any new retinopathy at follow-up, 34.6 % of the retinal patches that were initially free of retinopathy had abnormal mfERG ITs (defined as IT Z-scores ≥2) at baseline. Of these functionally abnormal patches, 34.9 % developed retinopathy at follow-up. In contrast, only 1.7 % of the retinal patches that had normal baseline IT developed retinopathy. Retinopathy development was, therefore, ~21 times more likely in patches with abnormal baseline mfERG ITs than in patches with normal baseline ITs (odds ratio = 31.4; p < .001). These findings established, for the first time, that localized functional abnormalities of the retina precede the development of clinical signs of new retinopathy at those locations [63].
Quantitative Models Predicting Diabetic Retinopathy and Edema
On the basis of the above findings, the next question is whether formal models based on the mfERG IT can be constructed to predict diabetic retinopathy. Our group has been actively working to find the answer. All of the subjects in our modeling studies had 20/25 visual acuity or better, clear optical media, no history of ocular surgery, and no eye disease other than NPDR. At baseline, medical history is taken, various clinical measurements are made, “standard” mfERGs are recorded, and dilated 50° stereoscopic fundus photographs are performed to image the tested retinal area. A follow-up examination is performed (usually 1 year later) that repeats the baseline exam. The fundus photographs are graded by a retina specialist who is masked to all of the examination results.
In our modeling studies, the 103 local mfERGs are measured as previously described. The mfERG ITs and Amps are converted to Z-scores based on normative data to account for normal differences that exist among the tested retinal locations. The 103 local IT and Amp Z-scores are arranged into 35 fixed zones made up of either two or three adjacent retinal locations. Each of these retinal zones is assigned the maximum IT and minimum Amp Z-scores occurring within it. The zone configurations are superimposed over a graded fundus photograph, and the retinopathic lesions are then mapped onto them.
We use retinal zones for a number of reasons. A retinal lesion that is visible in a fundus photograph could be smaller than the actual extent of the anatomical damage or the associated area of dysfunction. It is also possible that a visible lesion might not exactly spatially correspond to the location of a functional deficit. Lastly, using zones helps to reduce the consequences that would occur with minor mismatches between the retinal locations of the mfERG stimulus array elements and retinopathic lesions. In all of our predictive modeling studies except one, zones with any retinopathy present at baseline were excluded from further analysis.
Using stepwise logistic regression, we examine the association between baseline mfERG IT and Amp (plus additional potential risk factors) and the development of new retinopathy. The other potential baseline risk factors we have examined include age, gender, diabetes type, the duration of diabetes, blood glucose concentration at time of mfERG testing, HbA1c, diastolic and systolic blood pressures, baseline retinopathy status, and grade/severity of retinopathy. We apply generalized estimating equations with corrected (robust) estimation of the variance–covariance matrix for estimates of model coefficients to account for within-eye correlations of mfERG ITs and Amps among the retinal zones [64]. For further details regarding the model-building methods, please refer to [40, 65].
In our first predictive model-building project, 28 eyes of 28 patients with diabetes (12 with NPDR at baseline and 16 without baseline retinopathy) were studied at baseline and retested during a 1-year follow-up examination [66]. Out of 919 retinal zones that were free of retinopathy at baseline, 64 (7 %) had developed new retinopathy at follow-up. In a univariate model using baseline mfERG IT as the sole predictor, we found that a unit increase in baseline mfERG IT Z-score was associated with an increase of 23 % in the risk for development of new diabetic retinopathy at that retinal location. Receiver operating characteristic curve analysis of this simple model showed 73 % sensitivity and 77 % specificity, with an area under the curve (AUC, a measure of overall accuracy with a maximum of 1.0) of .80.
Six additional baseline risk factors were also examined: type of diabetes, duration of diabetes, gender, age, baseline retinopathy status, and concentration of blood glucose at mfERG recording [66]. The resulting multivariate model included the baseline variables mfERG IT Z-score, duration of diabetes, baseline retinopathy status, and blood glucose concentration. This model had 86 % sensitivity, 84 % specificity, and an AUC of .90. The multivariate model was then tested on 12 eyes of patients who were not included in the initial model-building project. Using the model and the criterion probability of new retinopathy development used in model making, we obtained 89 % sensitivity and 86 % specificity. For the first time, this study demonstrated that quantitative models based on mfERG IT can predict the development of diabetic retinopathy in specific retinal locations with high sensitivity, specificity, and accuracy [66].
