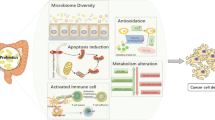
Abstract
We review the most recent data generated by studies in animal models and clinical trials on the role of probiotics in preventing colorectal cancer and the mechanisms proposed. Reduction of colonic carcinogenesis has been attributed to controlling colorectal neoplastic progression via an increased proportion of bacteria with proinflammatory characteristics. Studies in humans have examined the effect of oral administration of yogurt supplemented with probiotics on intestinal microbiota associated with colorectal cancer. A significant decrease in these cells was reported in the probiotic treatment group but not in the milk control group, implying the potential of probiotics for eliminating microbiota associated with colorectal cancer. An intervention study undertaken in the pouches of patients with familial adenomatous polyposis showed decreased cell proliferation and increased detoxification capacity after treatment with probiotics and sulindac/inulin. This mechanism and others were demonstrated experimentally in animals using a rat colon cancer model to examine colorectal tumorigenesis and DNA damage. In the future, with growing understanding of the human microbiome, probiotics may serve as chemoprotective agents for the prevention of colorectal cancer. However, more clinical trials in humans are needed to assess their protective effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third commonest cancer in men and the second commonest in women worldwide. There are estimated to be about 608,000 deaths from CRC annually, accounting for 8 % of all cancer deaths, making CRC the fourth commonest cause of death from cancer [1, 2]. However its cause is still uncertain. CRC has been shown to be strongly associated with certain hereditary gene mutations, but only 3-5 % of CRCs are due to these mutations alone [3, 4]. CRC is a major cause of morbidity and mortality in Western countries, resulting in a substantial economic burden to the population due to surgery, chemotherapy, and terminal care costs [5]. Intestinal bacteria may play a significant role in the disease process by producing possible carcinogens and/or promoters of CRC [6]. However, not all intestinal bacteria are deleterious; some are capable of competitive inhibition of carcinogen and mutagen formation, alteration of overall metabolism, or absorption and removal of toxic/mutagenic metabolites [7].
Probiotics are live microorganisms used as dietary supplements that can alter the balance of intestinal microbiota [8]. Among the colonic bacteria, bifidobacteria and lactobacilli are thought to generate beneficial effects on the human host [9]. The precise mechanisms by which probiotics inhibit CRC may involve multiple pathways, including anti-inflammatory and immune modulation properties, reduction of initial cancer cell growth, and a decrease in expression of toxic enzymes, apoptosis, and modification of the composition of the intestinal microbiota. In animals, probiotic ingestion was shown to prevent carcinogen-induced colonic cancer models [10]. In humans, a very recent intervention study [11•] among patients with familial adenomatous polyposis (FAP) with high risk of CRC found that ingestion of the commercially available probiotic formulation known as VSL#3 with inulin or with sulindac/inulin decreased (albeit with no statistical significance) cell proliferation and that detoxification capacity was increased after treatment with sulindac or VSL#3/inulin. However, combining both regimens did not generate an additive effect.
It has been reported that enterotoxigenic Bacteroides fragilis (ETBF) colonization in humans is associated with CRC [12–17]. In a recent open, randomized, parallel-group human study, subjects were requested to ingest yogurt supplemented with a probiotic strain, Bifidobacterium longum BB536. Milk was given to the control group [18••]. The cell numbers of ETBF were measured by a quantitative PCR method. Compared with baseline values, a significant decrease in the cell numbers of ETBF was reported in the probiotic group but not in the milk group. These results indicate the potential effect of probiotic yogurt in eliminating ETBF colonization found to be associated with CRC.
Hitherto, the hypothesis that probiotics may prevent CRC in humans is based mainly on experimental data. Within this context, it is postulated that the protective effects of probiotics may be due to the mechanisms described in this review and shown in Table 1.
