Abstract
Summary
To evaluate the influence of hard exudates on macular function in patients with diabetic retinopathy.
Methods
Thirty seven eyes from 27 diabetic patients, aged 57 ± 14 years, diabetes duration 12.5 ± 9 years, not previously treated with photocoagulation, underwent fundus photography, multifocal electroretinography (mfERG) and optical coherence tomography (OCT). Hard exudates were graded from fundus photography with superimposed OCT and a superimposed hexagonal pattern (mfERG) by one retinal specialist, unaware of mfERG and OCT results. We defined three groups; A = eyes with exudates in the analyzed zone, B = eyes with no exudates in the analyzed zone but elsewhere, and C = eyes with no exudates. The mfERG responses and OCT values from five defined areas in the macula were compared.
Results
MfERG showed that the implicit time was significantly prolonged in group A compared to group C in the central, middle and outer areas and in the nasal and temporal area (p = 0.045, 0.019, 0.017 and 0.035 and 0.016 respectively), in group B compared to group C in the central area (p = 0.016), and in group A compared to group B in the outer area (p = 0.035). Amplitude differed between group A and C in the middle area and outer area (14.2 ± 5.2 nV/deg2 vs 21.1 ± 8.7 nV/deg2, p = 0.037 and 14.1 ± 3.9 nV/deg2 vs 17.7 ± 7.1 nV/deg2 , p = 0.02 respectively), and between group B and C in the temporal area 14.5 ± 2.2 nV/deg2 vs 20.0 ± 8.7 nV/deg2, p = 0.017). Macular thickness assessed with OCT was similar between the groups.
Conclusions
In eyes with diabetic retinopathy, hard exudates prolong the implicit time assessed with mfERG, compared to eyes without hard exudates, and independently of macular thickness. These results indicate that the hard exudates in the macular region, even at a distance from the fovea centre, have a deleterious effect on macular function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clinically significant diabetic macular oedema is a thickening of the retina at or near the centre of the fovea as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) [1]. It is often accompanied with hard exudates which consist of lipoproteins leaking from the capillaries [2]. The presence of hard exudates has proven to be the strongest risk factor for the development of subretinal fibrosis [3], and hard exudates are independently associated with a higher risk of visual impairment [4].
Previous studies have shown that spontaneous regression of hard exudates in diabetic retinopathy may occur, but is not followed by visual recovery [5]. In addition, laser-treated eyes with diabetic macular oedema and hard exudates have a less favourable visual outcome than laser-treated eyes without hard exudates [4, 6]. Recently, it has been reported that intravitreal injection of triamcinolone reduces the amount of hard exudates, albeit only with little improvement in visual acuity [7].
In addition to visual acuity measurement, studies on fixation stability [8] and fundus microperimetry [9] in eyes with hard exudates have demonstrated decreased fixation stability and reduced function above circinate rings. However, the direct influence of hard exudates on retinal function is not fully understood. The development of mfERG, which was introduced by Sutter and Tran [10], has made it possible to study the macular region more accurately, and has made it possible for us to evaluate the extent of macular dysfunction in an objective way [11, 12].
The aim of the present study was to evaluate, with mfERG, the influence of hard exudates on macular function in patients with diabetic retinopathy. For this purpose, we analyzed mfERG amplitude and implicit time within the corresponding areas of the OCT`s central, middle and outer and nasal and temporal area.
Patients and methods
Patients
Thirty seven eyes from 27 consecutive patients with diabetic retinopathy, regularly attending the outpatient clinic at the Department of Ophthalmology, Lund University Hospital were included in this study. The other 17 eyes, from the 27 patients, either had cataract or had previously been treated with photocoagulation in the macular area.
Seven patients were classified as type I diabetes and the rest, who were older than 30 years at onset of diabetes, and did not require insulin treatment within 2 years of diagnosis, were classified as type II diabetes.
All examinations were performed between 08.00 a.m. and 11.00 a.m.
All patients gave written consent to participate in the study, which was approved by the Ethics Committee of Lund.
Classification of retinopathy
The level of diabetic retinopathy was diagnosed by a retina specialist using fundus photographs (45º Topcon fluoresceinangiography camera (FA) TRC 501X). All patients had nonproliferative diabetic retinopathy (NPDR) i.e., microaneurysm, haemorrhages, no IRMA, with or without hard exudates, according to definitions by the ETDRS [1]). No eye had signs of macular edema more than 6 months prior to the examination. None of the patients were previously treated with argon laser, anti-VEGF therapy or other intravitreal injections.