In our next study, 20 eyes of 20 adult diabetes patients were followed over a 2-year period, at year 1 (T1) and year 2 (T2) [40]. One- and 2-year multivariate predictive models based on mfERG IT Z-scores were constructed. The sensitivity, specificity, and AUC for the 1-year model were 95 %, 93 %, and .95, respectively, and for the 2-year model, they were 81 %, 82 %, and .88, respectively. These results suggested that prediction is less accurate over longer intervals than over shorter intervals [40].
The persistence of new diabetic retinopathy is very important. In a 3-year study of diabetic adults, Ng and colleagues showed that recurring or persistent retinopathy can be predicted using a multivariate model incorporating baseline mfERG IT Z-score, duration of diabetes, and baseline blood glucose concentration [65]. This model had a sensitivity of 88 %, a specificity of 98 %, and an AUC of .95. On the other hand, transient retinopathy was not accurately predicted. This suggests that significant changes in neuroretinal function are not associated with minor, transient signs of retinopathy.
The mfERG IT has also been used to predict the initial onset of diabetic retinopathy in 41 adults who had been examined annually by our lab for several years [67••]. The presence or absence of diabetic retinopathy at the final study visit was the outcome measure, and risk factor measurements from the previous examination were used for prediction. After considering seven potential factors, we derived a multivariate model with mfERG IT Z-score and diabetes type. Using fivefold cross-validation, we determined that this model had a sensitivity of 80 % and a specificity of 74 %. Thus, onset of diabetic retinopathy can be predicted within small retinal patches with impressive accuracy.
More recently, we determined whether diabetic retinal edema could be predicted within the central 45° on the basis of the mfERG in at-risk patients with retinopathy [68••]. Eleven potential factors were examined. Diabetic retinal edema developed in about 5 % of the retinal zones and 35 % of the eyes. Together, mfERG IT Z-score, mfERG Amp Z-score, systolic blood pressure, and male sex were predictive of diabetic retinal edema. When combined in a model, these variables had 84 % sensitivity and 76 % specificity. Notably, this is the only model in which mfERG Amp is a predictive factor. More important, this model predicts the onset of a condition, in specific retinal locations, that is potentially sight-threatening [68••]. All of the predictive models described above are summarized and compared in Table 1.
Conclusions
The mfERG has proven to be a valuable tool in the study of neuroretinal function in diabetes, diabetic retinopathy, and diabetic retinal edema. It has shown that small patches of the diabetic retina can exhibit abnormal function, even in eyes with no history of clinical retinopathy. More important, the implicit time of the mfERG allows for the prediction of diabetic retinopathy and edema with high accuracy when used in quantitative models incorporating other risk factors.
Predictive models such as those described in this review make it possible to identify individuals at risk for developing diabetic retinopathy and edema, as well as the specific retinal locations that are at risk. In the future, models such as these will allow for the identification of patients who should be followed more frequently, as well as patients who are good candidates for participation in trials of prophylactic treatments. In addition, the 1-year predictive time frame should make the mfERG-based models useful even in relatively short-term clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Society to Prevent Blindness. Visual problems in the U.S. definitions, data sources, detailed data tables, analyses, interpretation. New York: National Society to Prevent Blindness; 1980. p. 1–46.
Beckles GL, Chou CF. Diabetes - United States, 2006 and 2010. MMWR Surveill Summ. 2013;62 Suppl 3:99–104.
Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2003 [database on the Internet]. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2004. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2003.pdf. Accessed 8 Apr 2005.
Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes and prediabetes in the United States, 2011 [database on the Internet]. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2011. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 1 Apr 2014.
The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–20.
Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–63.
UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13.
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther. 2000;2:601–8.
Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res. 2005;24:355–77.
Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris 3rd FL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56.
Early Treatment Diabetic Retinopathy Study Research Group (ETDRS). Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33.
Early Treatment Diabetic Retinopathy Study Research Group (ETDRS). Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806.
Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–90.
Wolter JR. Diabetic retinopathy. Am J Ophthalmol. 1961;51:1123–41.
Bresnick GH. Diabetic retinopathy viewed as a neurosensory disorder. Arch Ophthalmol. 1986;104:989–90.
Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60.
Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11.
Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Curr Diab Rep. 2010;10:380–4.
Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103.
Sutter EE, Tran D. The field topography of ERG components in man–I. The photopic luminance response. Vis Res. 1992;32:433–46.
Kondo M, Miyake Y, Horiguchi M, Suzuki S, Tanikawa A. Clinical evaluation of multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1995;36:2146–50.