Inflammatory and Immune Modulatory Mechanisms
Inflammatory bowel diseases, including ulcerative colitis and Crohn’s disease, have been linked to CRC pathogenesis [19]. Marnett [20] suggested that chronic inflammation is a key predisposing factor of CRC in inflammatory bowel diseases. A commercially available probiotic formulation known as VSL#3 is composed of four strains of lactobacilli (Lactobacillus casei, L. plantarum, L. bulgaricus, and L. acidophilus), three strains of bifidobacteria (B. longum, B. breve, and B. infantis), and Streptococcus thermophilus [21]. The anti-inflammatory properties attributed to VSL#3 have been linked to changes in the microflora and local immune responses in both humans and animals [22, 23]. Several animal studies and the use of CRC cell lines have demonstrated the potential effectiveness of administering probiotics to prevent neoplastic changes [24].
Recently, Appleyard et al. [25] investigated the protective efficacy of probiotic VSL#3 in a colitis-associated CRC model. Chronic colitis was induced in rats by administering trinitrobenzene sulfonic acid. One group received VSL#3 in their drinking water from 1 week prior to colitis induction until they were killed. The colons were examined for damage and the presence of dysplasia or cancer. None of the probiotic-treated rats developed carcinoma and no high-grade dysplasia was found in either the proximal or the mid colon. In contrast, 29 % of the rats in the control group developed carcinoma in one or more regions of the colon. In this study, Appleyard et al. showed that pretreatment with probiotic VSL#3 can attenuate various inflammatory-associated parameters, thus delaying transition to dysplasia and cancer, and offering support for its potential therapeutic use in patients with long-standing colitis [26].
Probiotic bacteria produce immunoregulatory metabolites in vitro, such as conjugated linoleic acid (CLA), a polyunsaturated fatty acid with potent anticarcinogenic effects. CLA-producing bacterial strains can be found in VSL#3 [27]. In a very recent study, Bassaganya-Riera et al. [28•] investigated the ability of VSL#3 bacteria to modulate mucosal immune responses and thereby ameliorate colonic carcinogenesis in mouse models of inflammation-driven CRC. In mice treated with CLA or VSL#3, adenoma and adenocarcinoma formation was diminished by both treatments. VSL#3 increased the messenger RNA (mRNA) expression of TNF-α, angiostatin, and peroxisome-proliferator-activated receptor γ, whereas CLA decreased cyclooxygenase 2 levels. VSL#3-treated mice had increased IL-17 expression in mesenteric lymph node (MLN) CD4+ T cells, accumulation of lamina propria regulatory T cells and CD4+ memory T cells. Both CLA and VSL#3 suppressed colon carcinogenesis, although VSL#3 showed greater anticarcinogenic and antiinflammatory activities than CLA. Mechanistically, CLA modulated expression of cyclooxygenase 2 levels in the colonic mucosa, whereas VSL#3 targeted mucosal CD4+ regulatory T cell responses [29].
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant disease characterized by the progressive development of hundreds to thousands of adenomatous polyps in the colon, usually appearing during the second or third decade of life [30]. The treatment of choice to eliminate the risk of CRC in patients with FAP is a surgical prophylactic (procto) colectomy with an ileorectal anastomosis or ileal pouch anal anastomosis [31].
An intervention study in humans in which both probiotics and prebiotics were used was recently performed among 17 patients with FAP [11•]. In this single-center human study on patients with FAP, a 4-week intervention with (1) sulindac, (2) inulin/VSL#3, and (3) sulindac/inulin/VSL#3 was performed in order to define future chemoprevention strategies for treatment of colon adenomas or carcinomas with VSL#3. Prebiotics such as inulin are nondigestible carbohydrate compounds that induce beneficial changes, both in the composition and in the activity in the gastrointestinal microflora [32]. Primary end points were risk parameter cell proliferation and glutathione S-transferase (GST) detoxification capacity in the pouch mucosa; secondary end points were the short-chain fatty acid contents, pH, and cytotoxicity of fecal water. Cell proliferation was lower after treatment with sulindac or VSL#3/inulin; the combination of sulindac/inulin/VSL#3 showed the opposite effect. Glutathione S-transferase activity increased after treatment with sulindac or VSL#3/inulin; the combination treatment showed the opposite effect.
