Abstract
The emphasis of current cardiovascular imaging modalities is on the anatomic detection of coronary artery luminal narrowing. However, in the clinical setting, vulnerable plaques that are not flow limiting may account for the majority of cardiovascular events. Thus, the pursuit for developing noninvasive imaging techniques that target vulnerable plaques is a laudable goal. Recent studies have demonstrated the clinical feasibility of direct visualization and characterization of coronary and carotid artery plaques with 18F-fluorodeoxyglucose (FDG) positron emission tomography imaging. In experimental studies, the intensity of FDG uptake has been shown to correlate with macrophage density and inflammatory state of plaques. Vascular plaque FDG uptake has been linked to cardiovascular events such as myocardial infarction and stroke. Anti-inflammatory drugs and statins have been shown to attenuate FDG uptake in plaques. Thus, the identification of FDG uptake in vascular plaques may have important clinical implications for predicting and preventing future cardiovascular events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vulnerable atherosclerotic plaques typically have a necrotic lipid core with a thin fibrous cap and large amount of macrophages. When such vulnerable plaques rupture, they cause myocardial infarction, sudden death, or stroke. Current cardiovascular imaging modalities, such as coronary angiography and myocardial perfusion studies, identify coronary artery luminal narrowing and its downstream flow-limiting functional consequence in the left ventricular myocardium. These imaging techniques do not identify non-flow-limiting vulnerable plaques that tend to be small and imbedded within the walls of remodeled coronary arteries.
It has been reported that a significant majority of plaque ruptures occur in coronary arteries exhibiting none or only modest luminal narrowing on angiographic examination [1–3]. Epidemiologic studies have shown that a large proportion of patients who have suffered sudden cardiac events may in fact have no prior cardiovascular symptoms [4]. These data suggest that the biological composition and inflammatory state of an atherosclerotic plaque, rather than its degree of stenosis or size, may be the major determinants for acute clinical events. Thus, the pursuit for developing noninvasive imaging techniques that target vulnerable plaques is a laudable goal.
Recent studies reported on the potential use of intravascular ultrasonography, CT angiography, MRI, and metabolic 18F-fluorodeoxyglucose (FDG) imaging with positron emission tomography (PET) for this purpose [5–8]. A combined functional and structural whole-body imaging will likely afford the best potential for visualizing coronary atherosclerosis and plaque inflammation [8, 9].
Plaque FDG Uptake and Calcification
FDG is a glucose analogue that is mainly used in patients undergoing PET/CT studies for oncologic evaluation and in patients with ischemic cardiomyopathy for assessing myocardial viability. Beyond its role in identifying malignancy and myocardial viability, FDG also has been shown to accumulate in areas of infection and inflammatory sites. Uptake of FDG in patients with vasculitis of large arteries was first described in 1987 [10]. Among patients undergoing PET/CT studies for oncologic evaluation, it is not uncommon to visualize localization of FDG in the carotid arteries, aorta, and in the coronary arteries. Such vascular uptake of FDG has been shown to correlate with cardiovascular risk factors, such as age, hypercholesterolemia, and hypertension [11–13]. It is important to point out, however, that most FDG-positive plaques seen on PET show no calcifications on CT, and conversely, calcified lesions detected on CT do not avidly accumulate FDG. Thus, there is disparity between FDG uptake on PET and calcification detected on CT in large arteries. Congruent FDG uptake and calcification on CT were observed in only 2% to 14% of plaques in different studies [12–14]. The findings are entirely consistent with the biological process and progression of atherosclerosis. Plaque inflammation, as assessed by FDG uptake, is likely transient and may represent the vulnerable stages of a plaque, whereas calcification, as measured by CT, represents advanced lesions and may indicate stable plaques.
Plaque FDG Uptake Correlates with Macrophage Density and Inflammation
A large body of evidence in experimental animals and human subjects has linked FDG uptake to plaque macrophage density. In rabbit models, FDG was shown to be taken up by macrophage-rich atherosclerotic lesions in the aortic arch, and there was a correlation between FDG accumulation in the plaque with local macrophage density [15–18]. In humans, accumulation of 3H-deoxyglucose was shown in macrophage-rich areas of freshly isolated human carotid plaques [19] along with a significant correlation between FDG PET activity in carotid plaques and macrophage staining from the corresponding histologic sections of post-endarterectomy samples [20]. However, before the correlation of FDG uptake and macrophage density can be linked to plaque inflammation, it is important to determine conditions in which glucose utilization in macrophages is preferred over fatty acid, and the magnitude of macrophage content in inflammatory plaques.