Bearse Jr MA, Sutter EE. Imaging localized retinal dysfunction with the multifocal electroretinogram. J Opt Soc Am A Opt Image Sci Vis. 1996;13:634–40.
Sutter E. The interpretation of multifocal binary kernels. Doc Ophthalmol. 2000;100:49–75.
Sutter EE. Imaging visual function with the multifocal m-sequence technique. Vis Res. 2001;41:1241–55.
Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19:607–46.
Harrison WW, Bearse Jr MA, Ng JS, Barez S, Schneck ME, Adams AJ. Reproducibility of the mfERG between instruments. Doc Ophthalmol. 2009;119:67–78.
Sutter EE, Bearse Jr MA. The optic nerve head component of the human ERG. Vis Res. 1999;39:419–36.
Bearse Jr MA, Shimada Y, Sutter EE. Distribution of oscillatory components in the central retina. Doc Ophthalmol. 2000;100:185–205.
Hood DC, Bearse Jr MA, Sutter EE, Viswanathan S, Frishman LJ. The optic nerve head component of the monkey's (Macaca mulatta) multifocal electroretinogram (mERG). Vis Res. 2001;41:2029–41.
Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–85.
Hare WA, Ton H. Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Effects of APB, PDA, and TTX on monkey ERG responses. Doc Ophthalmol. 2002;105:189–222.
Bearse Jr MA, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:3259–65.
Shimada Y, Bearse Jr MA, Sutter EE. Multifocal electroretinograms combined with periodic flashes: direct responses and induced components. Graefes Arch Clin Exp Ophthalmol. 2005;243:132–41.
Bronson-Castain KW, Bearse Jr MA, Han Y, Schneck ME, Barez S, Adams AJ. Association between multifocal ERG implicit time delays and adaptation in patients with diabetes. Invest Ophthalmol Vis Sci. 2007;48:5250–6.
Palmowski AM, Sutter EE, Bearse Jr MA, Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1997;38:2586–96.
Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2638–51.
Hood DC, Li J. A technique for measuring individual multifocal ERG records. In: Yager D, editor. Non-invasive assessment of the visual system. Trends in optics and photonics, vol. 11. Washington, D.C.: Optical Society of America; 1997. p. 33–41.
Han Y, Bearse Jr MA, Schneck ME, Barez S, Jacobsen C, Adams AJ. Towards optimal filtering of "standard" multifocal electroretinogram (mfERG) recordings: findings in normal and diabetic subjects. Br J Ophthalmol. 2004;88:543–50.
Bearse Jr MA, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004;45:296–304.
Bearse Jr MA, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–48.
Klemp K, Larsen M, Sander B, Vaag A, Brockhoff PB, Lund-Andersen H. Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci. 2004;45:3812–9.
Yu M, Zhang X, Zhong X, Yu Q, Jiang F, Ma J, et al. Multifocal electroretinograms in the early stages of diabetic retinopathy. Chin Med J (Engl). 2002;115:563–6.
Kurtenbach A, Langrova H, Zrenner E. Multifocal oscillatory potentials in type 1 diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2000;41:3234–41.
Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S. Morphological and functional analyses of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol. 2001;239:96–101.
Shimada Y, Li Y, Bearse Jr MA, Sutter EE, Fung W. Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol. 2001;85:414–9.
Onozu H, Yamamoto S. Oscillatory potentials of multifocal electroretinogram retinopathy. Doc Ophthalmol. 2003;106:327–32.
Tyrberg M, Ponjavic V, Lovestam-Adrian M. Multifocal electroretinography (mfERG) in insulin dependent diabetics with and without clinically apparent retinopathy. Doc Ophthalmol. 2005;110:137–43.
Klemp K, Sander B, Brockhoff PB, Vaag A, Lund-Andersen H, Larsen M. The multifocal ERG in diabetic patients without retinopathy during euglycemic clamping. Invest Ophthalmol Vis Sci. 2005;46:2620–6.
Han Y, Adams AJ, Bearse Jr MA, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004;122:1809–15.
Schneck ME, Bearse Jr MA, Han Y, Barez S, Jacobsen C, Adams AJ. Comparison of mfERG waveform components and implicit time measurement techniques for detecting functional change in early diabetic eye disease. Doc Ophthalmol. 2004;108:223–30.
Greenstein VC, Holopigian K, Hood DC, Seiple W, Carr RE. The nature and extent of retinal dysfunction associated with diabetic macular edema. Invest Ophthalmol Vis Sci. 2000;41:3643–54.
Holm K, Ponjavic V, Lovestam-Adrian M. Using multifocal electroretinography hard exudates affect macular function in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:1241–7.