Nonsignificant decreased cell proliferation and increased detoxification capacity after treatment with sulindac or VSL#3/inulin was demonstrated, however, combining both regimens did not generate an additive effect, even in our center, which is specialized in hereditary CRC [11•]. Since FAP is a rare disorder, the main weakness of this study is the small number of patients included in a single-center fashion. More, multicenter studies are needed to confirm these results.
Reduction of Initial Colon Cancer Cell Growth
1,2-Dimethylhydrazine (DMH) [33] induces CRC in rats and is a valuable tool in investigating the relationship between aberrant crypt foci and CRC. It is also used to evaluate agents with potential chemopreventive properties prior to preclinical studies [34].
Recently, experimental approaches have employed these biomarkers to test the effect of probiotics on the early development of colon cancer. Chang et al. [35] orally administered L. acidophilus KFRI342 to F344 rats over a 10-week period to determine its effect on the growth of pathogenic bacteria and blood biochemical parameters. In addition, the effect of this L. acidophilus strain on the early development of cancer was examined by studying the formation of aberrant crypt foci in rats into which DMH had been injected. Oral administration of L. acidophilus KFRI342 inhibited the development of preneoplastic lesions and lowered the microbiota populations of both Escherichia coli and aerobic bacteria, which have been associated with carcinogenesis [36]. Treatment with L. acidophilus KFRI342 significantly decreased the number of E. coli bacteria in fecal samples and also decreased the ratio of the incidence of aberrant crypts to the incidence of aberrant crypt foci and the number of aberrant crypts in rats that received a high-fat diet containing the carcinogen and L. acidophilus KFRI342. The authors found that L. acidophilus demonstrated potential probiotic activity as an inhibitor of DMH-induced symptoms in live rats. In vivo studies have also indicated that L. acidophilus from kimchi may be a suitable probiotic for humans [35].
Foo et al. [37••] recently investigated the possibility of probiotics modulating immunity and the inhibition of colon carcinogenesis by evaluating the effect of B. longum and Lactobacillus gasseri on the development of DMH-induced colonic precancerous lesions and tumors in 70 male mice. Their results suggest that dietary consumption of B. longum and L. gasseri significantly inhibits DMH-induced aberrant crypt foci formation. Long-term (24-week) dietary consumption of probiotics resulted in a reduction of colon tumor multiplicity and the size of the tumors. This study showed that administration of B. longum and L. gasseri suppressed the rate of colonic mucosa cellular proliferation by correlating the inhibition of tumor induction with DMH [37••]. These findings correspond to the earlier model investigated in 1996 by Pool-Zobel et al. [38], who found that L. gasseri could inhibit the genotoxic effect of DMH.
Decreasing Expression of Cytochromes P450
Several studies have shown that the intake of probiotics can influence enzyme activities and can be linked with the risk of colon carcinogenesis. CYP1A1 and CYP2E1 are liver enzymes suspected as CRC risk factors [39, 40], whereas CYP3A9 is responsible for metabolizing toxins such as pyrrolizidine alkaloids [41]. Matuskova et al. [42] dissected sections of the duodenum, jejunum, ileum, cecum, and colon of 45 male Wistar rats and were able to monitor the expression of selected cytochromes P450 (CYP) determined by Western blotting and mRNA level using the real-time PCR method. In this recent study, the application of L. casei decreased the expression of the CYP1A1 enzyme in the proximal section of the jejunum and colon. CYP1A1 mRNA levels were decreased in the distal section of the jejunum, ileum, and cecum. Similarly, after L. casei treatment, decreased expression of cecal CYP2E1 mRNA and duodenal CYP3A mRNA was found. These results show that the L. casei probiotic might contribute to preventing CRC by decreasing levels of certain forms of xenobiotic-metabolizing enzymes. In general, there is a possibility of interactions with concomitantly taken pharmacotherapeutic agents.
