Abstract
Diagnosis of cardiac mechanical device or prosthesis valve infection, and more importantly accurate localization of the infection site, such as defibrillator pocket, pacemaker lead, annular or peri-annular valve ring abscesses remain clinically challenging. Inconclusive diagnosis can lead to delayed antibiotic therapy, device extraction or surgical intervention, which may have dire consequences to the patient. Among patients with suspected cardiac mechanical device or prosthetic valve infection, recent publications advocate the use of 18 F-fluoro-2-deoxyglucose positron emission tomography computed tomography (FDG PET/CT), particularly when anatomy based imaging studies, such as echocardiography or CT, are uncertain or negative. A potential advantage of FDG PET/CT is in its detection of inflammatory cells early in the infection process, before morphologic damages ensue. However, there are many unanswered questions in the literature. There is a need for standardization amongst the various imaging studies, such as dietary preparation, duration and timing of image acquisition, image processing with and without CT attenuation correction, and more importantly image interpretation criteria. The answer for these issues awaits well designed, prospective studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the introduction of 18 F-fluoro-2-deoxyglucose positron emission tomography computed tomography (FDG PET/CT), the main focus of its application has been in oncology, for early detection and staging of various malignancies. In cancer evaluation, false positive FDG uptake can occur at sites of infection as well as in a variety of non-infectious inflammatory disorders. The latter is a consequence of FDG targeting the inflammatory cells (macrophages, neutrophils, and lymphocytes) at the site of infection/inflammation. In non-cancer settings, however, the technique has been used clinically for the evaluation of a variety of infectious processes that include painful prosthetic joints, osteomyelitis, complicated diabetic foot, fever of unknown origin, and a variety of other non-infectious inflammatory diseases [1]. Given the high spatial and target-to-background contrast resolution of FDG PET/CT, recent publications in the literature support the application of this technique for detecting cardiac implantable pacemaker and defibrillator infections [2], as well as prosthetic valve endocarditis [3]. Other cardiovascular applications of FDG PET/CT include the assessment of myocardial perfusion and metabolism [4, 5], as well as coronary atherosclerosis and plaque inflammation [6, 7].
There has been significant increase in cardiac device implantation worldwide, particularly in the United States [8]. Unfortunately, the growth in cardiac device implantation has been accompanied by an even higher rate of increased device infection, partly due to the age of new device recipients and the higher rate of co-morbidities [9]. Device infection carries high risk of death, if not treated appropriately in this population. It has been reported that all-cause 12-week mortality could be as high as 35 % with cardiac device infection, especially for those with methicillin-resistant S. aureus infection [10]. The 1-year mortality after removal of an infected device was 12 % in pocket infection group and 17 % in the endovascular infection group [11]. Accurate diagnosis of cardiac device infection thus is critical for clinical decision making such as antibiotics alone or device extraction, but represents a challenge for current diagnostic methods. For example, local infection signs (i.e., erythema or external suppuration) on physical examination cannot localize the infection source relative to the device (i.e., superficial or deep to the pocket). Blood culture may not isolate the pathogen (particularly when antibiotics have been initiated), nor localize the infection site. Transthoracic or transesophageal echocardiography (TTE or TEE) may detect intra-cardiac vegetation but does not provide information regarding extra-cardiac portion of lead infection. Findings on anatomical imaging like CT are non-specific for infection and affected by artifacts from the metal device. Thus, there is a need of a new method in addition to existing tools for diagnosis of cardiac device and prosthesis valve infection.
Radiolabeled White Blood Cell Imaging
Unlike anatomic imaging, identifying inflammatory cells early at the site of cardiac device or prosthetic valve can prompt timely medical and/or surgical intervention before the development of morphologic damages from the infectious process. Given that inflammatory cells avidly migrate to an infection site, 111-Indium or 99m-Technetium radiolabeled autologous white blood cells (WBC) can be used to localize the site of infection by using planar imaging. Although radiolabeled WBC scintigraphy is used clinically for the evaluation of fever of unknown, painful prosthesis joint, and other possible causes of infection, its sensitivity can be significantly variable. Factors that may limit the sensitivity of radiolabeled WBC scan include the viability of the WBC after in vitro labeling process and the migration rate of the cells to the infection site. The latter becomes a particular concern in patients who are on antibiotic treatment, in whom cell chemotaxis is decreased. In addition, the WBC labeling process is time consuming, labor intensive, and costly. The imaging is performed 24 hours after injection of the radiolabeled WBC or longer, and the studies are often count-poor with low spatial resolution (2D planar images). There is potential risk of pathogen contamination during the in vitro labeling process and misadministration of radiolabeled WBC into a different patient. Thus, while radiolabeled WBC scintigraphy is relatively specific, it suffers from low spatial resolution (2D images) and low sensitivity which may limit its application in assessing cardiac device and prosthetic valve infection.
