Abstract
Purpose of the Review
Hyperactivity of sympathetic nervous system (SNS) plays a role in the development of arterial hypertension and heart failure, two co-morbidities frequently associated with type 2 diabetes (T2DM). This review aims at analyzing the effects of sodium-glucose cotransporter type 2 inhibitors (SGLT2is) on blood pressure and more especially on SNS activity in patients with T2DM.
Recent Findings
By enhancing glucosuria, natriuresis, and osmotic diuresis, SGLT2is improve glucose control, promote weight loss, lower arterial blood pressure, and reduce the risk of major cardiovascular events and hospitalization for heart failure. No rise of heart rate is detected despite reductions in blood pressure and plasma volume, which may suggest a dampening of SNS activity. Indeed, increasing experimental and clinical data demonstrated a reduction in SNS activity, including in key target organs such as the heart and the kidneys.
Summary
Of potential major interest, a better understanding of the mechanisms linking SGLT2 and SNS deserves further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
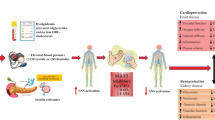
Autonomic nervous system, especially sympathetic nervous system (SNS) hyperactivity, plays a role in the pathophysiology of both arterial hypertension (HTN) [1,2,3] and heart failure (HF) [3,4,5], two comorbidities frequently associated with type 2 diabetes mellitus (T2DM) (Fig. 1) [6, 7]. Obesity plays also a major role in these complications [8, 9]. Overall, almost 80% of patients with T2DM are overweight or obese. Most of them have a so-called metabolic syndrome, i.e., an association of several cardiovascular (CV) risk factors, including elevated blood pressure (BP) [10]. The combination of essential HTN and T2DM resulted in the greatest sympathetic hyperactivity and level of plasma insulin to compensate for insulin resistance, and this hyperactivity could constitute a mechanism for the increased risks of this condition [11, 12].
Sodium-glucose cotransporter type 2 inhibitors (SGLT2is) are new glucose-lowering agents that specifically target the kidney and promote glucosuria, independent of the action of insulin. They improve glucose control, without inducing hypoglycemia, and with lower circulating plasma insulin concentrations. Besides this primary glucose-lowering effect, they also promote weight loss and induce osmotic diuresis and natriuresis [13]. These combined actions result in a significant reduction in arterial BP as shown in several randomized controlled trials (RCTs) whose results were summarized in systematic reviews and meta-analyses [14, 15, 16•, 17•]. Of major clinical importance, SGLT2is also reduce the risk of HF [18]. Several recent cardiovascular outcome trials (CVOTs) reported a reduction in major cardiovascular events (MACEs) and CV mortality and/or in the risk of hospitalization for HF in patients with T2DM and at high risk of CV disease as most patients had established CV disease already. They were treated with an SGLT2i compared with a placebo as added to standard therapy [19,20,21], and most important results have been summarized in a recent meta-analysis [22••]. The precise mechanisms underlying these beneficial effects are complex and remain a matter of discussion. They are most probably multifactorial, combining metabolic, endocrine, hemodynamic, and biochemical effects (Fig. 2) [23,24,25,26]. Furthermore, SGLT2is also modify the intra-renal hemodynamic pattern in hyperglycemic diabetic patients, presumably by restoring tubulo-glomerular feedback, a mechanism that contributes to renoprotective effects [27].
The mechanism of the diuretic effect of SGLT2is is rather different from that of other diuretic compounds [18, 28]. In contrast with classic diuretics that may be associated with increased SNS activity [29, 30]. SGLT2is reduce arterial BP without inducing a significant rise in heart rate, which may suggest an attenuation of the SNS activity (Fig. 2) [31]. Elevated heart rate is considered as an independent CV risk factor [32], including in patients with T2DM [33]. Of note, however, glucagon-like peptide-1 (GLP-1) receptor agonists (especially liraglutide), another class of glucose-lowering agents that recently showed a reduction in MACEs and CV mortality like SGLT2is [34], are associated with a significant rise in heart rate [35], an effect not observed with SGLT2is.
The aims of the present narrative review are to summarize the effects of SGLT2is on BP in T2DM patients and, more specifically, to analyze the effects of SGLT2is on the SNS. To what extent these effects may contribute to the reduction in CV events observed with this pharmacological class remains an open question [36].