Metabolic pathways in macrophages are tightly co-regulated with their proinflammatory or anti-inflammatory properties. In the setting of the classic activation pathway by proinflammatory Th1 cytokines such as interferon-γ, glycolysis is the dominant metabolic pathway. During alternative activation by anti-inflammatory Th2 cytokines, such as interleukin (IL)-4 and IL-13, fatty acid oxidation provides the main energy source for macrophages. In addition, transcription factors, such as peroxisome proliferator-activated receptors, which are expressed in plaque macrophages, regulate expression of genes involved in lipid metabolism, glucose homeostasis, and inflammatory response. All these factors could potentially affect macrophage glucose consumption in plaques.
Currently, there are no studies that examine FDG uptake in plaque macrophages with different activation pathways that link FDG uptake to plaque inflammation. Nevertheless, some studies have shown attenuation of plaque inflammation, as indicated by FDG uptake, with anti-inflammatory therapy. For example, the administration of an anti-inflammatory drug probucol in rabbits was associated with a reduction in FDG uptake and macrophage infiltration in the vulnerable atherosclerotic plaques [21]. In a prospective study of 43 subjects who were undergoing FDG PET cancer screening and had incidental FDG uptake in the thoracic aorta and/or the carotid arteries, patients were randomized to strict dietary management or administration of simvastatin in addition to dietary management for 3 months. Repeat FDG PET at 3 months showed significantly decreased FDG uptake in the atherosclerotic plaques in the simvastatin group, whereas no change was noted in the group with dietary management alone [22]. One of the major effects of statins is to promote plaque stability by decreasing plaque macrophage content and activity [23]. Similarly, among patients undergoing a series of FDG PET/CT studies and atherogenic risk reduction by lifestyle intervention, vascular FDG uptake was shown to improve with risk factor modification. The magnitude of improvement in FDG uptake correlated with increasing plasma high-density lipoprotein level [24]. These findings suggest that FDG PET/CT may not only identify atherosclerotic plaques but also monitor improvements in the inflammatory component of atherosclerotic lesions in response to risk modification or medical therapy.
Plaque FDG Uptake and Cardiovascular Events
Beyond identifying inflammatory atherosclerotic lesions, it is equally important to determine whether vascular FDG uptake is linked to cardiovascular events. Among patients with recent transient ischemic attack who had FDG PET imaging before carotid endarterectomy, the majority of patients exhibited high FDG uptake in the targeted lesion for endarterectomy or in nonstenotic lesions located in the vascular territory that was considered to be responsible for the presenting symptoms [25]. Others have reported that the degree of inflammation in the culprit carotid lesions could potentially be used to forecast future embolic events. Among patients with symptomatic carotid atherosclerosis and increased FDG accumulation, the subset of patients with intense FDG uptake suffered from subsequent death, recurrent nonfatal ipsilateral ischemic stroke, or restenosis after stenting [26].
Paulmier et al. [27•] retrospectively compared cardiovascular events in two groups of stable oncology patients, with and without detectable large arterial wall FDG uptake, who were otherwise matched for risk factors. Cardiovascular events were found to be significantly more frequent in the high-FDG uptake group compared with the low-FDG uptake group. A multivariate analysis showed that the extent of arterial FDG uptake was significantly related to the occurrence of a recent event. Conversely, vascular calcification was related to old cardiovascular events. Although these retrospective studies in a small number of subjects are promising, a prospective study is needed to provide unequivocal support of these preliminary observations.
Visualization of Coronary Artery Plaques
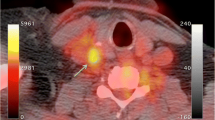
Atherosclerosis is a systemic inflammatory disease that involves multiple vessels in the body. Although plaque information from large arteries such as in carotid or major branches of aorta could potentially represent those in the coronary arteries and overall vascular systemic inflammatory status, there is a clinical necessity to directly visualize the vulnerable plaques in coronary arteries. Some studies have shown successful visualization of FDG uptake in coronary artery plaques (Fig. 1) [14, 28•]. However, several technical issues limit its application, such as 1) background myocardial FDG uptake, which decreases the target-to-background ratio; 2) cardiac motion during PET acquisition; and 3) partial volume effect due to small coronary artery and the size of plaques. All of these factors reduce the sensitivity of PET for coronary plaque identification.