Radiolabeled 18 F-fluoro-2-deoxyglucose PET/CT Imaging
Different from radiolabeled WBC scintigraphy, FDG PET/CT imaging is based on in vivo FDG labeling of the pre-existing inflammatory cells at the infection site. With the stimulation of cytokines, these cells (macrophages, neutrophils, and lymphocytes) overexpress the glucose transporter 1 and accumulate FDG with high concentration. Thus, FDG PET/CT is expected to have a higher sensitivity than radiolabeled WBC scintigraphy and particularly useful for detecting chronic infection in patients on antibiotic treatment. The method of FDG PET/CT itself has several practical advantages over radiolabeled WBC scintigraphy, such as (1) superior tomographic images with high spatial and target-to-background contrast resolution, (2) completion of the examination within a short period of time (1.5 to 2 hours), (3) not labor intensive, and (4) low radiation dose (2 to 3 times lower than that of 111In-WBC scintigraphy).
In patients with joint prosthesis, FDG PET/CT has been shown to have superior spatial and contrast resolution in detecting infection when compared to radiolabeled WBC scintigraphy [12]. Recent studies have expanded the potential application of FDG PET/CT to other mechanical or prosthetic devices, such as cardiac pacemaker, defibrillator, and prosthetic valve infections [2, 3]. The preliminary data suggest that FDG PET/CT is highly accurate in diagnosing cardiac device pocket infection, while it may have a relatively lower sensitivity for lead infection and vegetation evaluation. Nonetheless, FDG PET/CT can provide incremental information to anatomic based imaging studies, such as echocardiography and CT, particularly when clinical suspicion in concert with inflammatory biomarkers and blood cultures is incongruent with the anatomic based imaging findings.
Cardiac Implantable Electronic Device Infection
The cardiac implantable electronic device (CIED) system (mainly implantable cardioverter-defibrillators and pacemakers) consists of a pocket (usually placed in the subcutaneous region or below a muscle in the upper chest wall) and leads (placed intravascularly or sometimes in epicardial surface of the heart). Severe deep pocket infection or lead infection needs complete extraction of the device, while superficial soft tissue infection not connected to the pocket may be managed by antibiotics alone. The diagnosis appears straightforward in the presence of local infection such as erythema or external suppuration over the chest wall pocket, associated with bacteremia, and vegetation attached to the leads at either TTE or TEE. However, real clinical cases are more complicated with unclear blood culture or TEE findings. FDG PET/CT can localize the infection site, and may be highly helpful when vegetation is not detected by TEE.
Pocket Infection
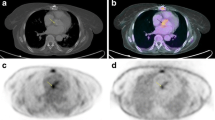
In patients with clinical suspicion of cardiac device pocket infection, FDG PET/CT can accurately diagnose infection [13•, 14•] as well as distinguish deep pocket infection from superficial soft tissue infection [15••]. On the other hand, local infection signs such as skin redness or drainage on physical examination cannot localize the source of infection. Findings on CT such as local fluid or gas collection are non-specific and accurate evaluation may further be affected by the metal artifact. Figure 1a showed a case with deep pocket infection on FDG PET/CT. Deep pocket infection necessitates extraction. Focal FDG uptake limited to superficial tissues without direct contact with the pocket indicates superficial skin infection which could be treated by antibiotics with sustained clinical improvement [15••].