No eyes had any media opacity, a history of ocular surgery or other eye diseases than diabetic retinopathy.
Visual acuity
The best-corrected visual acuity was measured using the ETDRS Charts [13], and given as logMAR.
Multifocal ERG
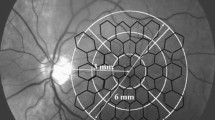
Multifocal electroretinograms were recorded using a visual evoked response imaging system (VERIS 4; EDI, San Mateo, CA, USA). The stimulus matrix consisted of 103 hexagonal elements that were displayed on a screen in an IR camera, driven at 75 Hz frame rate. The sizes of the hexagons were scaled with eccentricity to elicit approximately equal amplitude responses at all locations. At a viewing distance of 5 cm, the radius of the stimulus array subtended approximately 20–25 degrees. The luminance of each hexagon was independently alternated between black and white according to a pseudorandom binary m-sequence at 75 Hz. The maximum luminance was 138.0 cd/m2 and the minimum luminance was 3.5 cd/ m2, resulting in a mean luminance of approximately 70.8 cd/ m2. Pupils were maximally dilated with tropicamide and phenylephrine hydrochloride. A gold ground electrode was attached to the forehead. Retinal activity was recorded with a gold bipolar contact lens (Hansen Ophthalmic, Iowa City, IA, USA), which was placed on the anesthetized (oxibuprocain) cornea. The fixation was controlled using a fundus camera and illumination with infrared light from the recording electrode, with visualization of the hexagonal elements over the retina [14]. P1 amplitude and implicit time were calculated according to the guidelines for basic mfERG [14]. The central area was defined as the summed responses from the first and second ring of the standard mfERG hexagonal pattern. The middle area was defined as the third ring and the outer area was defined as the fourth ring of the standard mfERG hexagonal pattern. Amplitude and implicit time were analyzed within the four innermost (13°) of the six concentric rings, registered by the mfERG, which correspond to the three areas measured by the OCT (Ø 6.0 mm). (Fig. 1a). The nasal and temporal area was defined from the summed response from the corresponding inner and outer nasal, and inner and outer temporal OCT areas (Fig. 1b) .
a Central area of the OCT and the central ring (summed response of ring 1+2) of mfERG. 2 Middle area of the OCT and the ring 3 of mfERG. 3 Outer area of the OCT and the ring 4 of mfERG. b The summed response from the nasal and temporal mfERG response, corresponding to the inner and outer nasal and temporal areas on the OCT areas were recorded and further analyzed
For further calculations we divided the eyes into two groups; eyes with no exudates and eyes with exudates. We analyzed and compared amplitude and implicit time.
Optical coherence tomography
The retinal mapping was performed using the OCT 2 (Zeiss Humphrey Instruments, Dublin, CA, USA) standard program for retinal mapping. An examination with six radial lines with a length of 6.0 mm centred at the fovea, and with 30 degrees displacement from each other was performed. The mean macular thickness calculation was from the OCT retinal thickness software program.
Calculating macular thickness, mfERG response and grading hard exudates
For each patient, in a standardized way, a skilled ophthalmologic assistant superimposed the OCT pattern over the fundus photography. The distance between the foveola and the temporal border of the optic disk is considered as half the length of the radial line (3 mm) used at the OCT examination.
The ophthalmologic assistant also superimposed the hexagonal pattern from the mfERG examination, corresponding to the three OCT areas, providing that 1 mm of the radial line corresponds to 4º of the mfERG rings (Fig. 2). [15, 16].
We studied three different macular areas defined from the OCT examination; the central area (corresponding to the summed response from the two innermost rings of the mfERG pattern), the middle area (corresponding to the summed response from the third ring of the mfERG pattern), and the outer area (corresponding to the summed response from the fourth ring of the mfERG pattern) (Fig. 1a). In addition, we further analyzed the summed response from the nasal and temporal mfERG corresponding to the inner and outer nasal and inner and outer temporal OCT areas (Fig. 1b).
Hard exudates were graded in the three circular macula areas and in the nasal and temporal areas as present or not present. No hard exudates had an extension of more than 1/3 optic disc diameter.
