Abstract
Purpose of Review
This review will serve to highlight the clinical rationale used in the selection of sodium-glucose cotransporter 2 inhibitors (SGLT2-i) or glucagon-like peptide 1 receptor agonists (GLP1-ra).
Recent Findings
SGLT2-i and GLP1-ra are the first anti-hyperglycemics to demonstrate significant cardiovascular benefit in multiple cardiovascular outcomes trials (CVOTs), with benefits that are consistent across class of medication.
Summary
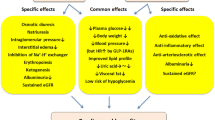
Diabetes is a major risk factor for morbidity and mortality from cardiovascular disease. Sodium-glucose cotransporter 2 inhibitors (SGLT2-i) and glucagon-like peptide 1 receptor agonists (GLP1-ra) are the first anti-hyperglycemics to demonstrate significant cardiovascular benefit. Given the unique side effect and benefit profiles, appropriate consideration of these agents with a focus on cardiovascular risk reduction requires an individualized approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a major cause of cardiovascular morbidity and mortality, both in the USA and globally. Eighty percent of individuals with type 2 diabetes mellitus (DM) will ultimately succumb to death from a cardiovascular cause compared with 30% of the non-diabetic population [1]. Unfortunately, until recently, the role of glycemic control using anti-hyperglycemic medications in prevention of cardiovascular disease (CVD) and macrovascular disease had not shown a clear benefit in preventing these outcomes [2,3,4].
This paradigm was challenged with the EMPA-REG OUTCOMES and LEADER trials, which ushered in a new era of diabetes management, particularly in those at highest risk for cardiovascular disease. The sodium-glucose cotransporter 2 inhibitors (SGLT2-i) and glucagon-like peptide 1 receptor agonists (GLP1-ra) have demonstrated reduction in cardiovascular events and mortality in multiple trials, likely through mechanisms that have little to do with their glucose-lowering effects. These findings have led to an extension in the paradigm of cardiovascular risk reduction with a recognition that diabetes is a metabolic disease exerting its pathologic effects through pathways beyond hyperglycemia. Clinicians should be aware of this evolving approach to care and understand the important role that these novel therapies have in risk reduction for their patients with diabetes.
SGTLT2 Inhibitors
The primary mechanism of action for SGLT-2 inhibitors is the inhibition of the sodium-glucose cotransporter 2 in the kidney, leading to glycosuria and osmotic diuresis (Table 1). There have been a number of cardiovascular outcomes trials (CVOTs) that have demonstrated the benefit of these medications in individuals with diabetes and established CVD or at high risk of CVD (Table 2).
As noted in Table 2, the major cardiovascular benefits of this medication class are in the reduction of cardiovascular mortality and heart failure endpoints. In EMPA-REG OUTCOME (Empagliflozin, Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus), a 38% reduction in cardiovascular mortality, 32% reduction in all-cause mortality, and a 35% reduction in heart failure hospitalizations were observed in those randomized to empagliflozin [5••]. CANVAS (CANagliflozin cardiovascular Assessment Study) also similarly showed a 33% reduction in heart failure hospitalizations in those patients randomized to canagliflozin [6]. These benefits appeared to be present in both individuals with and without heart failure at baseline, although slightly greater benefit was seen in the endpoints of CV death and heart failure hospitalization for those with baseline heart failure [7, 8]. The particular importance of this medication class in heart failure was highlighted in DECLARE-TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events), which had data on left ventricular ejection fraction available [9•]. Dapagliflozin reduced hospitalizations for heart failure in patients with and without reduced ejection fractions, but reduced CV death and all-cause mortality only in those with an ejection fraction < 45%. The implication of this is that the ejection fraction may be an important measure in identifying those who have the greatest benefit of SGLT-2 inhibitors, with a strikingly low number needed to treat of 16 to prevent 1 death in those with heart failure with reduced ejection fraction and type 2 diabetes mellitus. Importantly, this benefit was seen in patients already treated with standard contemporary medications including angiotensin-converting enzyme-inhibitors/angiotensin II receptor blockers, beta-blockers, and diuretics.