Blood Pressure Lowering Effects
Factors Modulating BP-Lowering Effects of SGLT2is
Treatment with SGLT2is is consistently associated with a lowering of arterial BP in T2D patients with or without hypertension [37,38,39]. This effect has been confirmed with all SGLT2is and extensively analyzed in several meta-analyses [14, 15, 16•, 17•]: canagliflozin [40, 41], dapagliflozin [42,43,44], empagliflozin [45, 46], ertugliflozin [47], and ipragliflozin [48]. The contribution of the BP-lowering effect of SGLT2is in the overall cardiovascular [36] and renal [49] protective effects of this pharmacological class remains, however debatable.
Dose-Response
Canagliflozin, but not dapagliflozin or empagliflozin, showed a significant dose-response relationship with systolic BP reduction, canagliflozin 300 mg once daily being more potent than 100 mg once daily [14, 15, 40]. However, the overall difference in BP-lowering effect between the two doses of 25 mg versus 10 mg canagliflozin is rather modest (systolic BP − 5.0 versus − 4.3 mmHg in the overall population and − 14.2 versus − 12.8 mmHg in patients with baseline levels ≥ 140 mmHg) [41]. In T2DM patients with HTN, empagliflozin showed only a sligthly greater reduction in systolic (− 4.16 versus − 3.44 mmHg) and diastolic (− 1.72 versus − 1.36 mmHg) mean 24-h ambulatory BP with the daily dose of 25 mg compared with 10 mg, respectively [45]. In the EMPA-REG OUTCOME CVOT, the reductions observed with the two doses of empagliflozin were almost similar throughout the 3-year follow-up, also resulting in a comparable CV protection [19].
Systolic Versus Diastolic BP
Generally, the SGLT2i-associated reduction in systolic BP was greater (almost twofold) than the reduction in diastolic BP. This difference was observed when BP was controlled in seated position in the investigator office (− 3.96 mmHg for systolic BP versus − 1.59 mmHg for diastolic BP [14] or during 24-h ambulatory monitoring (− 3.76 mmHg for systolic BP versus − 1.83 mmHg for diastolic BP) [17•], as shown in meta-analyses of RCTs that compared the effects of SGLT2is versus placebo or another glucose-lowering agent.
Central BP Measurement
An experimental study compared the effects of three SGLT2is (canagliflozin, empagliflozin, and luseogliflozin) on BP and showed similar reductions in brachial and central BP [50]. In another study in T2DM patients treated with dapagliflozin for 6 weeks, central systolic and diastolic BP values were significantly lower, by 3.0 and 2.2 mmHg, respectively, compared with placebo [51].
24-h Ambulatory Monitoring Versus Office BP Measurement
The BP reduction observed in office conditions [14, 16•] has been confirmed during 24-h ambulatory monitoring with all SGLT2is: canagliflozin [40], dapagliflozin [42, 43], empagliflozin [45, 52] and ertugliflozin [47]. According to a meta-analysis of six RCTs having compared an SGLTT2i with a placebo using 24-h ambulatory BP monitoring [17•], SGLT2is were associated with a significant reduction in both daytime (− 4.34 mmHg, 95% CI − 5.09 to − 3.58) and nighttime (− 2.61 mmHg, 95% CI − 3.08 to − 2.14) BP levels. A similar trend for a slightly lower reduction during nighttime compared with daytime was also observed for diastolic BP. No significant differences were recorded between the various SGLT2is included in this meta-analysis, in agreement with a class effect [17•]. It has been suggested that circadian BP rhythm may represent a possible key target of SGLT2is used for the treatment of T2DM [53].
Baseline High Versus Normal BP
Overall, the average BP reduction reported with SGLT2is may appear rather modest. However, it is noteworthy that most trials with SGLT2is were performed in T2DM patients with well-controlled BP at baseline. Post hoc subgroup analyses have shown that greater BP reduction may be achieved in patients with higher BP levels at baseline (> 140/90 mmHg) [14, 37, 38, 44, 54]. Even in patients with normal BP levels, the risk of orthostatic hypotension when adding an SGLT2i to standard therapy was considered to be low [44].
Background Antihypertensive Therapy
The BP lowering effect was also observed in patients already treated with a combination antihypertensive therapy [43], including renin-angiotensin blockers [55]. However, the antihypertensive effect seems to be less marked when an SGLT2i is added to a diuretic. For instance, the reduction in seated systolic BP was almost twofold lower when dapagliflozin was added to a diuretic agent than when it was added to a beta-blocker or a calcium-channel blocker. Nevertheless, such a difference almost vanished when 24-h ambulatory systolic BP measurements were compared [43]. In another study using 24-h ambulatory BP monitoring in patients with T2DM and HTN, empagliflozin for 12 weeks reduced systolic and diastolic BP versus placebo, irrespective of the number of antihypertensive agents and use of diuretics or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers [56]. The use of thiazide diuretics remains very popular for the treatment for T2DM patients with HTN or even HF [57]. It has been shown that switching from low-dose thiazide diuretics to SGLT2i improves various metabolic parameters without affecting BP in T2DM patients with HTN [58].