18F-fluorodeoxyglucose uptake in the aortic arch (arrowheads in a) and ascending aortic root (arrowhead in b), as well as in the left main coronary artery (arrow in b), which corresponds to a noncalcified plaque in coronary CT angiography images (arrow in c). (From Alexanderson et al. [28•]; with permission.)
Depending on the dietary condition, myocardial glucose utilization can be prominent and the myocardial activity of FDG can overwhelm focal activities within the walls of the coronary arteries. Theoretically, increasing the target-to-background ratio improves PET sensitivity, which is more relevant in plaque detection than the size of the inflamed plaque. Background myocardial FDG uptake may be suppressed by a strict high-fat diet with low carbohydrate for a day before the scan [29•]. Cardiac motion may be attenuated by β blockade and the use of cardiac gating [30]. The current PET/CT has a usual spatial resolution of about 4 to 6 mm, which is likely the major challenge of identifying coronary plaque. With the development of PET/CT with high spatial resolution, or a specialized cardiac PET/CT, direct visualization of plaques less than 5 mm may become feasible.
The current PET/CT imaging protocol requires that patients are imaged 1 h after FDG injection, which is optimized for oncologic evaluation. Several studies have suggested that plaques may be visualized better when images are acquired 1.5 to 3 h after the injection of FDG [20, 31], although a recent study showed no significant advantage in imaging at 3 h over 1 h after FDG injection [32]. Standardization of imaging protocols (fast and diet preparation, FDG dose, data acquisition mode, and particularly imaging time) is essential and would incrementally facilitate the appropriate use of FDG PET/CT in the evaluation of plaques.
New Emerging Targets for Plaque Imaging
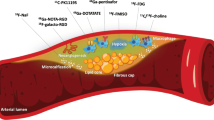
Beyond FDG, there are other emerging tracers that target specific pathways and molecules in atherosclerotic lesions. The three major targets that are currently under investigation are apoptosis, αvβ3 integrin, and matrix metalloproteinase (MMP). Different from FDG, targeting these pathways may result in a significantly decreased background myocardial uptake, and thereby higher lesion-to-background ratio and increased sensitivity to detect coronary artery plaques. However, atherosclerosis is a dynamic and complex inflammatory process, mediated by multiple factors. Targeting a single molecule or pathway in the progression of atherosclerosis may not necessarily be a representative signal for the vulnerable atherosclerotic plaque. Focusing the target to the final pathway or end point of plaque formation, rather than all earlier pathways leading up the final end point, may be the ultimate goal for imaging the vulnerable plaque.
Apoptosis Imaging
As an atherosclerotic lesion matures, macrophage apoptosis contributes to the development of plaque necrotic core. Apoptotic macrophages are the major source of proinflammatory cytokines such as tumor necrosis factor-α and IL-6, which further promote plaque inflammation. It is believed that progressive loss of smooth muscle cells through apoptosis could account for the weakening of the fibrous cap of the plaque. Plaque rupture and the ensuing intraplaque hemorrhage allows binding of apoptotic agent Annexin V (which can be radiolabeled with single photon emission radionuclides, such as 99mTc and indium-111, or positron emission radionuclides, such as 124I, 64Cu, and 18F) to the externalized phosphatidylserine of the red blood cells, which is normally confined exclusively in the inner cell membrane.
The first evidence of apoptosis imaging in atherosclerosis plaque came from single photon emission computed tomography (SPECT) imaging of 99mTc-labeled Annexin V in animal models [33]. Annexin V uptake was clearly visualized in apolipoprotein E knockout mouse aorta lesions and correlated with the histologic extent of plaques [33]. In a subsequent study, 99mTc-labeled Annexin V was found to co-localize with FDG uptake in atherosclerotic lesions in mice [34]. In a pilot clinical study of two patients with recent transient ischemic attacks, 99mTc-labeled Annexin V uptake was shown to be substantial in the carotid lesions that were considered to be responsible for the presenting symptoms and correlated with histopathologic assessment of the endarterectomy specimens taken from the patients [35]. In contrast, among the two control patients with a remote history of transient ischemic attack, no significant Annexin V uptake was identified.