Fused FDG PET/CT images (a) showed a focal uptake (arrow) deep to the pocket indicating pocket infection, (b) mild residual activity at the level of right pre-pectoral area in a case with resent implant of device, indicating inflammation, (c) intense focal uptake in the left lateral chest wall compatible with lead infection, and (d) complete absence of uptake along the cardiac device system without evidence of infection and inflammation. (Reproduced with permission from: Sarrazin JF et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59:1616-25) [15••]
While focal increased FDG uptake within or immediately adjacent to the CIED pocket is the hallmark of infection, the significance of non-specific FDG uptake related to inflammation (not infection) needs to be addressed. It is anticipated that FDG activity in a recently implanted CIED pocket is mild rather than intense, and in a diffuse pattern along the entire pocket, rather than limited to a focal area [15••]. Figure 1b showed mild residual activity at the level of right pre-pectoral area in a case with resent implant of device, indicating inflammation. Clearly, further prospective studies are needed in order to develop image interpretation criteria to differentiate true pocket infection from inflammation.
Lead Infection
In contrast to the cardiac device pocket, FDG PET/CT appears less reliable for lead infection evaluation with a reported sensitivity and specificity of 60 % and 100 %, respectively [13•]. The lower sensitivity of FDG PET/CT may be attributed to the small size of the lead, and/or ongoing antibiotic treatment. Nevertheless, for clinical management purposes, an abnormal FDG PET/CT finding of a lead necessitates extraction. On the other hand, despite clinical suspicion of infection, if the FDG signal is negative along the lead, then the system can be left in place without ongoing clinical observation over the ensuing year, looking for objective signs of active lead endocarditis [14•]. Figure 1c showed an infection in the left lateral chest wall lead on FDG PET/CT. Figure 1d showed complete absence of uptake along the cardiac device system without evidence of infection and inflammation.
Quantitative assessment of regional metabolic activity, assessed by standard uptake value (SUV), has been shown to be higher in infected CEID pockets than control, but not in lead infection [13•]. For lead infection, a slightly increased focal uptake over the lead seems to be more valuable for the diagnosis of infection than the overall SUV value. Although further studies are needed to define whether a cut-off value of SUV can be used to differentiate an infection or not, it appears that the pattern of uptake rather than the intensity of SUV plays a more critical role in defining an infection.
FDG PET/CT for Prosthesis Valve Endocarditis
Early diagnosis of prosthetic valve endocarditis is challenging. Mortality from endocarditis remains high, if not appropriately treated [16]. The final diagnosis is made on the basis of the Duke Endocarditis Service criteria [17]. TTE or TEE and blood culture results are critical for the Duke criteria. However, factors like thickened valves, nodules, or valvular calcifications can lead to a false-positive TEE diagnosis in about 15 % of patients [17]. Acoustic shadowing of the metal prosthesis ring may obscure detection of vegetation and peri-annular extension (abscess/mycotic aneurysm) [18]. Furthermore, vegetation revealed by anatomical imaging may not necessarily be actively infected [19]. Yield of blood culture can be affected by many factors particularly antibiotic treatment. In some instances, blood cultures or echocardiography is inconclusive, thus leading to a high proportion of unconfirmed cases of suspected endocarditis based on the Duke criteria. Indeed, up to 24 % of the patients with pathologically proven endocarditis were misclassified as having possible endocarditis based on the Duke criteria alone [20]. Modifications to these criteria have been proposed by various organizations such as the American Heart Association [16] and The European Society for Cardiology [21] for diagnostic classification. The modified Duke criteria are the current gold standard for endocarditis diagnosis, which provides a diagnosis probability classified as definite, possible or rejected endocarditis [22]. However, the modified Duke criteria are not optimal for accurate diagnosis of early endocarditis, as it was initially developed to define endocarditis for epidemiological studies, conducted at the end of follow up period [22]. Recent efforts have aimed in developing new imaging techniques to facilitate early diagnosis of the endocarditis. For example, radiolabeled WBC scan has been shown to improve the performance of the Duke criteria [23].