Lovestam-Adrian M, Holm K. Multifocal electroretinography amplitudes increase after photocoagulation in areas with increased retinal thickness and hard exudates. Acta Ophthalmol. 2010;88:188–92.
Greenstein VC, Chen H, Hood DC, Holopigian K, Seiple W, Carr RE. Retinal function in diabetic macular edema after focal laser photocoagulation. Invest Ophthalmol Vis Sci. 2000;41:3655–64.
Tan W, Wright T, Dupuis A, Lakhani E, Westall C. Localizing functional damage in the neural retina of adolescents and young adults with type 1 diabetes. Invest Ophthalmol Vis Sci. 2014.
Lakhani E, Wright T, Abdolell M, Westall C. Multifocal ERG defects associated with insufficient long-term glycemic control in adolescents with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:5297–303.
Bronson-Castain KW, Bearse Jr MA, Neuville J, Jonasdottir S, King-Hooper B, Barez S, et al. Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina. 2012;32:92–102. The results of this study indicate that adolescents with type 2 diabetes have greater dysfunction and vascular changes in the retina of the eye than adolescents with type 1 diabetes.
Laron M, Bearse Jr MA, Bronson-Castain K, Jonasdottir S, King-Hooper B, Barez S, et al. Interocular symmetry of abnormal multifocal electroretinograms in adolescents with diabetes and no retinopathy. Invest Ophthalmol Vis Sci. 2012;53:316–21.
Bronson-Castain KW, Bearse Jr MA, Neuville J, Jonasdottir S, King-Hooper B, Barez S, et al. Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina. 2009;29:618–26.
Tyrberg M, Lindblad U, Melander A, Lovestam-Adrian M, Ponjavic V, Andreasson S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc Ophthalmol. 2011;123:193–8.
Laron M, Bearse Jr MA, Bronson-Castain K, Jonasdottir S, King-Hooper B, Barez S, et al. Association between local neuroretinal function and control of adolescent type 1 diabetes. Invest Ophthalmol Vis Sci. 2012;53:7071–6. The findings reported here show, in adolescents with type 1 diabetes, that there is a correlation between long-term blood glucose concentration (HbA1c) and neuroretinal function measured with the mfERG.
Dhamdhere KP, Bearse Jr MA, Harrison W, Barez S, Schneck ME, Adams AJ. Associations between local retinal thickness and function in early diabetes. Invest Ophthalmol Vis Sci. 2012;53:6122–8. This paper describes the lack of association, in patients with diabetes and no retinopathy, between local retinal function measured with the mfERG and changes in total retinal thickness. The functional changes do not appear to have a measureable anatomical correlate.
Han Y, Bearse Jr MA, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:948–54.
Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60.
Ng JS, Bearse Jr MA, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49:1622–8.
Han Y, Schneck ME, Bearse Jr MA, Barez S, Jacobsen CH, Jewell NP, et al. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:4106–12.
Harrison WW, Bearse Jr MA, Ng JS, Jewell NP, Barez S, Burger D, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011;52:772–7. This paper describes the first model based on the mfERG to predict the initial onset of diabetic retinopathy in adults. The model was cross-validated and demonstrates impressive accuracy.
Harrison WW, Bearse Jr MA, Schneck ME, Wolff BE, Jewell NP, Barez S, et al. Prediction, by retinal location, of the onset of diabetic edema in patients with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:6825–31. This report describes the formulation of the first mfERG-based model to predict the onset of diabetic retinal edema in adults. The cross-validated model demonstrates impressive accuracy.
Acknowledgments
We thank Anthony Adams, Marilyn Schneck, Nicholas Jewell, Kevin Bronson-Castain, Kavita Dhamdhere, Michal Laron, Wendy Harrison, Shirin Barez, Jason Ng, Ying Han, Brian Wolff, and Ken Huie for their contributions and valuable assistance. Marcus A. Bearse Jr. reports grants from JDRF and the National Eye Institute. Glen Y. Ozawa reports grants from JDRF.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Marcus A. Bearse Jr. and Glen Y. Ozawa declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Microvascular Complications—Retinopathy
Rights and permissions
About this article
Cite this article
Bearse, M.A., Ozawa, G.Y. Multifocal Electroretinography in Diabetic Retinopathy and Diabetic Macular Edema. Curr Diab Rep 14, 526 (2014). https://doi.org/10.1007/s11892-014-0526-9
Published:
DOI: https://doi.org/10.1007/s11892-014-0526-9