Chronic Kidney Disease
Chronic kidney disease (CKD) and HTN are closely related, and CKD is a recognized risk factor for developing or aggravating not only HTN but also HF [59]. Because of their specific mechanism of action, SGLT2is lose part of their glucose-lowering efficacy in patients in CKD when estimated glomerular filtration rate (eGFR) falls < 60 ml/min/1.73 m2 [60]. However, it has been demonstrated that in those patients, dapagliflozin still significantly reduced BP (and body weight), while it did not improve glycemic control [61]. Furthermore, subgroups analyses of CVOTs showed that the CV protection reported with SGLT2is was maintained in T2DM patients with moderate CKD (eGFR 30–60 ml/min/1.73 m2) [22]. These findings have been recently confirmed in CREDENCE [62]. This study compared canagliflozin 100 mg with placebo and, in contrast to previous CVOTs, specifically recruited patients with T2DM and albuminuric CKD. All the patients were treated with renin-angiotensin system blockade and had an eGFR of 30 to < 90 ml/min/1.73 m2 and a ratio of albumin (mg)/creatinine (g) (> 300 to 5000). The relative risk of the primary outcome [composite of end-stage kidney disease (dialysis, transplantation, or a sustained estimated GFR of < 15 ml/min/1.73 m2), a doubling of the serum creatinine level, or death from renal or CV causes] was 30% lower in the canagliflozin group than in the placebo group (hazard ratio 0.70; 95% confidence interval [CI] 0.59 to 0.82; P = 0.00001). The relative risks of the renal-specific composite (excluding CV death) (P < 0.001) and of the major CV events (cardiovascular death, myocardial infarction, or stroke: P = 0.01) were also significantly reduced in the canagliflozin group compared to the placebo group. On average, BP levels were lower in the canagliflozin group, yet the difference was modest (3.3 mmHg for systolic BP and 0.95 mmHg for diastolic BP) [62].
Ethnic Group
In African-Americans with T2DM and HTN (mean BP 146.3/89.4 mmHg), empagliflozin significantly reduced BP and its BP-lowering effect increased from 12 to 24 weeks, suggesting a full antihypertensive effect takes ≥ 6 months to be achieved. At week 24, the placebo-subtracted BP effect was impressive (systolic BP − 8.39 mmHg [95% CI − 13.74, − 3.04; P = 0.0025], similar to standard antihypertensive monotherapies [63]. In a meta-analysis, comparison of the efficacy in SGLT2i treatment between Asian and non-Asian T2DM patients showed no significant difference in BP reduction, similarly to the effects observed regarding HbA1c, body weight, and all-cause mortality [64].
BP-Lowering Mechanisms Related to SGLT2is
Several mechanisms could explain the reduction in BP observed with SGLT2is (Fig. 2) [65,66,67].
Weight Loss
In various studies, including RCTs and real-world observational studies, patients treated with SGLT2is have reported weight loss of around 1 to 3 kg [68]. Although fluid loss may somewhat contribute, dedicated studies having measured body composition showed that most of the weight reduction is explained by loss of fat mass [68]. It is well-known that interventions that cause weight loss have a positive impact on cardiovascular risk factors [69], including improved BP control [70]. However, the rather limited amount of weight loss associated with SGLT2i therapy seems insufficient to markedly influence BP in patients with T2DM. A detailed analysis of a 24-week placebo-controlled RCT concluded that weight loss of 2 kg associated with dapagliflozin contributes to 28% of the overall systolic BP reduction, and 24% of the overall diastolic BP reduction [71].
Natriuresis and Osmotic Diuresis
After the publication of EMPA-REG OUTCOME [19], the role of a natriuretic effect [72] or a diuretic effect [73] of empagliflozin was put forward to explain the favorable CV outcomes with the SGLT2i compared with placebo. It is likely that plasma volume contraction due to natriuresis and osmotic diuresis in response to SGLT2 inhibition is at least in part responsible for the reduction in the risk of HF observed within the first few weeks in all CVOTs. However, the natriuretic/diuretic effects of SGLT2is are quite different from those described with classic diuretics [28]. The effects on natriuresis and diuresis appeared rather modest when properly measured over 5 days after an acute administration of empagliflozin 25 mg [74]. Further, if present, they were generally observed transiently without changing long-term overall fluid balance [75, 76]. Of note, however, the spill-over of glucose and sodium beyond the proximal nephron following SGLT2 inhibition triggers dynamic and reversible realignment of energy metabolism, renal filtration, and plasma volume without relying on losses into the urine. All these processes are observed in the absence of significant glucosuria or ongoing natriuresis [77].
