Abstract
The use of injectable bulking agents is a well-established approach to management of patients with stress urinary incontinence (SUI). No single bulking agent to date has been shown to be superior or consistently durable in the literature. Novel therapeutic strategies, including the use of injectable, muscle-derived stem cell therapy, have shown promising results in investigational stages. Urethral bulking agent therapy can be helpful in the early management of men with SUI following radical prostatectomy, and in women with SUI due to intrinsic sphincter deficiency, urethral hypermobility, or in the setting of failed midurethral sling placement. Despite their widespread use historically, biocompatible agents have been supplanted in recent years by synthetic agents secondary to their potentially improved durability and nonimmunogenic profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress urinary incontinence (SUI) can be defined as the involuntary loss of urine during a period of increased abdominal pressure in the absence of detrusor activity [1]. SUI affects about 0.3% to 1.1% of men and 26% to 31% of women in the United States [2, 3]. The annual direct costs of treating incontinence are estimated at 15 billion dollars in the older adult population alone [4]. Urinary incontinence is associated with significant reductions in health-related quality of life [5]. The most common cause of SUI in men is iatrogenic (radical prostatectomy [RP] or transurethral resection of the prostate) [6]. SUI in women can be a result of urethral hypermobility, intrinsic sphincter deficiency (ISD), or a combination of both. ISD is related to an abnormal urethral sphincteric mechanism caused by neurological disease, prior surgery, denervation, or muscle damage during childbirth [1]. Several invasive surgical methods for the correction of SUI have been described in the literature. Urethral bulking agent injection is one of the newer, less invasive, but well-established techniques used in the treatment of SUI, particularly among female patients [1]. In this article, the proposed mechanisms through which bulking agents are thought to act; the ideal characteristics of urethral bulking agents; injection approaches; and available agents, including outcome data, novel injection therapies, and patient selection, are reviewed (Table 1).
Mechanism of Action
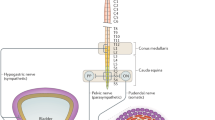
Although the mechanisms through which bulking agents improve SUI have not been completely elucidated, data exist that implicate their direct role in patients with ISD. It has been suggested that urethral mucosal coaptation is crucial to the maintenance of urinary continence. This coaptation is thought to be secondary to three main factors: 1) inherent properties of the mucosa itself, 2) functionality of the smooth muscles comprising the urethra, and 3) mucosal tissue compression via submucosal vascular cushions. Aberrations in any of these factors can result in the onset of symptomatic SUI. Submucosal urethral injection of bulking agents has been proposed to augment inward compressive forces toward the urethral lumen. This may lead to improved urethral coaptation and restoration of continence during periods of increased abdominal pressure [1, 7–9].
Ideal Profile of a Urethral Bulking Agent
The ideal injectable bulking agent should be biocompatible and hypoallergenic. Its active particles should be large enough to prevent distal migration away from the site of injection (diameter >80 μm). In addition, it should cause minimal inflammatory or foreign body reaction, be durable, and be easy to prepare and inject [7, 10].
Injection Technique
Bulking agents may be injected in a transurethral or periurethral fashion in women. Both approaches may be performed under local anesthesia with or without sedation; however, two main principles should be adhered to irrespective of injection technique: 1) the bulking agent should be injected slowly under direct vision into the proximal urethra; and 2) the injecting instrument should not be advanced beyond previously injected zones, as this may result in compression or extrusion of the bulking material. Although the two approaches have shown equivalence in efficacy when compared, periurethral injection has been associated with higher rates of acute urinary retention (AUR) and other adverse events [7, 9, 11].
Clinically Available Injectable Bulking Agents
Several bulking agents are available for clinical use in the management of SUI. For the sake of this review, these agents have been categorized as autologous, biocompatible, or synthetic.
Autologous Materials
Autologous Blood
The use of autologous blood was reported in a small series of 14 women. A total of 30 mL of blood collected from the antecubital vein into a heparinized syringe was injected into patients, rendering them continent within two treatment sessions. This effect, however, lasted for only about 10 to 17 days; thus, use of this agent has since been aborted [10].
Autologous Fat
The technique of fat harvesting for the use of injectable urethral bulking was first reported by Gonzalez de Garibay et al. [12] in ten women. Fat cells were harvested from the abdominal wall by liposuction, washed, and resuspended prior to injection [10]. In a study comparing the outcomes of periurethral injection of autologous fat versus saline in 68 women, the authors found that 22% of patients reported a cure or improvement in symptoms at 3 months, while 78% did not. Of the patients cured, many required multiple injections. Two deaths were also noted in the treatment group, one secondary to particle migration and subsequent fat embolism to the lung [7, 13]. The durability of autologous fat injection has been poor. As much as 60% of the autologus fat graft has been shown to be lost only 3 weeks following injection secondary to its rapid rate of resorption [14, 15]. As a result of its poor durability and potential association with systemic embolization and death, the use of autologus fat injection in the management of SUI has been discouraged [1, 7, 13].