The hard exudates were graded by a skilled ophthalmologist who was unaware of the mfERG and OCT results.
Three groups of eyes
-
Group A:
eyes with hard exudates within the analyzed area.
-
Group B:
eyes with no hard exudates within the analyzed area but elsewhere.
-
Group C:
eyes without hard exudates.
Medical parameters
At baseline, we measured the glycosylated hemoglobin levels. We recorded diabetes duration, blood pressure, type of diabetes, type of diabetes treatment, and whether the patients used antihypertensive drugs or not.
Analytical techniques
The upper normal reference range for HbA1c was < 5.3%.
Statistics
Values are given as means ± SD or median and range. Student's t-test was used for normal distributed data and Mann–Whitney U-test for skewed data. Spearman's rho was used to calculate correlations between the parameters. Visual acuity was given as logMAR. The Kruskal–Wallis one-way analysis of variance test for multiple independent samples was used as an extension of the Mann–Whitney. SPSS version 16 was used for the calculations.
Results
Patient characteristics and visual acuity
The age difference was not statistically significant between the patients with and without hard exudates (59 ± 13 vs 57 ± 15 years), but patients without hard exudates had a longer diabetes duration (15 ± 10 vs 8 ± 6 years; p = 0.034). Mean HbA1c levels (7.1 ± 1.8 %; Fig. 3) and systolic and diastolic blood pressure (135 ± 22 and 78 ± 11 mmHg respectively) were similar between the two groups.
Mean visual acuity score on the ETDRS chart, in the central area, was 81 ± 7 letters in Group A, 82 ± 7 letters in group B, and 80 ± 10 letters in group C. Corresponding figures in logMAR were in group A −0.18 ± 0.2, in group B −0.09 ± 0.2, and in group C −0.13 ± 0.2. There was no statistical difference between the three groups.
Multifocal ERG between group A, B and C in the different macula areas
The Kruskal–Wallis test demonstrated different implicit time between the three groups (A, B and C) in the central, outer and temporal area, p = 0.027, p = 0.033, and p = 0.032 respectively. Different amplitude was calculated in the middle and temporal area, p = 0.041 and p = 0.045 respectively.
Implicit time was significantly prolonged in group A compared to group C in the central, middle and outer areas and in the nasal and temporal areas; p = 0.045, 0.019, 0.017 and 0.035 and 0.016 respectively (Table 1). A significantly prolonged implicit time was also seen in group B compared to group C in the central area; p = 0.016, and group A compared to group B in the outer area; p = 0.035.
Amplitude differed between groups A and C in the middle area and the outer area; 14.2 ± 5.2 nV/deg2 vs 21.1 ± 8.7 nV/deg2 (p = 0.037) and 14.1 ± 3.9 nV/deg2 vs 17.7 ± 7.1 nV/deg2 (p = 0.02) respectively and between groups B and C in the temporal area, 14.5 ± 2.2 nV/deg2 vs 20.0 ± 8.7 nV/deg2 (p = 0.017) (Table 2).
Macular thickness assessed with OCT was similar between the groups (Table 3).
Multifocal ERG between eyes with and without exudates in the different areas
For further evaluation of the mfERG of the central macular are, we compared the 16 eyes with no exudates with the 21 eyes with exudates.
Amplitude and implicit time were analyzed. Eyes with exudates demonstrated significant lower amplitude (21 ± 7 vs 28 ± 12 nV/deg2, p = 0.037) and prolonged implicit time (32 ± 3 vs 29 ± 5 ms, p = 0.021) compared to eyes without exudates.
Discussion
Several studies have reported the influence of macular thickness, assessed with OCT, on visual acuity and retinal function [17, 18]. Hard exudates often accompany diabetic macular edema. They are also known to be the strongest risk factor for subretinal fibrosis and a poor visual outcome [3, 6]. Increasing amounts of exudates appear to be associated with an increased risk of visual impairment [4]. However, the influence of hard exudates on retinal function has to our knowledge not previously been studied.
Former studies have shown a local predictive power of the mfERG implicit time, for diabetic retinopathy, in areas without diabetic retinopathy [16, 19, 20].
The findings that implicit time is prolonged in diabetic eyes can be referred to the fact that diabetic retinopathy is largely caused by defects of retinal capillaries in the inner nuclear layer, where the cellbodies of the bipolar cells, the primary generators of the mfERG, are located [21].