Similar results have also been reported by Matuskova et al. [43], who employed the live probiotic E. coli Nissle 1917 O6:K5:H1 (EcN) to test the expression of CYP in rat intestines daily for 7 days. The rat control group was stressed by oral application of a saline solution daily for 7 days as well. Sections of the duodenum, jejunum, ileum, cecum, and colon were removed from each experimental rat. Changes in the expression of CYP enzymes along the intestine were found. CYP1A1, CYP2B1/2 and CYP2E1 were present mainly in the duodenum and jejunum, CYP2C6 was expressed mainly in the cecum and colon, whereas CYP3A was found throughout the rat intestine. The results of this in vivo study showed a significant increase in the expression of CYP3A after the rats had been treated with E. coli Nissle 1917 O6:K5:H1. However, human studies are needed to test its effect on the colon.
Enhancing Apoptosis in Colon Tumor Cells
Probiotics may retard colon carcinogenesis by stimulating tumor cell apoptosis. Apoptosis is an active cellular process in which individual cells are triggered for self-destruction. It has been well documented that tumor cell apoptosis blocks tumor progression [44]. To determine whether probiotics influence colon carcinogenesis, Chen et al. [45] used a CT-26 colon carcinoma animal model. The CT-26 cells were N-nitroso-N-methylurethane-induced murine colon adenocarcinoma cells derived from BALB/cByJ mice. CT-26 cells are ideal for a colon cancer model both in vivo and in vitro [46, 47]. In this study, mice were preinoculated with the probiotic L. acidophilus NCFM for 14 days. Subcutaneous dorsal-flank tumors and segmental orthotopic colon cancers were implanted into mice using CT-26 murine colon adenocarcinoma cells. On day 28 after tumor initiation, the lamina propria of the colon, MLNs, and spleen were harvested and purified for flow cytometry and mRNA analyses. L. acidophilus NCFM preinoculation reduced tumor volume growth by more than half compared with untreated mice 28 days after tumor implants [45]. Inoculation with L. acidophilus NCFM reduced the severity of colonic carcinogenesis caused by CT-26 cells, such as the level of colonic involvement and structural abnormality of epithelial/crypt damage. L. acidophilus NCFM enhanced apoptosis of CT-26 cells both in dorsal-flank tumors and in segmental orthotopic CRC compared with untreated mice [45]. L. acidophilus NCFM preinoculation significantly downregulated the CXCR4 mRNA expressions in the colon, MLNs, and extraintestinal tissue compared with untreated mice. These findings suggest that the probiotic L. acidophilus NCFM may play a role in attenuating tumor growth during CT-26 cell carcinogenesis. The downregulated expression of CXCR4 mRNA and MHC class I, as well as increasing apoptosis in tumor tissue, indicates that L. acidophilus NCFM may be associated with modulating the cellular response triggered by colon carcinogenesis [45].
Modification of Carcinogenic Intestinal Microflora
One of the most important pathways for controlling colorectal neoplastic progression is altered composition of colonic microbiota, with an increased proportion of bacteria with proinflammatory characteristics. Probiotics are recognized modifiers of the numbers and types of intestinal microbes and have been reported to reduce CRC experimentally. It has been suggested that ETBF colonization is associated with CRC. Toprak et al. [12] isolated fecal ETBF from 38 % of 56 Turkish patients with CRC and from only 12 % of 40 sex- and age-matched concurrent controls. Studies at Johns Hopkins University demonstrated that ETBF secretes a 20-kDa zinc-dependent metalloprotease toxin (B. fragilis toxin, BFT) that stimulates the proliferation and migration of human colon cancer cells in vitro [13] and greatly induces colonic tumors in multiple intestinal neoplasia mice [14]. BFT is known to bind to colonic epithelial cells and stimulate E-cadherin cleavage, thus increasing intestinal barrier permeability. BFT has been reported to induce colon IL-17 via pathways dependent on STAT3 and T helper type 17 cells in inflammation-induced cancer. Three isomers of BFT have been identified—BFT-1, BFT-2, and BFT-3 [15–17]—which differ in their ability to induce colon IL-17 and promote colon tumors.