Reduction in Arterial Stiffness
Several studies have reported a significant reduction in arterial stiffness in T2DM patients treated with dapagliflozin [78], canagliflozin [79], or empagliflozin [54]. This effect has been shown to be associated with improved endothelial dysfunction [78]. Both reduction in arterial stiffness and improvement of endothelial function could contribute to lower BP in T2DM patients, especially those with HTN, and possibly to the improvement of CV outcomes.
In patients with type 1 diabetes, deterioration of autonomic nervous function is associated with an increase in arterial stiffness, which, in turn, is associated with, and may cause, increased systolic BP and pulse pressure [80]. In young patients with type 1 diabetes, empagliflozin was associated with a decline in arterial stiffness; however, heart rate variability and circulating adrenergic mediators (plasma norepinephrine and epinephrine levels) remained unchanged under both clamped euglycemic and hyperglycemic conditions [81].
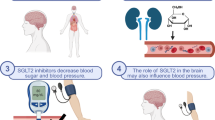
Effects of SGLT2is on Sympathetic Nervous System
Renin-angiotensin system (RAS) plays a major role in regulating BP and body fluids, and RAS blockers are key players in the treatment of both HTN and HF. Available data indicate that treatment with SGLT2is, by causing polyuria and natriuresis, transiently activates the systemic RAS in T2DM, but not the intrarenal RAS [82]. SNS also plays an important role in controlling BP and the pathophysiology of HTN [1, 9]. It is also activated in HF, an effect potentially deleterious [4, 5]. Animal [31] and human [83•] data suggest that SGLT2is are able to reduce SNS hyperactivity.
Intriguingly, experimental in vitro and in vivo studies provide evidence for a cross-talk between the SNS and SGLT2 regulation (Fig. 3) [84••]. Sympathetic nerves innervate the proximal tubules of the kidney where they have been shown to regulate the expression of transporters such as the sodium hydrogen exchanger 3, but also SGLT2 [85].
SGLT2i, Adrenergic Activity, and HTN
Experimental animal data investigated the effects of SGLT2is on BP and SNS activity in salt-treated obese and metabolic syndrome rats, who develop HTN with an abnormal circadian rhythm of BP, a non-dipper type of HTN, and do not exhibit a circadian rhythm of SNS activity. Treatment with SGLT2is significantly decreased BP and normalized circadian rhythms of both BP and SNS activity, but did not change heart rate [31].
Similarly, in patients with T2DM, the reduction in BP induced by SGLT2 inhibitors is not accompanied by a significant increase in heart rate [54]. Treatment with any of the three SGLT2is with the largest experience (canagliflozin, dapagliflozin, and empagliflozin) results in sustained systolic and diastolic BP reduction, in part via minimal natriuresis and possible reductions in sympathetic tone [66]. After 4 days of treatment with empagliflozin in patients with T2DM, no significant changes in muscle sympathetic nerve activity were apparent despite a numerical increase in urine volume, numerical reductions in BP, and significant weight loss. There were no clinically relevant changes in heart rate [83•], confirming previous observations [54]. Thus, empagliflozin is not associated with clinically relevant reflex-mediated sympathetic activation in contrast to increases observed with diuretics in other studies. These human data confirm data from animal models [31] and suggest that SGLT2is can affect autonomic CV regulation [83•].
SGLT2i, Adrenergic Activity, and HF
An elevation of sympathetic activity not only contributes to the development of HTN but also aggravates HF [4, 5]. The reduction by SGLT2is in sympathetic overactivity seen in patients with HF may contribute to the reduction in hospitalization for HF consistently reported with SGLT2is [86••]. In an elderly patient with severe refractory HF, ipragliflozin treatment improved cardiac sympathetic nerve activity evaluated with (123)I-metaiodobenzylguanidine cardiac-scintigraphy, an effect that might be one of the mechanisms of the cardio-protection reported with SGLT2is [87]. These observations are of interest as a working hypothesis postulates that DPP-4 inhibitors (saxagliptin) may increase the risk of HF events by activating the SNS to stimulate cardiomyocyte cell death as recently discussed considering findings from both experimental studies and clinical trials [71].