Biocompatible Materials
Glutaraldehyde Cross-Linked Bovine Collagen
Glutaraldehyde cross-linked bovine collagen (GAX-collagen [Contigen; Bard Nordic, Helsingborg, Sweden]) is a biocompatible, biodegradable suspension of bovine collagen cross-linked with glutaraldehyde. It was initially approved by the US Food and Drug Administration (FDA) for the treatment of ISD in the latter part of 1993 and to date has been the most widely used and studied bulking agent. It contains at least 95% type I collagen and 1% to 5% type III collagen. Despite being rapidly degraded in vivo (within 19 months), urethral collagen injection results in the recruitment of host fibroblasts that ultimately contribute to the preservation of continence within hosts [16]. A skin test must be performed prior to injecting GAX-collagen to detect hypersensitivity reaction.
Given the variability in definitions of success, pooled analysis of GAX-collagen injection has been hampered. It has yielded mixed short-term results and poor durability. Cured or improved rates combined at a minimum of 1-year mean follow-up have ranged from 26% to 95% [9]. A review of 17 studies with a pooled population of 867 patients revealed short-term cure rates defined as complete dryness ranging from 30% to 78%. The average overall success rate was 76% [8, 17, 18]. Long-term data revealed that patients observed for up to 50 months after initial injection had a 30% and 40% cure and improved rate, respectively [19]. Additional studies examining long-term results (up to 2 years) following collagen injection suggest a continuous decline in cure and success rates over time, as well as the need for reinjection [8].
Complications associated with collagen injection from most to least common include de novo urgency (seen in 13% of patients), AUR (2% of patients), and sterile abscess formation. Despite being the most popular and well-studied urethral bulking agent, GAX-collagen will no longer be available for use.
Porcine Dermal Implant
Porcine dermal implant (Permacol; Covidien AG, Dublin, Ireland) is made up of nonreconstituted porcine dermal collagen. In contrast to GAX-collagen, a skin test need not be performed, as this product is nonallergenic. No studies have compared Permacol urethral injection with placebo control. A single study, however, compared the efficacy of Permacol with that of the synthetic agent Macroplastique (Uroplasty, Minneapolis, MN) in women with SUI. Fifty patients, 25 of whom received Permacol and 25 of whom received Macroplastique, were assessed at 6 weeks and 6 months. Mean age of patients was 61 years (range, 28–80 years). No statistical difference was noted in objective tests or improvement in symptoms of incontinence when the two groups were compared. Subjective tests, however, tended to favor Permacol. Complications associated with Permacol urethral injection include AUR (8% of patients) and urge incontinence (4%) [7, 20].
Synthetic Materials
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE [Teflon/polytef; DuPont, Wilmington, DE]) was initially described for use in the correction of SUI in the 1970s [10, 21]. It is produced via the pyrolysis of Teflon and comes packaged as a paste composed of PTFE particles, glycerin, and polysorbate [7]. PTFE particles are typically less than 50 μm in size, predisposing them to the proclivity for migration through phagocytosis into the reticuloendothelial system. This migration results in the formation of foreign body granulomas locally or distally. Locally, urethral fibrosis, diverticulum, and periurethral abscess resulting in bladder outlet obstruction have been reported [9, 22]. PTFE has been shown to have varying degrees of clinical effectiveness in the management of SUI. Lopez et al. [23] reported on the largest cohort of patients with long-term follow-up (mean, 31 months). Analyzing their 30-year experience using Teflon in a series of 128 women with SUI, the authors found an overall cure rate of 54.3%, with 73% of patients improved after injection [9, 24]. Continuing safety concerns surrounding this agent have limited its use [9].
Silicone Particles (Macroplastique)
Silicone particle microimplants were initially developed as an “off-the-shelf” alternative to Teflon [9]. The brand form is composed of highly textured polydimethylsiloxane macroparticles suspended within a bioexcretable, water-soluble carrier hydrogel. Particles typically range in size from 100 to 300 μm. Despite the large size, distal migration to sites such as the lungs, kidneys, brain, and lymph nodes has been demonstrated in canines 4 months following injection [9]. Although similar to Teflon with regard to particle migration, silicone microimplants do not cause a granulomatous response in patients. Overall rates of short-term success (<6 months) have ranged from 68% to 75% [9]. Several studies have reported on the durability of silicone’s efficacy in the management of SUI. Most recently, silicone was shown to yield sustained success in 84% of patients with SUI primarily due to ISD, who were dry or improved at 1 year (56 of 67 patients) [25•]. Despite its efficacy, concerns regarding particle migration and the possible association with the development of some types of collagen vascular disorders persist [1].