Odamaki et al. [18••] very recently studied the efficacy of a probiotic bifidobacterial strain, B. longum BB536, in yogurt on the cell numbers of ETBF. In this study, fecal samples from 420 adult volunteers (151 men, 269 women) were analyzed for ETBF. Thirty-eight subjects were found to be ETBF carriers (prevalence of approximately 9 %). Among them, 32 were enrolled in an open, randomized, parallel-group study for 8 weeks to ingest yogurt supplemented with the probiotic strain B. longum BB536 [18••]. Milk was given to the control group (milk group). The cell numbers of ETBF and the dominant species of the B. fragilis group were measured by a quantitative PCR method. Compared with the baseline values, a significant decrease in the cell numbers of ETBF at week 8 in the group that received yogurt supplemented with B. longum BB536 was observed. However, in the milk group, no decrease was noted [18••]. Linear mixed model analysis for longitudinal data revealed a significant difference in the changes of ETBF cell numbers between the two groups during the intervention phase. These results imply that the potential of probiotic yogurt in eliminating ETBF colonization associated with CRC [18••].
Recently, Vannucci et al. [48], implied that chronic inflammation in response to adverse bacterial flora promotes a neoplastic progression, thus causing CRC. It was found that during inflammation in ulcerative colitis patients, the amount of different members of the family Enterobacteriaceae and different Clostridium species increased in parallel with a decrease in the amount of bifidobacteria and lactobacilli [49, 50]. This change leads to an imbalance between potentially beneficial and adverse bacteria and can possibly contribute to the pathogenesis of CRC.
Recently, Hakansson et al. [51••] evaluated the influence of probiotics on colorectal carcinogenesis and subsequent liver damage by combining blueberry husks with the probiotics. Colorectal tumors were induced in rats by a cyclic treatment of dextran sulfate sodium. A mixture of three probiotic strains (B. infantis DSM 15159, L. gasseri, DSM 16737, and Lactobacillus plantarum DSM 15313) and blueberry husks supplemented a basic diet fortified with oats. Blueberries are rich in phenolics, which have been shown to inhibit CRC and cell proliferation and induce apoptosis in vitro [52]. The probiotic mixture decreased the fecal viable count of Enterobacteriaceae, increased that of lactobacilli, and mitigated hepatic injuries by decreasing parenchyma infiltration and the incidence of stasis and translocation. These results demonstrate a dietary option of combining a probiotic mixture with blueberry husks in order to delay colonic carcinogenesis and hepatic injuries in a rat model.
Conclusion
Probiotics have been shown to deactivate genotoxic carcinogens, thus preventing DNA damage. Chemopreventive systems may be stimulated in vivo in colon tissues. Probiotics can inhibit the inflammatory process by enhancing host immune responses, modifying the intestinal microflora in the colon, and impacting the gut metabolome. Probiotics may have antitumor properties through direct antiproliferative activity on tumor cells. Further experimental studies in animal models and clinical trials in humans are needed to quantify the effect and elucidate the mode of action of probiotics in prophylaxis and treatment of CRC.
References
Paper of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;15:2893–917.
Senter L. Genetic testing by cancer site: colon (nonpolyposis syndromes). Cancer J. 2012;18:334–7.
Kaz AM, Brentnall TA. Genetic testing for colon cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:670–9.
Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–424.
Rowland I. Gut microflora and cancer. In: Leeds AR, Rowland IR, editors. Gut flora and health-past, present and future. London: Royal Society of Medicine; 1996. p. 19–25.
De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res. 2011;55:46–57.
Orrhage K, Nord CE. Bifidobacteria and lactobacilli in human health. Drugs Exp Clin Res. 2000;26:95–111.
Fernández L, Langa S, Martín V, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2012. doi:10.1016/j.phrs.2012.09.001.
Pool-Zobel BL, Neudecke C, Domizlaff I, et al. Lactobacillus- and Bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26:365–80.