An important finding is that benefit appeared early in each of the trials, with the difference in cardiovascular endpoints between the placebo and intervention arms evident within the first few weeks. Possible mechanisms to explain this finding include an osmotic diuresis effect leading to improved cardiac hemodynamics by reduction in left ventricular preload, lowering of body weight due to both calorie and fluid losses, and lowering of blood pressure through reduction in circulating plasma volume [10,11,12]. Heart failure hospitalizations were reduced in the SGLT-2 inhibitor arm for all three trials, likely due to this diuretic effect. Another proposed mechanism of cardiovascular benefit may be a shift in metabolism in myocardial cells via increased ketosis (with a rare but serious adverse event of non-hyperglycemic ketoacidosis). Ketones are a preferred substrate for cardiac tissue, as opposed to free fatty acids [13]. This improvement in myocardial fuel energetics is theorized to translate to CVD benefit. Additionally, SGLT-2 inhibitors are associated with weight reduction early after initiation of treatment, with weight loss generally seen from 1 to 2 kilograms (kg) [14].
Another important finding seen in these studies was the improvement in renal outcomes. Although a benefit in renal outcomes was observed in secondary analyses in the CVOTs mentioned above, the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial was the first to study renal outcomes as a primary outcome in a population at risk for renal failure and end-stage renal disease [15]. Within the group randomized to canagliflozin, a 30% reduction in the primary endpoint of end-stage kidney disease, doubling of serum creatinine, or death from renal or cardiovascular causes was observed. The mechanism for improved renal outcomes is unclear, although is thought to be due to decreases in intraglomerular pressure and hyperfiltration as a consequence of decreased plasma volume [12, 16].
Importantly, these findings have been shown to be consistent in the general population as well, outside of the setting of a randomized controlled trial. The multinational observational study, CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors Study), showed that in 306,156 adults the findings shown in the previously mentioned CVOTs were confirmed, with a lower risk of death in individuals with and without CVD (HR 0.56, 95% CI 0.44–0.70; HR 0.56, 95% CI 0.50–0.63) as well as a reduction in heart failure hospitalizations (HR 0.72, 95% CI 0.63–0.82; HR 0.61, 95% CI 0.48–0.78) [17]. These findings appear to show a class benefit, as benefit was seen with all three SGLT2 inhibitors included in the study (empagliflozin, canagliflozin, and dapagliflozin).
GLP1 Receptor Agonists
GLP-1 receptor agonists exert their anti-hyperglycemic effects through the incretin effect, an effect characterized by potentiating insulin secretion, decreasing postprandial glucagon, delaying gastric emptying, and promoting weight loss (Table 1) [12, 18]. Several trials have shown cardiovascular benefit with this class of medications, including LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcomes Results-A Long-Term Evaluation), SUSTAIN-6 (Semaglutide in Subjects with Type 2 Diabetes), and HARMONY (Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease) (Table 2) [19•, 20, 21].
There are several important considerations within these GLP1-ra trials. The reduction in the primary end point of CV death, non-fatal MI, and non-fatal stroke was driven by significantly lower CV mortality in LEADER. While there was numerical reduction in MI and stroke rates, this did not reach statistical significance. There was composite CV event reduction with semaglutide in SUSTAIN-6 which was driven by a significant reduction in stroke (1.6 vs. 2.7% in placebo) without a reduction in CV mortality. The HARMONY trial did not demonstrate any mortality benefit with albiglutide as compared with placebo, but did show a statistically significant reduction in MI. Neither liraglutide, albiglutide, nor semaglutide had any significant effect on HF hospitalizations, in contrast with the substantial benefit seen with SGLT2 inhibitors. The theorized mechanism of benefit seen with these medications is thought to be an anti-atherothrombotic effect given the endpoints improved are primarily driven by atherosclerosis (MI, stroke) as well as the timing of the benefit seen later in the trials (months to years) in contrast with SGLT-2 inhibitors (weeks). However, given the magnitude of the CV mortality improvement seen in LEADER, a more convincing reduction in MI or stroke would have been expected (neither were significantly reduced in LEADER). Other potential effects like blood pressure lowering, weight reduction, and avoidance of hypoglycemia seen in all 3 trials may be contributory to improved CV outcomes [22].