Similarly, recent studies have shown that FDG PET/CT can facilitate the diagnosis of prosthesis valve endocarditis. Inclusion of abnormal FDG uptake at the site of the prosthetic valve, as a novel major criterion, can significantly increase the sensitivity of the modified Duke criteria from 70 to 97 %, without compromising specificity [24••]. This improvement is the consequence of a significant reduction in the number of cases that were classified as “possible” by the modified Duke criteria, after inclusion of FDG PET/CT. By reclassifying the majority of “possible” cases to definite, FDG PET/CT may have a significant impact on patient management decisions and clinical outcome. It is important to point out, however, that the latter has not been demonstrated by a large well-designed prospective study. In the current literature, the contribution of FDG PET/CT has been predominantly in patients with a clinical suspicion of endocarditis, in whom the initial TEE was negative or indeterminate [24••, 25]. The results emphasize the power of FDG PET/CT in early diagnosis before significant infectious damage occurs on the valve, which may subsequently be discovered on a repeated second or third TEE (Fig. 2).
Example of positive FDG PET/CT in patient with prosthesis valve endocarditis with initial normal echocardiography. The first transesophageal echocardiogram did not show any abnormalities around the aortic bioprosthetic annulus (b). The second transesophageal echocardiogram, performed at the end of antibiotic treatment (1 month later), showed a large periprosthetic abscess (c, red arrow). The FDG PET/CT performed 3 days after the first echocardiogram showed an abnormal uptake around the aortic prosthesis (a, yellow arrow) and another hyperintensity in the left colon, revealing a cancer (a, green arrow) that was confirmed by colonoscopy. The FDG PET/CT images are presented as fused (PET + CT) and attenuation-corrected maximal intensity (MIP) projection images. (Reproduced with permission from: Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18 F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374-82) [24••]
It would be important to discuss the false positive rate of FDG PET/CT as well. The false positive cases could be related to inflammatory activity, particularly when the FDG PET/CT is performed too early after the implantation of the prosthesis, or uptake related to the surgical adhesive used to seal the aortic root graft [26]. On the other hand, false negative FDG PET/CT cases may be related to small size of vegetation or prior antibiotic therapy. Thus, further studies are needed to determine the impact of non-specific uptake from inflammation (which may be minimized by optimal timing for performing FDG PET/CT after prosthesis implantation or the onset of antibiotic treatment), and the performance of FDG PET/CT for small size vegetation. In addition, physiologic myocardial FDG uptake and cardiac motion could also interfere with the proper evaluation of prosthetic valve endocarditis.
Approximately half of prosthetic valve endocarditis cases are complicated by peri-annular extensions (abscesses/mycotic aneurysms) which need urgent surgical intervention [18]. TEE may fail to recognize this potentially fatal complication. Although ECG-gated computed tomography angiography (CTA) can improve the diagnostic accuracy in some cases, it is also purely anatomic technique [27]. The incremental value of FDG PET/CT to the findings on TEE or CTA has been shown in observational case series [28•]. Although these results are encouraging, FDG PET/CT is not advocated as a “first-line” or confirmatory imaging study for detecting prosthetic valve endocarditis. Rather, it should be reserved for patients with clinical and microbiological suspicion of endocarditis but indeterminate or negative TEE [2, 29].
Current Challenges and Future Research Directions
Evidence thus far regarding FDG PET/CT in the diagnosis of cardiovascular device infection is mainly from studies with a relatively small cohort of patients, particularly in the CIED group. Imaging protocol and data acquisition processes in these studies are not completely consistent (for example, the time of imaging after FDG injection). It should be emphasized that any “positive” FDG uptake seen on the attenuation corrected images should be confirmed on the non-attenuation corrected images. When applying CT based PET attenuation correction in patients with metal device, such as CIED, the metal creates an artifact of “apparent” increased FDG uptake adjacent to the metal device that mimics infection (Fig. 3). Despite aggressive dietary preparations, physiological myocardial glucose utilization cannot always be completely suppressed on FDG PET/CT studies. In such cases, the evaluation of intra-cardiac portion of the device, such as lead-related vegetation or prosthetic valve endocarditis may be difficult to assess. Cardiac and valve motion could also decrease the sensitivity of FDG PET/CT, especially when the valve vegetation is rather small. Whether ECG or respiratory gated imaging can improve the performance of FDG PET/CT in this setting has not been tested. In addition to these technique related issues, standardized interpretation criteria are particularly important. It appears that the distribution and pattern of FDG uptake rather than the intensity is more reliable for a positive diagnosis of infection. A focal uptake favors a true infection while mild diffuse activity along the device or the lead may favor non-specific inflammatory change.