• Friederich P, Verschuur J, van Heumen BW, et al. Effects of intervention with sulindac and inulin/VSL#3 on mucosal and luminal factors in the pouch of patients with familial adenomatous polyposis. Int J Colorectal Dis. 2011;26:575–82. This intervention study in patients with FAP showed decreased cell proliferation and increased detoxification capacity (albeit not statistical significant) after treatment with sulindac or VSL#3/inulin; combining both regimens did not show an additional effect on carcinogenesis.
Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–6.
Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392–400.
Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22.
Chung GT, Franco AA, Wu S, et al. Identification of a third metalloprotease toxin gene in extraintestinal isolates of Bacteroides fragilis. Infect Immun. 1999;67:4945–9.
Franco AA, Mundy LM, Trucksis M, Wu S, Kaper JB, Sears CL. Cloning and characterization of the Bacteroides fragilis metalloprotease toxin gene. Infect Immun. 1997;65:1007–13.
Kato N, Liu CX, Kato H, et al. A new subtype of the metalloprotease toxin gene and the incidence of the three bft subtypes among Bacteroides fragilis isolates in Japan. FEMS Microbiol Lett. 2000;182:171–6.
•• Odamaki T, Sugahara H, Yonezawa S. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012;18:14–8. This is a well-designed open, randomized, parallel-group human study indicating the potential effect of probiotic yogurt in eliminating ETBF colonization found to be associated with CRC.
Pozza A, Scarpa M, Ruffolo C, et al. Colonic carcinogenesis in IBD: Molecular events. Ann Ital Chir. 2011;82:19–28.
Marnett LJ. Inflammation and cancer: chemical approaches to mechanisms, imaging, and treatment. J Org Chem. 2012;77:5224–38.
Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–46.
Ng SC, Plamondon S, Kamm MA, et al. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis. Inflamm Bowel Dis. 2010;16:1286–98.
Wang X, O'Gorman MR, Bu HF, Koti V, Zuo XL, Tan XD. Probiotic preparation VSL#3 alters the distribution and phenotypes of dendritic cells within the intestinal mucosa in C57BL/10 J mice. J Nutr. 2009;139:1595–602.
Rafter J. The effects of probiotics on colon cancer development. Nutr Res Rev. 2004;17:277–84.
Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1004–13.
Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–9.
Hofmanová J, Ciganek M, Slavík J, et al. Lipid alterations in human colon epithelial cells induced to differentiation and/or apoptosis by butyrate and polyunsaturated fatty acids. J Nutr Biochem. 2012;23:539–48.
• Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Hontecillas R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 2012;7:e34676. This study describes the effect of combining probiotics and polyunsaturated fatty acid in diminishing adenoma and adenocarcinoma formation.
Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol. 2010;3:209–12.
Bulow S, Alm T, Fausa O, Hultcrantz R, Jarvinen H, Vasen H. Duodenal adenomatosis in familial adenomatous polyposis: DAF profect group. Int J Colorectal Dis. 1995;10:43–6.
Vasen HFA, Duijvendijk P, Buskens E, et al. Decision analysis in the surgical treatment of patients with familial adenomatous polyposis: A Dutch-Scandinavian collaborative study including 659 patients. Gut. 2001;49:231–5.
Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137 Suppl 2:S830–7.
Demarzo MM, Garcia SB. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004;216:31–4.
Wargovich MJ, Chen CD, Harris C, Yang E, Velasco M. Inhibition of aberrant crypt growth by non-steroidal anti-inflammatory agents and differentiation agents in the rat colon. Int J Cancer. 1995;60:515–19.
Chang JH, Shim YY, Cha SK, Reaney MJ, Chee KM. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J Med Microbiol. 2012;61:361–8.
Chang J-H, Shim YY, Cha S-K, et al. Probiotic characteristics of lactic acid bacteria isolated from kimchi. J Appl Microbiol. 2010;109:220–30.