Strategies for Selection
In patients with diabetes and established CVD or at high risk for CVD, initiation of an SGLT2-I or GLP1-ra should be considered for cardiovascular risk reduction based on available evidence [23••]. The distinct clinical benefit profiles of each class should be considered in the decision-making process for individual patients (Table 3).
Considerations for Using an SGLT2 Inhibitor
As noted in the outcomes trials, the predominant cardiovascular benefit of SGLT2 inhibitors is likely reduction of heart failure events. As evidenced in the DECLARE-TIMI 58 study, diabetic patients with a reduced ejection fraction may have a significant degree of benefit from these medications and an SGLT2 inhibitor should be strongly considered in this patient population. As demonstrated across trials, there has been an improvement in renal outcomes as well with these medications, particularly in those with albuminuria. Other patient-specific considerations (i.e., fear of needles or preference for daily oral medication) can also sway clinicians to using an SGLT2 inhibitor rather than a GLP1 receptor agonist (although the GLP1-ra semaglutide will soon be available orally). Currently, empagliflozin is the only medication among the SGLT2 inhibitors to receive an FDA-approved indication of reducing risk of CV death in adults with type 2 diabetes and CVD.
Contraindications for Using an SGLT2 Inhibitor
There are several important side effects of the SGLT2 inhibitors for the provider and patients to be aware. Amputation is an important but albeit rare adverse event noted with the use of canagliflozin in CANVAS. The exact mechanism of the higher rates of lower limb amputation is unknown but may be due to volume depletion leading to circulatory failure in distal peripheral arterial beds [12, 24]. This has led to an FDA black-box warning to clinicians to avoid using canagliflozin in individuals at risk for amputation (history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers). Out of caution, clinicians should avoid all SGLT2 inhibitors in those at particularly high risk of amputation. In those with risk factors for peripheral arterial disease, a screening ankle-brachial index can be considered prior to initiation of therapy.
A significantly increased rate of genital mycotic infections is seen throughout this class of medications and, although a nuisance to the patient, generally tends to be easily treated with a brief course of anti-fungal agents. However, the occurrence of this adverse event is quite common (~ 5% in males and ~ 10% in females) and may contribute to discontinuation of the medication by patient or provider. Volume depletion, dehydration, and increased urinary tract infections have been associated with these medications and are also likely secondary to the mechanism of action of this class (glucosuria leading to osmotic diuresis) [25]. Additionally, patients may require adjustment of their diuretic regimen to avoid hypotension due to volume depletion after being started on an SGLT2 inhibitor. Also, although these medications have benefit in delaying renal outcomes, they are contraindicated in significant renal disease (GFR < 45 ml/min/1.73 m2) and should be stopped in the context of acute kidney injury.
Euglycemic diabetic ketoacidosis is another rare but serious adverse effect of the medication. Briefly, the mechanism is a result of persistent glycosuria leading to a series of metabolic changes leading to a reduction in insulin production and an increase in glucagon secretion, which in turn stimulates ketogenesis [26]. This potential adverse event presents a challenge diagnostically given the absence of significant hyperglycemia can lead to delayed or missed diagnosis of diabetic ketoacidosis. These medications should not be used in type 1 diabetes or insulin-dependent diabetics with a history of diabetic ketoacidosis.
Canagliflozin was also associated with a small but statistically significant increase in the risk of bone fractures, so use of SGLT-2 inhibitors in those with osteoporosis or at risk of falls should be done with caution.