SGLT2i, Adrenergic Activity, and Sudden Death
Cardiac sympathetic hyperactivity is related to poor prognosis and fatal arrhythmias, especially in patients with coronary insufficiency. SGLT2is potentially reduce SNS activity that is augmented in part due to the stimulatory effect of hyperglycemia. In EMPA-REG OUTCOME, empagliflozin was associated with a reduction in sudden death [19]. The precise reason for this effect remains unknown, yet a potential contribution of reduced malignant arrhythmias is likely. Cardiac autonomic neuropathy is a frequent complication of diabetes mellitus, and diabetic patients are at higher risk for developing arrhythmias and sudden cardiac death [88]. Higher sympathetic tone and lower parasympathetic tone promote fatal arrhythmias by multiple mechanisms including reduction of ventricular refractory period and ventricular fibrillation threshold, thereby promoting triggered activity and automaticity [89]. Whether a reduction in SNS tone by SGLT2is may contribute to reduce sudden death in patients with established CV disease remains an open question. The ongoing EMBODY placebo-controlled trial carried out in Japan is designed to determine whether the suppression of cardiac sympathetic activity induced by SGLT2 inhibition is accompanied by protection against adverse CV outcomes. Sudden cardiac death surrogate markers will be assessed after 24 weeks of empagliflozin therapy such as heart variability, heart rate turbulence, T-wave alternans, late potentials, and (123)I-MIBG scintigraphy imaging [90].
SGLT2i, Adrenergic Activity, and Microangiopathy
Microangiopathy (nephropathy, retinopathy) and macroangiopathy (coronary artery disease, stroke, peripheral artery disease) should not be viewed as entirely separate entities, but rather as a continuum of the widespread vascular damage determined by diabetes mellitus [51, 91]. Increasing evidence suggests that overactive SNS could play a role not only in CV disease but also in diabetes-associated CKD and retinopathy, yet the underlying mechanisms appear multifactorial [92]. For instance, there is the complex interaction between RAS activation, vascular reactive oxygen species (ROS) generation, and increased sympathetic outflow in HTN, especially when associated to T2DM [93].
Postganglionic sympathetic fibers innervate renal vasculature, tubules, and juxtaglomerular apparatus. Renal sympathetic activation in juxtaglomerular apparatus induces renin release and activates the RAS system, which further leads to sodium reabsorption and fluid retention, thereby contributing to HTN and HF [59]. Furthermore, local sympathetic hyperactivity in the kidney may induce proteinuria, glomerulosclerosis, and finally renal fibrosis, through the activation of proinflammatory/profibrotic markers [94]. It has been suggested that the SNS hyperactivity [92] is one of the potential mechanisms involved in the development not only of diabetic cardiomyopathy but also of chronic renal dysfunction associated with diabetes [59].
Hypoxia and oxidative stress contribute to the development of diabetic retinopathy [95]. Sympathetic activation in diabetic patients may lead to peripheral vasoconstrictor responses and be associated with rheological disorders, thereby leading to deleterious hypoxia [96]. As SGLT2is may downregulate the SNS activity in the heart and kidneys [84••], it is plausible that SGLT2 inhibition may also alleviate detrimental retinal changes that may be underpinned by local hyperactivation of the SNS [97]. In a study that evaluated changes in retinal capillary flow and arteriole remodeling using scanning laser Doppler flowmetry, dapagliflozin for 6 weeks improved parameters associated with the early stages of vascular remodeling [51].
Underlying Mechanisms and Contribution of Weight Loss
The relationships between SGLT2 and SNS appear rather complex and still poorly understood. Reciprocal relationships have been reported, SGLT2 inhibition reducing SNS activity, while SNS may also regulate SGLT2 expression (Fig. 3) [84••]. SGLT2is consistently reduce body weight and fat mass [68]. Several studies also showed that weight loss is associated with a reduction of SNS activity [98, 99]. Caloric restriction is capable of significantly improving essential HTN, fast heart rate, low heart rate variability, SNS dominance over parasympathetic, arterial stiffness, endothelial dysfunction, and poor flow-mediated arterial dilatation [100]. Insulin stimulates SNS activity, especially in the context of insulin resistance associated with obesity [101]. To what extent the rather modest reduction in body weight associated with SGLT2is contributes to the reduction in SNS activity as well as the potential role of other underlying mechanisms remain to be investigated.