•• Foo NP, Ou Yang H, Chiu HH, Chan HY, Liao CC, Yu CK, Wang YJ. Probiotics prevent the development of 1, 2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through suppressed colonic mucosa cellular proliferation and increased stimulation of macrophages. J Agric Food Chem. 2011;59:13337–45. This provides important information for every cancer researcher showing that dietary consumption of probiotics significantly inhibits initial colon cancer cell growth in a CRC model.
Pool-Zobel BL, Neudecker C, Domizlaff I, et al. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer. 1996;26:365–80.
Jin JQ, Hu YY, Niu YM, et al. CYP1A1 Ile462Val polymorphism contributes to colorectal cancer risk: a meta-analysis. World J Gastroenterol. 2011;17:260–6.
Zhou GW, Hu J, Li Q. CYP2E1 PstI/RsaI polymorphism and colorectal cancer risk: a meta-analysis. World J Gastroenterol. 2010;16:2949–53.
Mei N, Guo L, Liu R, Fuscoe JC, Chen T. Gene expression changes induced by the tumorigenic pyrrolizidine alkaloid riddelliine in liver of Big Blue rats. BMC Bioinforma. 2007;8 Suppl 7:S4.
Matuskova Z, Siller M, Tunkova A, et al. Effects of Lactobacillus casei on the expression and the activity of cytochromes P450 and on the CYP mRNA level in the intestine and the liver of male rats. Int J Immunopathol Pharmacol. 2011;1(Suppl):45S–50S.
Matuskova Z, Tunkova A, Anzenbacherova E, et al. Effects of probiotic Escherichia coli Nissle 1917 on expression of cytochromes P450 along the gastrointestinal tract of male rats. Euro Endocrinol Lett. 2010;31 Suppl 2:46–50.
Butler LM, Hewett PJ, Fitridge RA, et al. Deregulation of apoptosis in colorectal carcinoma: theoretical and therapeutic implications. Aust N Z J Surg. 1999;69:88–94.
Chen CC, Lin WC, Kong MS. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br J Nutr. 2012;107:1623–34.
Plotnikov A, Tichler T, Korenstein R, et al. Involvement of the immune response in the cure of metastatic murine CT-26 colon carcinoma by low electric field-enhanced chemotherapy. Int J Cancer. 2005;117:816–24.
Cho KH, Lee HS, Ku SK, et al. Decrease in intestinal endocrine cells in Balb/c mice with CT-26 carcinoma cells. J Vet Sci. 2008;9:9–14.
Vannucci L, Stepankova R, Grobarova V, et al. Colorectal carcinoma: importance of colonic environment for anti-cancer response and systemic immunity. J Immunotoxicol. 2009;6:217–26.
Mylonaki M, Rayment NB, Rampton DS, Hudspith BN, Brostoff J. Molecular characterization of rectal mucosa-associated bacterial flora in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:481–7.
Bullock NR, Booth JC, Gibson GR. Comparative composition of bacteria in the human intestinal microflora during remission and active ulcerative colitis. Curr Issues Intest Microbiol. 2004;5:59–64.
•• Håkansson A, Bränning C, Molin G, et al. Blueberry husks and probiotics attenuate colorectal inflammation and oncogenesis, and liver injuries in rats exposed to cycling DSS-treatment. PLoS One. 2012;7:e33510. This is a well-designed study relating to dietary options of combining a probiotic mixture with blueberry husks in order to delay colonic carcinogenesis and hepatic injuries in induced colorectal tumors.
Yi WG, Fischer J, Krewer G, Akoh CC. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J Agric Food Chem. 2005;53:7320–9.
Acknowledgments
The authors thank Phyllis Curchack Kornspan for her editorial services. The study was supported in part by the Stanley Steyer Institute for Cancer Epidemiology and Research.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shmuely, H., Domniz, N. & Cohen, D. Probiotics in the Prevention of Colorectal Cancer. Curr Colorectal Cancer Rep 9, 31–36 (2013). https://doi.org/10.1007/s11888-012-0153-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-012-0153-2