Considerations for Using GLP-1 Receptor Agonists
GLP-1 receptor agonists should also be considered in diabetic patients with CVD. Given the difference in benefit profile, the patient population that may derive most benefit is likely different than those deriving maximal benefit from SGLT2 inhibitors. Therefore, in individuals without heart failure history but at risk for MI/stroke, GLP1 receptor agonists may be the appropriate option. Additionally, in individuals in whom SGLT2 inhibitors should not be used (prior amputation or ulceration/infection due to peripheral arterial disease), GLP1 receptor agonists should be considered.
These medications have also shown significant reduction in weight loss, greater than what was observed with SLGT-2 inhibitors. For example, liraglutide was associated with a 2.3 kg weight loss as compared with placebo in LEADER. Similarly, albiglutide was associated with a 0.83 kg weight loss greater than placebo and semaglutide was associated with an impressive 2.9 kg reduction in the 0.5 mg arm, with a 4.3 kg body weight reduction in 1.0 mg arm compared with placebo. Given this substantial benefit, these medications should be considered in obese patients for both CV risk reduction and weight loss.
There are currently five GLP-1 agonists that are available for clinical use. Thus far, only liraglutide has been approved by the FDA with an indication for reducing the risk of cardiovascular events in individuals with type 2 diabetes and CVD.
Cautions on the Use of GLP-1 Receptor Agonists
The most common side effects with GLP-1 receptor agonists are gastrointestinal in nature, including diarrhea, nausea, and vomiting. These side effects usually occur early, tend to be transient, and can often be mitigated by gradual dose titration [27]. However, if symptoms do no resolve, patients may not be able to tolerate these medications. Gastrointestinal (GI) complaints were a frequent occurrence in the trials. For example, in SUSTAIN-6, 50% of patients treated with semaglutide had GI complaints, as compared with 35% on placebo. GI complaints were the leading reason for discontinuation of a GLP1-ra, with 3–5% of patients discontinuing GLP1-ra therapy compared with < 1% on placebo. Additionally, as GLP1 receptor agonists delay gastric emptying, caution should be used in patients with prior gastric surgery or known gastroparesis. Although post-marketing case reports have suggested possible associations between GLP1-ra and acute pancreatitis, there was not increased risk of pancreatitis or pancreatic cancer in a large meta-analysis of available placebo-controlled studies [28].
GLP1-ra have been associated with medullary thyroid cancer in rodents, and thus for now should be avoided or used with caution in individuals who have a history of medullary thyroid cancer or multiple endocrine neoplasia 2 [29]. The mechanism of this is thought to be due to stimulation of the GLP-1 receptor in thyroid C cells which, upon longer-term exposure, is accompanied by C cell proliferation and the formation of C cell adenomas and medullary thyroid carcinomas. Although increased risk for thyroid cancer has not been shown in humans, long-term consequences of sustained GLP-1 receptor activation in the human thyroid remain unknown and require further investigation and follow-up.
Additionally, in SUSTAIN-6, semaglutide was associated with a higher rate of worsening diabetic retinopathy (HR 1.76, 95%CI 1.11–2.78). This has been noted with rapid improvement in glycemic control with insulin as well, and thought to be due to worsening of pre-existing diabetic retinopathy associated with these rapid and large changes in glucose, and likely not a complication specific to semaglutide [12, 30].
Given that this class of medications is renally excreted, a GFR < 30 ml/min/1.73 m2 is a contraindication and thus GLP1-ra should be used with caution in individuals with significant renal disease and avoided in those with end-stage renal disease.
Combination Treatment with SGLT-2 Inhibitors and GLP-1 Receptor Agonists
There have been no cardiovascular outcomes trials examining the use of these classes of medications in combination. The DURATION-8 trial did show greater BP and weight reductions with a combination of exenatide and dapagliflozin [31]. The study, however, was not designed to demonstrate a difference in cardiovascular outcomes, although additional data is expected from ongoing investigations to better inform the decision to use these drug classes in combination. There are no obvious contraindications to combining these medications, although there is a clear need for further examination of this question prior to adoption of this practice.