Conclusion
The mechanisms contributing to the cardiovascular and renal protective effects of SGLT2is appear multifactorial and remain a matter of discussion. These agents exert an antihyperglycemic affect, reduce body weight, lower arterial BP, and reduce fluid overload, without increasing heart rate. The latter observation suggests a possible dampening of SNS activity associated with SGLT2is. Recent experimental and clinical dedicated studies confirm a lowering of SNS activity. This effect may contribute to a better control of BP, especially in T2DM patients with HTN, and to the reduction in the risk of HF, two complications associated with SNS hyperactivity. The underlying mechanisms by which SGLT2is reduce SNS activity remain to be better understood, yet a contribution of reduction of hyperglycemia with lower insulin circulating levels and weight loss may play a role.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vecchione C, Argenziano L, Fratta L, et al. Sympathetic nervous system and hypertension in diabetic patients. Diabetes Nutr Metab. 2000;13:327–31.
Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–90.
Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–66.
Toschi-Dias E, Rondon M, Cogliati C, et al. Contribution of autonomic reflexes to the hyperadrenergic state in heart failure. Front Neurosci. 2017;11:162.
van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D, et al. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the translational research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2017;19:1361–78.
Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–10.
Ofstad AP, Atar D, Gullestad L, Langslet G, Johansen OE. The heart failure burden of type 2 diabetes mellitus-a review of pathophysiology and interventions. Heart Fail Rev. 2018;23:303–23.
Ortega-Loubon C, Fernandez-Molina M, Singh G, et al. Obesity and its cardiovascular effects. Diabetes Metab Res Rev. 2019;35:e3135.
Lim K, Jackson KL, Sata Y, Head GA. Factors responsible for obesity-related hypertension. Curr Hypertens Rep. 2017;19:53.
Schlaich M, Straznicky N, Lambert E, Lambert G. Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol. 2015;3:148–57.
Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DASG. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–101.
Masuo K, Rakugi H, Ogihara T, Esler M, Lambert G. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev. 2010;6:58–67.
Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59.
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–75 e9.
Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–94.
• Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6:e004007 This systematic review of prospective studies shows that treatment with SGLT2 inhibitors has beneficial off-target effects on blood pressure in patients with type 2 diabetes mellitus.
• Baker WL, Buckley LF, Kelly MS, et al. Effects of sodium-glucose cotransporter 2 inhibitors on 24-hour ambulatory blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005686 According to this meta-analysis, the diurnal effect of SGLT2 inhibitors on 24-hour ambulatory blood pressure is a class effect and may contribute to their favorable effects on cardiovascular outcomes.
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643–58.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
•• Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9 SGLT2i-associated moderate benefits on atherosclerotic major adverse cardiovascular events in patients with established atherosclerotic disease, but robust benefits on reducing hospitalisation for heart failure and progression of renal disease regardless of existing atherosclerotic disease or a history of heart failure.
Scheen AJ. Reduction in cardiovascular and all-cause mortality in the EMPA-REG OUTCOME trial: a critical analysis. Diabetes Metab. 2016;42:71–6.
Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27–38.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–25.
Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–17.
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39.
Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in EMPA-REG OUTCOME: comparison with classic diuretics. Diabetes Metab. 2016;42:224–33.
Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des. 2004;10:3579–89.
Rabbia F, Martini G, Cat Genova G, et al. Antihypertensive drugs and sympathetic nervous system. Clin Exp Hypertens. 2001;23:101–11.
Wan N, Rahman A, Hitomi H, Nishiyama A. The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol (Lausanne). 2018;9:421.
Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis. 2009;52:6–10.
Hillis GS, Woodward M, Rodgers A, Chow CK, Li Q, Zoungas S, et al. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia. 2012;55:1283–90.
Scheen AJ. Cardiovascular outcome studies in type 2 diabetes: comparison between SGLT2 inhibitors and GLP-1 receptor agonists. Diabetes Res Clin Pract. 2018;143:88–100.
Lorenz M, Lawson F, Owens D, Raccah D, Roy-Duval C, Lehmann A, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16:6.
Scheen AJ. Effects of reducing blood pressure on cardiovascular outcomes and mortality in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Res Clin Pract. 2016;121:204–14.
Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8:330–9.
Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens. 2015;9:48–53.
Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33:2185–97.
Townsend RR, Machin I, Ren J, Trujillo A, Kawaguchi M, Vijapurkar U, et al. Reductions in mean 24-hour ambulatory blood pressure after 6-week treatment with canagliflozin in patients with type 2 diabetes mellitus and hypertension. J Clin Hypertens (Greenwich). 2016;18:43–52.