Hard exudates are most typically located in the outer retina [22].
Compared to the five areas with no exudates in the examined area or elsewhere in the macular region(group C), we found a prolonged implicit time in all five areas with exudates (group A).
From interest in the matter, we also found a prolonged implicit time in the central area without exudates but elsewhere in the macular region (group B). Furthermore, this was not found in the central area without exudates (group C). Our results imply that a retina with hard exudates is in all respects sicker than a retina without exudates, even in areas without fundus pathology. This is also in agreement with former studies, which found hard exudates themselves to be related to visual loss even when other retinal lesions are mild [23, 24].
We found a significant reduction of the amplitudes in eyes with hard exudates only in the middle and outer area. However, in all areas the amplitudes were highest in eyes without exudates, albeit not reaching statistical significance. This is in agreement with a morphological study which found degeneration of photoreceptors due to hard exudates [25].
Fortune et al. (1999) showed that the amplitude is not associated with diabetes retinopathy in the same way as implicit time. One reason for this might be the higher inter-subject variability of local mfERG amplitude among control subjects [26, 22].
Macular thickness assessed with OCT did not differ between areas with or without hard exudates. Thus, macular thickness could not have influenced the mfERG results, which is in agreement with ETDRS report no 22, which describes hard exudates as being independently associated with the risk of visual loss even after adjusting for the extent of macular edema [4].
In the present study, patients with eyes with hard exudates had a shorter duration. Recent HbA1c did not differ between the groups, but it has been shown that metabolic control years earlier determines the degree of complications later [27, 28]. This could be an alternative explanation to the functional differences between the group with and the group without hard exudates. Another difficulty is that the onset of diabetes and the date of diagnosis may differ. In former studies [19, 20] where a predictive model for development of diabetic retinopathy was made, retinopathy level at baseline was shown to be a strong predictor for the development of diabetic retinopathy, and we had minor information about the retinopathy grade at baseline. Another explanation is that this could be an accidental significance because of the limited number of patients, and could therefore not be further evaluated.
Hard exudates, which consist of extra vascular lipoproteins, and are most typically located between the small retinal vessels in the outer retina [22], might spread to the subretinal space when long-standing [29]. Although we could not determine the exact duration of the macular oedema, no oedema had a longer duration than 6 months. Therefore, it is not likely that the hard exudates had moved subretinally.
In the present study, the hard exudates could be located anywhere within the central, middle, or outer area, and it may be argued that the mean recordings from the mfERG areas corresponding to the OCT rings did not reflect the hard exudates in a proper way. However, when analyzing the temporal area we found that eyes presenting hard exudates in the temporal area had a prolonged implicit time compared to eyes without exudates in that specific location. The OCT areas did not correspond perfectly well to the mfERG areas, which might influence the results,. However, we found statistical difference between Group A (eyes with exudates in the analyzed area) and Group C (no exudates) in all five areas.
We only recorded the HbA1c. Klemp et al. [30] have shown that blood glucose may influence the mfERG result. However, Klemp's study included only diabetic patients without retinopathy. This effect has not been demonstrated in diabetics with retinopathy, the group selected here.
The results indicate that the occurrence of hard exudates in the macular region, even at a distance to the fovea, has a deleterious effect on macular function.
The influence of peripherally located hard exudates on central foveal function might extend the indications for laser treatment of diabetic macular edema, which according to the ETDRS is retinal thickening with or without hard exudates within 500 micron [13].
We need further research, including more patients, making it possible to study the influence of hard exudates on macular function.