αvβ3 Integrin Imaging
One of the hallmarks of atherosclerosis is the development of microvascular networks of vasa vasorum extending deeply from the adventitia and outer media toward the intima. The integrin αvβ3, which has been extensively investigated for tumor neovascularization, also plays an important role in plaque vasa vasorum neoangiogenesis. The αvβ3 is highly expressed in atherosclerotic plaque intimal smooth muscle cells and by endothelial cells of angiogenic microvessels, as well as in plaque macrophages.
Imaging of plaque vasa vasorum neoangiogenesis was first performed by MRI using a nanoparticle agent targeting αvβ3 integrin [36]. Recently, a new 18F-labeled radiotracer (18F-galacto-RGD) was shown to be specifically taken up in atherosclerotic lesions of mouse aorta, and its uptake was associated with macrophage density [37]. Whether this signal of plaque vasa vasorum neoangiogenesis and inflammation can ultimately be used to evaluate atherosclerotic lesions in patients remains untested.
MMP Imaging
MMPs are expressed in atherosclerotic plaque and play an important role in plaque stability. MMPs mediate vascular remodeling after the breakdown of extracellular matrix. Gradual dissolution of matricial content by MMPs in the fibrous cap results in cap thinning and renders the atherosclerotic plaque unstable. Among patients with carotid lesions who were undergoing stent placement, baseline and post-stenting MMP levels were shown to be higher in patients with carotid lesions exhibiting high FDG uptake [38].
Imaging of MMP activity can be achieved by two ways. The first approach is to use MMP substrate linked to a radioligand, which can be cleaved and activated only in lesions expressing active MMP. The second approach is to use radiolabeled MMP inhibitors, which bind specifically to the lesions with significant MMP activity. Studies in animal models have shown clear visualization of 99mTc-MMP inhibitor in mouse aortic lesions by micro-SPECT. 18F-labeled MMP inhibitor (CGS 27023A) has also been synthesized for PET imaging.
Conclusions
Plaque macrophage has been the main target for atherosclerosis imaging. FDG is a nonspecific tracer and has been shown to accumulate in inflammatory sites, such as vulnerable atherosclerotic plaque. Preliminary studies in a small number of subjects have suggested a potential role for FDG PET/CT imaging in identifying vulnerable atherosclerotic plaques and monitoring treatment response. However, the limitations of the partial volume effect of small plaques, low target-to-background ratio of FDG uptake, and cardiac motion make direct visualization of atherosclerotic plaques in coronary arteries with the current PET/CT technology rather challenging. Nevertheless, in the near future, with the development of a PET/CT technology that is optimized for cardiovascular imaging, along with progress in standardization of cardiac imaging protocol (e.g., dietary preparation, acquisition mode, acquisition time after the injection of the radiotracer, and cardiac gating), direct visualization of coronary artery plaques may become clinically feasible. Identifying FDG uptake in vascular plaques may have clinical implications for medical therapy or intervention as well as preventing adverse cardiovascular events in the future. Although there are other emerging tracers that target specific pathways and molecules in atherosclerotic lesions, such as apoptosis, αvβ3 integrin, and MMP, they remain predominantly in the experimental phase.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance
Ambrose JA, Tannenbaum MA, Alexopoulos D, et al.: Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988, 12:56–62.
Little WC, Constantinescu M, Applegate RJ, et al.: Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988, 78:1157–1166.
Fuster V, Badimon L, Badimon JJ, et al.: The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 1992, 326:242–250.
Myerburg RJ: Sudden cardiac death in persons with normal (or near normal) hearts. Am J Cardiol 1997, 79:3–9.
Glagov S, Weisenberg E, Zarins CK, et al.: Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987, 316:1371–1375.
Motoyama S, Kondo T, Sarai M, et al.: Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007, 50:319–326.
Raman SV, Winner MW 3rd, Tran T, et al.: In vivo atherosclerotic plaque characterization using magnetic susceptibility distinguishes symptom-producing plaques. JACC Cardiovasc Imaging 2008, 1:49–57.
Chen W, Bural GG, Torigian DA, et al.: Emerging role of FDG-PET/CT in assessing atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging 2009, 36:144–151.
Dilsizian V, Narula J: Putting the face to a name: concurrent assessment of vascular morphology and biology. J Am Coll Cardiol Imaging 2009, 2:1243–1244.