Conclusion: Challenges and Future Directions
Evolving clinical trial data indicate SGLT2 inhibitors and GLP1-ra independently improve CV outcomes and appear to do so via separate mechanisms. Further research is needed to better define the ideal population for these medications. Given the magnitude of benefit noted, there has been interest in exploring the use of these medications for primary prevention, as well as in non-diabetics, as the major effect of these medications appears independent of their glucose-lowering effects. While these new diabetes drugs have ushered in an exciting new era of managing cardiovascular risk, additional trials are needed as the utility and safety of these medications in a larger-scale population is still largely unknown. At present, these medications are powerful tools for reducing CV morbidity and mortality in individuals with diabetes at high risk or with established CVD and should be utilized by clinicians caring for these patients in the absence of contraindications.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cubbon RM, Wheatcroft SB, Grant PJ, Gale CP, Barth JH, Sapsford RJ, et al. Temporal trends in mortality of patients with diabetes mellitus suffering acute myocardial infarction: a comparison of over 3000 patients between 1995 and 2003. Eur Heart J. 2007;28(5):540–5.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group Lancet (London, England) 1998;352(9131):837–53.
Effects of intensive glucose lowering in type 2 diabetes. 2008;358(24):2545–59.
Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. 2008;358(24):2560–72.
•• Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28 This study was the first cardiovascular outcomes trial to show a benefit of an anti-hyperglycemic medication in cardiovascular outcomes. Empagliflozin demonstrated a large and surprising benefit in cardiovascular endpoints as compared with placebo.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. 2017;377(7):644–57.
Fitchett D, investigators tE-ROt, Zinman B, investigators tE-ROt, Wanner C, investigators tE-ROt, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37(19):1526–34.
Rådholm K, Figtree G, Perkovic V, Solomon Scott D, Mahaffey Kenneth W, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018;138(5):458–68.
• Kato Eri T, Silverman Michael G, Mosenzon O, Zelniker Thomas A, Cahn A, Furtado Remo HM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–36. This study showed that the benefit of the SGLT2 inhibitors is particularly evident in heart failure with reduced ejection fraction.
Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119–26.
Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–8.
Dhindsa DS, Sandesara PB, Shapiro MD. The intersection of diabetes and cardiovascular disease—a focus on new therapies. Frontiers in Cardiovascular Medicine. 2018;5:160.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22.
Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–23.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–97.
Kosiborod M, Cavender Matthew A, Fu Alex Z, Wilding John P, Khunti K, Holl Reinhard W, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation. 2017;136(3):249–59.
Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. 2017;3(1):3–14.
• Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–22 This study was the first to show cardiovascular benefit among the GLP1 receptor agonists, showing a reduction in cardiovascular mortality in those randomized to liraglutide as compared with placebo.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29.
Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181–7.
•• Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease. A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200–23. Major statement by the American College of Cardiology urging the consideration and use of SGLT-2 inhibitors and GLP-1 receptor agonists among cardiologists.
Tanaka A, Node K. Increased amputation risk with canagliflozin treatment: behind the large cardiovascular benefit? Cardiovasc Diabetol. 2017;16(1):129.
Trujillo JM, Nuffer WA. Impact of sodium-glucose cotransporter 2 inhibitors on nonglycemic outcomes in patients with type 2 diabetes. Pharmacotherapy. 2017;37(4):481–91.
Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638–42.
Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279–84.
Storgaard H, Cold F, Gluud LL, Vilsboll T, Knop FK. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906–8.
Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–86.
Vilsboll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simo R, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20(4):889–97.
Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Devinder S. Dhindsa, Anurag Mehta, Pratik B. Sandesara, Aneesha Thobani, Stephen Brandt, and Laurence S. Sperling declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Cardiovascular Disease
Rights and permissions
About this article
Cite this article
Dhindsa, D.S., Mehta, A., Sandesara, P.B. et al. Strategies for Appropriate Selection of SGLT2-i vs. GLP1-RA in Persons with Diabetes and Cardiovascular Disease. Curr Cardiol Rep 21, 100 (2019). https://doi.org/10.1007/s11886-019-1197-6
Published:
DOI: https://doi.org/10.1007/s11886-019-1197-6