Carbon Beads
Carbon beads (Durasphere; Carbon Medical Technologies, St. Paul, MN) were approved by the FDA in 1999. The original formulation was a synthetic, nonbiodegradable, radiopaque product composed of pyrolytic, carbon-coated zirconium oxide beads suspended in a polysaccharide, water-based carrier gel. Particles in this formulation range in size from 251 to 550 μm, essentially prohibiting migration. The original formulation was similar to Teflon with regard to viscosity, requiring a greater injection force of this agent into the urethra. Recent concerns regarding migration after injection were raised based on the fact that direct embolization of this material could occur through high-pressure injection, resulting in material displacement into vascular and lymphatic spaces. This issue was addressed with the development of Durasphere-EXP, which contains smaller particles ranging in size from 95 to 200 μm [26, 27]. In a randomized, multicenter, double-blind study comparing GAX-collagen with Durasphere among 355 women with ISD, Durasphere was shown to be as effective as GAX-collagen at 12 months, with approximately 66% of patients gaining symptomatic benefit. The mean number of injections was similar for both groups (Durasphere, 1.69; bovine collagen, 1.55). Of note, there was a nearly 1.5-fold increased incidence of AUR in the Durasphere group [28]. Chrouser et al. [29] examined the long-term efficacy of Durasphere. They compared 56 women treated with Durasphere to age-matched patients treated with GAX-collagen between 1996 and 2000 to determine patient satisfaction and urinary continence after extended follow-up. Forty-three of the original 56 patients completed extended follow-up. At 24 and 36 months, Durasphere remained effective in 33% and 21% of patients, compared with 19% and 9% for GAX-collagen, respectively. However, at 51-month follow-up, only 21% of patients treated with Durasphere reported that their treatment remained effective [29]. Overall, Durasphere demonstrates short-term continence rates similar to those of GAX-collagen without the need for preinjection antigenic testing. However, its effects decline significantly over time.
Ethylene Vinyl Alcohol Copolymer
Ethylene vinyl alcohol copolymer (EVOH [Uryx/Tegress; Bard Nordic, Helsingborg, Sweden]) is an injectable solution of ethylene vinyl alcohol copolymer dissolved in a dimethyl sulfoxide carrier [30]. This agent has a distinctive ability to transition from a liquid to solid state within 60 s on contact with bodily tissues or fluid through the diffusion of dimethyl sulfoxide away from the copolymer. Preclinical animal studies showed no evidence of migration after injection [9, 30]. This agent has been approved by the FDA only for use via a transurethral approach, as a multicenter, prospective study of patients injected with EVOH via periurethral injection showed a higher rate of adverse events [31]. EVOH has been shown to be at least as effective as GAX-collagen [30]. Kuhn et al. [32] evaluated the long-term efficacy (median follow-up, 51 months) of 33 female patients with SUI as confirmed by urodynamics who were managed with EVOH. On follow-up, the patients were asked to use a visual analogue scale to measure their satisfaction in addition to undergoing uroflowmetry and cough testing. At the end of follow-up, 69% of patients considered themselves completely continent, while 69% were satisfied or very satisfied. Pad test was positive in 54.5% of patients, and cough test was positive in 60.6%. The authors concluded that EVOH has a 4.5-year success rate of 45%. Surprisingly, patient satisfaction did not correlate with objective dryness [32]. Long-term efficacy data for EVOH are sparse. Recent reports have demonstrated significant complication rates, with 37% of patients in one case series experiencing urethral erosion [31, 33].
Novel Urethral Bulking Agent Therapies
Calcium Hydroxylapatite
Calcium hydroxylapatite (CaHA [Coaptite; Boston Scientific, Natick, MA]) is a radiopaque synthetic material composed of calcium hydroxyl apatite spherical particles in an aqueous gel carrier. In animal models, this material has been shown to be biocompatible and nonimmunogenic [10]. In a recent multicenter, prospective, randomized trial, the safety and efficacy of CaHA were compared with that of GAX-collagen for the treatment of SUI in women due purely to ISD over 12 months. Using the Stamey Urinary Incontinence Scale to grade improvement as the primary end point of the study, the authors noted that 63.4% of patients treated with CaHA—compared with 57% of patients treated with GAX-collagen—showed improvement of one Stamey grade or more (P = 0.34) at 12 months. It was also noted that more CaHA patients required only one injection (38.0%, compared with 26.1% in the GAX-collagen group). In this study, CaHA was not associated with adverse events [34]. There has since been at least one report of a large urethral prolapse that occurred after a CaHA injection in a 67-year-old patient with two prior anti-incontinence surgeries [35]. Long-term data for this agent are limited.