References
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular oedema: ETDRS report number 1. Arch Ophthalmol 103:1796–1806
Toussaint D, Cogan DG, Kuwabarat T (1962) Extra vascular lesions of diabetic retinopathy. Arch Opthhalmol 67:42–47
Early Treatment Diabetic Retinopathy Study Research Group (1997) Sub retinal fibrosis in diabetic macular edema. ETDRS report No 23. Arch Ophthalmol 115:873–877
Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D (1996) Association of elevated serum lipid levels with retinal hard exudates in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 114:1079–1084
King RC, Dobree JH, Kok D, Foulds WS, Dangerfield WG (1963) Exudative diabetic retinopathy: spontaneous changes and effects of a corn oil diet. Br J Ophthalmol 47:666–672
Lövestam-Adrian M, Agardh E (2000) Photocoagulation of diabetic macular edema-complications and visual outcome. Acta Ophthalmol Scand 78:667–671
Larsson J, Kifley A, Zhu M, Wang JJ, Mitchell P, Sutter FK, Gillies MC (2009) Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol 87(3):275–280
Möller E, Bek T (2003) The relation between visual acuity, fixation stability, and the size and location of foveal hard exudates after photocoagulation for diabetic maculopathy. Graefes Arch Clin Exp Ophthalmol 241:458–462
Rohrschneider K, Bültmann S, Glück R, Kruse FE, Fendrich T, Völcker HE (2000) Scanning laser ophthalmoscope fundus perimetry before laser photocoagulation for clinical significant diabetic macular oedema. Am J Ophthalmol 129:27–32
Sutter E, Tran D (1992) The field topography of ERG components in man—I. The photopic luminance response. Vision Res 32:433–446
Bearse MA, Sutter EE (1996) Imaging localized retinal dysfunction with the multifocal electreoretinogram. J Opt Soc Am Association 13:634–640
Greenstein VC, Holopigian K, Hood DC, Seiple W, Carr RE (2000) The nature and extent of retinal dysfunction associated with diabetic macular oedema. Invest Ophthalmol Vis Sci 41:3643–3654
Early Treatment Diabetic Retinopathy Study Research Group (1991) Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology 98:766–785
Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y, International Society for Clinical Electrophysiology of Vision (2003) Guidelines for basic multifocal electroretinography (mfERG). Doc Ophthalmol 106:105–115
Holm K, Larsson J, Lovestam-Adrian M (2007) In diabetic retinopathy, foveal thickness of 300 µm seems to correlate with functionally significant loss of vision. Doc Ophthalmol 114(3):117–124
Bearse MA, Jr AJ, Adams YH, Schneck ME, Ng J, Bronson-Castain K, Barez S (2006) A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res 25:425–448
Hee MR, Puliafito CA, Duker JS, Reichel E, Coker JG, Wilkins JR, Schuman JS, Swanson EA, Fujimoto JG (1998) Topography of diabetic macular edema with optical coherence tomography. Ophthalmology 105:360–370
Otani T, Kishi S, Maruyama Y (1999) Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmology 127:688–693
NG JS, Bearse MA Jr, Schneck ME, Barez S, Adams AJ (2008) Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci 49(4):1622–1628
Han Y, Schneck ME, Bearse MA Jr, Barez S, Jacobsen CH, Jewell NP, Adams AJ (2004) Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci 45(11):4106–4112
Hood DC, Frishman LJ, Saszik S, Viswanathan S (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43:1673–1685
Cusick M, Chew EY, Chan CC, Kruth HS, Murphy RP, Ferris FL 3rd (2003) Histopathology and regression of retinal hard exudates in diabetic retinopathy after reduction of elevated serum lipid levels. Ophthalmology 11:2126–2133
Aiello LM, Rand LI, Briones JC, Wafai MZ, Sebestyen JG (1981) Diabetic retinopathy in Joslin Clinic patients with adult-onset diabetes. Ophthalmology 88:619–623
Klein R, Klein BE, Moss SE (1984) Visual impairment in diabetes. Ophthalmology 91:1–9
Bloodworth JMB (1962) Diabetic retinopathy. Diabetes 11:1–22
Fortune B, Schneck ME, Adams AJ (1999) Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci 40:2638–2651
Lind M, Odén A, Fahlén M, Eliasson B (2009) The true value of HbA1c as a predictor of diabetic complications: simulations of HbA1c variables. PLoS One 4(2):e4412
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589
Takagi H, Otani A, Kiryu J, Ogura Y (1999) New surgical approach for removing massive foveal hard exudates in diabetic macular edema. Ophthalmology 106:249–257
Klemp K, Larsen M, Sander B, Vaag A, Brockhoff PB, Lund-Andersen H (2004) Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci 45:3812–3819
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holm, K., Ponjavic, V. & Lövestam-Adrian, M. Using multifocal electroretinography hard exudates affect macular function in eyes with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 248, 1241–1247 (2010). https://doi.org/10.1007/s00417-010-1347-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1347-4