Théron J, Tyler JL: Takayasu’s arteritis of the aortic arch: endovascular treatment and correlation with positron emission tomography. AJNR Am J Neuroradiol 1987, 8:621–626.
Yun M, Jang S, Cucchiara A, et al.: 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Semin Nucl Med 2002, 32:70–76.
Tatsumi M, Cohade C, Nakamoto Y, et al.: Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 2003, 229:831–837.
Ben-Haim S, Kupzov E, Tamir A, et al.: Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med 2004, 45:1816–1821.
Dunphy MP, Freiman A, Larson SM, et al.: Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med 2005, 46:1278–1284.
Ogawa M, Ishino S, Mukai T, et al.: (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 2004, 45:1245–1250.
Tawakol A, Migrino RQ, Hoffmann U, et al.: Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005, 12:294–301.
Zhang Z, Machac J, Helft G, et al.: Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: a histopathological correlation. BMC Nucl Med 2006, 6:3–10.
Davies JR, Izquierdo-Garcia D, Rudd JH, et al.: FDG-PET can distinguish inflamed from non-inflamed plaque in an animal model of atherosclerosis. Int J Cardiovasc Imaging 2009 Sep 22 (Epub ahead of print).
Rudd JH, Warburton EA, Fryer TD, et al.: Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002, 105:2708–2711.
Tawakol A, Migrino RQ, Bashian GG, et al.: In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006, 48:1818–1824.
Ogawa M, Magata Y, Kato T, et al.: Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med 2006, 47:1845–1850.
Tahara N, Kai H, Ishibashi M, et al.: Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006, 48:1825–1831.
Crisby M, Nordin-Fredriksson G, Shah PK, et al.: Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001, 103:926–933.
Lee SJ, On YK, Lee EJ, et al.: Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008, 49:1277–1282.
Davies JR, Rudd JH, Fryer TD, et al.: Identification of culprit lesions after transient ischemic attack by combined 18F Fluorodeoxyglucose positron-emission tomography and high-resolution magnetic resonance imaging. Stroke 2005, 36:2642–2647.
Arauz A, Hoyos L, Zenteno M, et al.: Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography. Pilot study. Clin Neurol Neurosurg 2007, 109:409–412.
• Paulmier B, Duet M, Khayat R, et al.: Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol 2008, 15:209–217. This retrospective study showed that vascular FDG uptake can be linked to cardiovascular events in humans.
• Alexanderson E, Slomka P, Cheng V, et al.: Fusion of positron emission tomography and coronary computed tomographic angiography identifies fluorine 18 fluorodeoxyglucose uptake in the left main coronary artery soft plaque. J Nucl Cardiol 2008, 15:841–843. This is an excellent case report showing coronary artery plaque FDG uptake.
• Wykrzykowska J, Lehman S, Williams G, et al.: Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT inpatients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 2009, 50:563–568. This paper shows evidence that myocardial background uptake can be suppressed by a high-fat, low-carbohydrate diet.
Buther F, Dawood M, Stegger L, et al.: List mode-driven cardiac and respiratory gating in PET. J Nucl Med 2009, 50:674–681.
Rudd JH, Myers KS, Bansilal S, et al.: (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007, 50:892–896.
Menezes LJ, Kotze CW, Hutton BF, et al.: Vascular inflammation imaging with 18F-FDG PET/CT: when to image? J Nucl Med 2009, 50:854–857.
Isobe S, Tsimikas S, Zhou J, et al.: Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med 2006, 47:1497–1505.
Zhao Y, Kuge Y, Zhao S, et al.: Comparison of 99mTc-annexin A5 with 18F-FDG for the detection of atherosclerosis in ApoE-/- mice. Eur J Nucl Med Mol Imaging 2007, 34:1747–1755.
Kietselaer BL, Reutelingsperger CP, Heidendal GA, et al.: Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med 2004, 350:1472–1473.
Winter PM, Morawski AM, Caruthers SD, et al.: Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation 2003, 108:2270–2274.
Laitinen I, Saraste A, Weidl E: Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging 2009, 2:331–338.
Wu YW, Kao HL, Chen MF, et al.: Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J Nucl Med 2007, 48:227–233.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Dilsizian, V. 18F-Fluorodeoxyglucose PET Imaging of Coronary Atherosclerosis and Plaque Inflammation. Curr Cardiol Rep 12, 179–184 (2010). https://doi.org/10.1007/s11886-010-0095-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-010-0095-8