Weir MR, Januszewicz A, Gilbert RE, Vijapurkar U, Kline I, Fung A, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16:875–82.
Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–20.
Sjostrom CD, Johansson P, Ptaszynska A, et al. Dapagliflozin lowers blood pressure in hypertensive and non-hypertensive patients with type 2 diabetes. Diab Vasc Dis Res. 2015;12:352–8.
Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–8.
Zhao D, Liu H, Dong P. Empagliflozin reduces blood pressure and uric acid in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Hum Hypertens. 2019;33:327–39.
Amin NB, Wang X, Mitchell JR, Lee DS, Nucci G, Rusnak JM. Blood pressure-lowering effect of the sodium glucose co-transporter-2 inhibitor ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805–8.
Kashiwagi A, Yoshida S, Kawamuki K, Nakamura I, Kazuta K, Ueyama E, et al. Effects of ipragliflozin, a selective sodium-glucose co-transporter 2 inhibitor, on blood pressure in Japanese patients with type 2 diabetes mellitus: a pooled analysis of six randomized, placebo-controlled clinical trials. Diabetol Int. 2017;8:76–86.
Scheen AJ, Delanaye P. Effects of reducing blood pressure on renal outcomes in patients with type 2 diabetes: focus on SGLT2 inhibitors and EMPA-REG OUTCOME. Diabetes Metab. 2017;43:99–109.
Takenaka T, Ohno Y, Suzuki H. Impacts of sodium-glucose co-transporter type 2 inhibitors on central blood pressure. Diab Vasc Dis Res. 2018;15:154–7.
Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, et al. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol. 2017;16:26.
Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation. 2018;published on line;139:2089–97. https://doi.org/10.1161/CIRCULATIONAHA.118.037076.
Tamura K, Wakui H, Azushima K, Uneda K, Umemura S. Circadian blood pressure rhythm as a possible key target of SGLT2 inhibitors used for the treatment of type 2 diabetes. Hypertens Res. 2016;39:396–8.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93.
Heerspink HJ, Johnsson E, Gause-Nilsson I, et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–7.
Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, et al. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. 2016;68:1355–64.
Scheen AJ. Type 2 diabetes and thiazide diuretics. Curr Diab Rep. 2018;18:6.
Kimura T, Sanada J, Shimoda M, Hirukawa H, Fushimi Y, Nishioka M, et al. Switching from low-dose thiazide diuretics to sodium-glucose cotransporter 2 inhibitor improves various metabolic parameters without affecting blood pressure in patients with type 2 diabetes and hypertension. J Diabetes Investig. 2018;9:875–81.
Komici K, Femminella GD, de Lucia C, Cannavo A, Bencivenga L, Corbi G, et al. Predisposing factors to heart failure in diabetic nephropathy: a look at the sympathetic nervous system hyperactivity. Aging Clin Exp Res. 2019;31:321–30.
Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Clin Pharmacokinet. 2015;54:691–708.
Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. CREDENCE trial investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Ferdinand KC, Izzo JL, Lee J, Meng L, George J, Salsali A, et al. Antihyperglycemic and blood pressure effects of empagliflozin in African Americans with type 2 diabetes and hypertension. Circulation. 2019;139:2098–109.
Cai X, Gao X, Yang W, Chen Y, Zhang S, Zhou L, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: a meta-analysis. J Diabetes Investig. 2018;9:850–61.
Reed JW. Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure. Vasc Health Risk Manag. 2016;12:393–405.
Briasoulis A, Al Dhaybi O, Bakris GL. SGLT2 inhibitors and mechanisms of hypertension. Curr Cardiol Rep. 2018;20(1):1.
Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, et al. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharmacol Toxicol. 2017;18:23.
Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19:1630–41.
Zomer E, Gurusamy K, Leach R, Trimmer C, Lobstein T, Morris S, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016;17:1001–11.
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–84.
Sjostrom CD, Hashemi M, Sugg J, et al. Dapagliflozin-induced weight loss affects 24-week glycated haemoglobin and blood pressure levels. Diabetes Obes Metab. 2015;17:809–12.
Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int. 2016;89:524–6.
McMurray J. EMPA-REG - the “diuretic hypothesis”. J Diabetes Complicat. 2016;30:3–4.
Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, et al. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin Ther. 2016;38:2248–64 e5.
Yasui A, Lee G, Hirase T, Kaneko T, Kaspers S, von Eynatten M, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018;9:863–71.
Tanaka H, Takano K, Iijima H, Kubo H, Maruyama N, Hashimoto T, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–51.
Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61:2098–107.
Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138.
Ramirez AJ, Sanchez MJ, Sanchez RA. Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J Hypertens. 2019;37:636–42.
van Ittersum FJ, Schram MT. Van der Heijden-Spek JJ, et al. autonomic nervous function, arterial stiffness and blood pressure in patients with type I diabetes mellitus and normal urinary albumin excretion. J Hum Hypertens. 2004;18:761–8.
Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28.
Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20:pii: E629.
• Jordan J, Tank J, Heusser K, Heise T, Wanner C, Heer M, et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J Am Soc Hypertens. 2017;11:604–12 This experimental study showed that empagliflozin is not associated with clinically relevant reflex-mediated sympathetic activation in contrast to increases observed with diuretics in other studies.
•• Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35:2059–68 In-vitro and in-vivo studies showed an important cross-talk between the sympathetic nervous system and SGLT2 regulation that may potentially contribute to cardiovascular and renal protection observed with SGLT2 inhibitors.
Elliott RH, Matthews VB, Rudnicka C, Schlaich MP. Is it time to think about the sodium glucose co-transporter 2 sympathetically? Nephrology. 2016;21:286–94.
•• Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71:471–6 This review discusses novel insights into cardiovascular protection by SGLT2is, focusing on the interaction between SGLT2 and the sympathetic nervous system in diabetic patients with hypertension or heart failure.
Kiuchi S, Hisatake S, Kabuki T, Fujii T, Oka T, Dobashi S, et al. Long-term use of ipragliflozin improved cardiac sympathetic nerve activity in a patient with heart failure: a case report. Drug Discov Ther. 2018;12:51–4.
Vasiliadis I, Kolovou G, Mavrogeni S, Nair DR, Mikhailidis DP. Sudden cardiac death and diabetes mellitus. J Diabetes Complicat. 2014;28:573–9.
Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res. 2015;116:2005–19.
Kubota Y, Yamamoto T, Tara S, Tokita Y, Yodogawa K, Iwasaki Y, et al. Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: rationale. Diabetes Ther. 2018;9:2107–16.
Avogaro A, Fadini GP. Microvascular complications in diabetes: a growing concern for cardiologists. Int J Cardiol. 2019;published on line. https://doi.org/10.1016/j.ijcard.2019.02.030.
Kaur J, Young BE, Fadel PJ. Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int J Mol Sci. 2017;18:E1682.
Masi S, Uliana M, Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vasc Pharmacol. 2019;115:13–7.
Pop-Busui R, Kirkwood I, Schmid H, Marinescu V, Schroeder J, Larkin D, et al. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004;44:2368–74.
Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304.
Valensi P, Smagghue O, Paries J, et al. Peripheral vasoconstrictor responses to sympathetic activation in diabetic patients: relationship with rheological disorders. Metabolism. 1997;46:235–41.
Herat LY, Matthews VB, Rakoczy PE, et al. Focusing on sodium glucose cotransporter-2 and the sympathetic nervous system: potential impact in diabetic retinopathy. Int J Endocrinol. 2018;2018:9254126.
Lambert EA, Rice T, Eikelis N, Straznicky NE, Lambert GW, Head GA, et al. Sympathetic activity and markers of cardiovascular risk in nondiabetic severely obese patients: the effect of the initial 10% weight loss. Am J Hypertens. 2014;27:1308–15.
Costa J, Moreira A, Moreira P, Delgado L, Silva D. Effects of weight changes in the autonomic nervous system: a systematic review and meta-analysis. Clin Nutr. 2019;38:110–26.
Nicoll R, Henein MY. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int J Mol Sci. 2018;19:E751.
Landsberg L. Insulin resistance, energy balance and sympathetic nervous system activity. Clin Exp Hypertens A. 1990;12:817–30.
Funding
This paper was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No conflicts of interest are directly relevant to the content of this manuscript.
A.J. Scheen has received lecturer/advisor fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, NovoNordisk, Sanofi, and Servier. He also worked as clinical investigator in the three cardiovascular outcome trials with SGLT2 inhibitors, EMPA-REG-OUTCOME, CANVAS-R, and DECLARE-TIMI 58.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension
Rights and permissions
About this article
Cite this article
Scheen, A.J. Effect of SGLT2 Inhibitors on the Sympathetic Nervous System and Blood Pressure. Curr Cardiol Rep 21, 70 (2019). https://doi.org/10.1007/s11886-019-1165-1
Published:
DOI: https://doi.org/10.1007/s11886-019-1165-1