CT attenuation correction PET image (a) showed a curvilinear FDG uptake along the pocket (arrow) in the left upper chest wall, which was not clearly seen on the non attenuation corrected PET image (b), indicating a false positive FDG PET/CT findings due to attenuation correction artifact. Middle panel: corresponding CT images and lower panel: fused PET/CT images (unpublished data)
Quantitative assessment of regional metabolic activity, assessed by SUV uptake, may appear increased in infection sites compared to controls. However, there is no sufficient data in the literature to establish a cut-off SUV value that differentiates infection from inflammation. Similarly, there is no data on the sensitivity of FDG uptake in relation to the infectious pathogen isolated from the device. It is also unclear whether antibiotic treatment could affect the sensitivity of FDG PET/CT or not in detecting device infection, and whether FDG PET/CT can be used to monitor the antibiotic treatment effect.
Conclusion
FDG PET/CT is an accurate technique for identifying CIED pocket infection. It can pin point the site and extension of the infection, such as superficial versus deep pocket, which guides proper clinical management. FDG PET/CT appears to be less reliable for the evaluation of device lead and prosthetic valve vegetation infection, particularly in patients with long term antibiotics therapy and small vegetation size. Nevertheless, FDG PET/CT information may guide the clinical management of cardiac device infection, i.e., deep pocket and lead infection necessitates complete extraction, while superficial soft tissue infection not connected to the pocket may be treated with antibiotics without necessitating extraction of the device. However, there are many unanswered questions in the literature. There is a need for standardization amongst the various imaging studies, such as dietary preparation, duration and timing of image acquisition, image processing with and without CT attenuation correction, SUV and image interpretation criteria. The answer for these issues awaits well designed, prospective studies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Basu S, Zhuang H, Torigian DA, et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124–45.
Brinker J. Imaging for infected cardiac implantable electronic devices: a new trick for your pet. J Am Coll Cardiol. 2012;59:1626–8.
Bertagna F, Bisleri G, Motta F, et al. Possible role of F18-FDG-PET/CT in the diagnosis of endocarditis: preliminary evidence from a review of the literature. Int J Cardiovasc Imaging. 2012;28:1417–25.
Dilsizian V, Bacharach SL, Beanlands SR, et al. ASNC Imaging Guidelines for Nuclear Cardiology Procedures: PET Myocardial Perfusion and Metabolism Clinical Imaging J Nucl Cardiol. 2009;16:651, ISSN 1532–6551 (Online) doi:10.1007/s12350-009-9094-9.
Dilsizian V, Taillefer R. Journey in Evolution of Nuclear Cardiology: Will There Be Another Quantum Leap with the F-18 labeled Myocardial Perfusion Tracers? J Am Coll Cardiol Imaging. 2012;5:1269–84.
Chen W, Dilsizian V. Fluorodeoxyglucose PET Imaging of Coronary Atherosclerosis and Plaque Inflammation. Curr Cardiol Rep. 2010;12:179–84.
Chen W, Dilsizian V. Targeted PET/CT Imaging of Vulnerable Atherosclerotic Plaques: Microcalcification with Sodium Fluoride and Inflammation with Fluorodeoxyglucose. Curr Cardiol Rep. 2013;15:364–9.
Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013–27.
Kurtz SM, Ochoa JA, Lau E, et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993-2006. Pacing Clin Electrophysiol. 2010;33:705–11.
Chu VH, Crosslin DR, Friedman JY, et al. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med. 2005;118(1416):e19–24.
Tarakji KG, Chan EJ, Cantillon DJ, et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–7.
Pill SG, Parvizi J, Tang PH, et al. Comparison of fluorodeoxyglucose positron emission tomography and (111)indium-white blood cell imaging in the diagnosis of periprosthetic infection of the hip. J Arthroplasty. 2006;21:91–7.
Bensimhon L, Lavergne T, Hugonnet F, et al. Whole body [(18) F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: a preliminary prospective study. Clin Microbiol Infect. 2011;17:836–44. The study prospectively evaluated the usefulness of FDG PET/CT for the diagnosis of device infection in 21 patients with clinically suspected device infection, and 14 controls free of infection. Compared to the gold standard of device culture after extraction or clinical follow-up, FDG PET/CT showed a high sensitivity and specificity for diagnosis of device infection, superior for pocket infection than lead infection evaluation.
Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm. 2011;8:1478–81. The study showed the potential value of FDG-PET/CT scanning in the diagnosis of pacing lead endocarditis in 10 difficult cases with absence of valvular or lead vegetations on transthoracic and transesophageal echocardiography. FDG PET/CT showed lead infection in 6 cases which were confirmed on subsequent culture of lead after extraction. In the 4 cases with a negative FDG PET/CT scan, the pacing system was left in place without objective signs of active lead endocarditis during follow-up. The study showed that FDG PET/CT may guide the clinical decision making for difficult cases with clinically suspected cardiac device infection.
Sarrazin JF, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59:1616–25. The study first showed that FDG PET/CT can differentiate device infection from recent post-implant inflammation changes. FDG PET/CT findings can guide clinical management of infected cases by localizing the infection sites of superficial or deep pocket infection.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434.
Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–9.
Hill EE, Herijgers P, Claus P, et al. Abscess in infective endocarditis: the value of transesophageal echocardiography and outcome: a 5-year study. Am Heart J. 2007;154:923–8.
Mathew J, Anand A, Addai T, et al. Value of echocardiographic findings in predicting cardiovascular complications in infective endocarditis. Angiology. 2001;52:801–9.
Habib G, Derumeaux G, Avierinos JF, et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33:2023–9.
Habib G, Hoen B, Tornos P, et al. ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–413.
Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8.
Erba PA, Conti U, Lazzeri E, et al. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. 2012;53:1235–43.
Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18 F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–82. The study prospectively studied 72 consecutive patients suspected of having prosthesis valve endocarditis. The results showed that FDG PET/CT can detect prosthesis valve infection earlier than the echocardiography with a high sensitivity and specificity. Inclusion of abnormal FDG uptake at the site of the prosthetic valve, as a novel major criterion, can significantly increase the sensitivity of the modified Duke criteria, without compromising specificity.
Saby L, Le Dolley Y, Laas O, et al. Early diagnosis of abscess in aortic bioprosthetic valve by 18 F-fluorodeoxyglucose positron emission tomography-computed tomography. Circulation. 2012;126:e217–20.
Schouten LR, Verberne HJ, Bouma BJ, et al. Surgical glue for repair of the aortic root as a possible explanation for increased F-18 FDG uptake. J Nucl Cardiol. 2008;15:146–7.
Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol. 2012;22:2407–14.
Tanis W, Scholtens A, Habets J, et al. Fusion of Cardiac Computed Tomography Angiography and 18 F-Fluorodesoxyglucose Positron Emission Tomography for the Detection of Prosthetic Heart Valve Endocarditis. J Am Coll Cardiol Imaging. 2013. doi:10.1016/j.jcmg.2013.07.004. This was an “iPix-Imaging Vignette” in the journal with typical images showing that FDG PET/CT could provide unique and incremental information over echocardiography and CTA in diagnosing prosthesis valve endocarditis and its complication of peri-annular abscess. Meanwhile, FDG PET/CT could confirm the diagnosis of infection by echocardiography and CTA, but provide additional information of extra-cardiac infection sites.
Dilsizian V, Achenbach S, Narula J. On Adding Versus Selecting Imaging Modalities for Incremental Diagnosis: A Case-Study of 18 F-Fluorodeoxyglucose PET/CT in Prosthetic Valve Endocarditis. Imaging: J Am Coll Cardiol; 2013. doi:10.1016/j.jcmg.2013.07.005.
Compliance with Ethics Guidelines
Conflict of Interest
Wengen Chen, Jongho Kim, Olga P. Molchanova-Cook, and Vasken Dilsizian declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Chen, W., Kim, J., Molchanova-Cook, O.P. et al. The Potential of FDG PET/CT for Early Diagnosis of Cardiac Device and Prosthetic Valve Infection Before Morphologic Damages Ensue. Curr Cardiol Rep 16, 459 (2014). https://doi.org/10.1007/s11886-013-0459-y
Published:
DOI: https://doi.org/10.1007/s11886-013-0459-y