Hyaluronic Acid and Dextranomer Microspheres
Deflux (Oceana Therapeutics, Edison, NJ) is a suspension of dextranomer microspheres in a viscous sodium hyaluronan carrier with an average particle size of 120 μm. This agent is nonimmunogenic and biodegradable [36, 37]. Local recruitment of fibroblasts, inflammatory cells, and blood vessels with subsequent deposition of collagen, resulting in the formation of an endogenous mass of soft fibrous tissue long after this agent is reabsorbed is responsible for urethral coaptation after injection. A total of 77% of the implant volume has been shown to be retained up to 1 year after implantation in animal models, and there has been no evidence to date of distant migration after injection [38]. One report from Europe examined the short-term follow-up data for this agent. Twenty women with SUI underwent transurethral injection of Deflux under local anesthesia. Outcomes were assessed subjectively with a visual analogue scale and questionnaire and objectively with short-term and 48-hour pad test. Eighty-five percent and 80% of patients were cured or improved, respectively, by subjective and objective criteria at a mean follow-up of 6 months. There were no complications related to the material itself. In 2009, Lightner et al. [39] demonstrated nonequivalence of dextranomer (Zuidex; Q-Med, Uppsala, Sweden) when compared with GAX-collagen in a multicenter trial. In addition, this agent was associated with a high complication rate when used in women with SUI secondary to ISD. Injection techniques varied between the agents (proximal vs midurethral), making it difficult to ascertain whether the equivalence and higher complications rates were secondary to injection strategy or inherent properties of the material [39]. The authors recently explored whether a cystoscopically directed proximal injection technique would result in fewer complications. In a retrospective case series of 56 women with SUI, 35 were found to have ISD. Four of these 35 women developed pseudoabscess formation with outlet obstruction requiring multiple operative interventions. Per validated questionnaire, the efficacy of this agent among patients with ISD was poor. The authors concluded that complications with cystoscopically injected dextranomer at the bladder neck occurred at a high rate [40•]. At present, with long-term follow-up data from Europe pending, this agent has not been widely recommended in the treatment of SUI.
Tissue Engineered Agents
It has been shown that the density of striated muscle cells in the rhabdosphincter gradually decreases with age in humans secondary to constant loss from apoptosis, which can lead to SUI [41]. A direct correlation was found between age and the decrease in volume and density of striated muscle cells; striated cells represented 87.6% and 34.2% of the rhabdosphincter in a newborn and a 92-year-old woman, respectively [42]. In addition, it has been shown that continent women on average had higher volumes of sphincter muscle tissue seen on three-dimensional ultrasound than those who were incontinence (3.75 vs 1.09 cm3) [43].
Stem cell therapy involving the use of selective autologus cell transplantation to create new, functional, nonimmunogenic tissue with durable survival in vivo has served as the basis for research encompassing this technology for the management of SUI in women caused by ISD. Donor tissue is harvested and separated into individual cells that are then attached to a support matrix and injected back into the host [9, 44, 45]. To date, autologous chondrocytes and muscle-derived stem cells (MDSCs) have been investigated in this regard.
Autologus Chondrocytes
In a human study, auricular cartilage was harvested and expanded in cell culture from women with documented ISD. After culture expansion, 32 patients received a single outpatient injection of harvested cells just distal to the bladder neck. Outcome measures, including voiding diary, quality-of-life scores, incontinence severity grading, and pad weight testing, were noted. Incontinence grading indicated that 32% of patients were dry, while 31% were improved at 12 months. In addition, quality-of-life scores improved significantly after treatment. There was a decrease in incontinence impact scores in all categories. Based on these findings, the authors concluded that endoscopic treatment of ISD with autologous chondrocytes is safe, effective, and durable, with 50% of patients dry 12 months after one injection. Eighty-one percent of study patients who were dry or improved at 3 months after the injection maintained this effect at 1-year follow-up [46].
Muscle-Derived Stem Cells
Two reports from Europe have described the harvesting and use of MDSCs in the management of SUI caused by ISD. In both reports, muscle biopsies were taken from the biceps of human hosts, separated into myoblasts and fibroblasts, and then allowed to expand in cell culture for 6 to 8 weeks. After adequate cell expansion, harvested cells were transferred to sterile syringes containing myoblasts or fibroblasts. Myoblasts and fibroblasts were suspended in two different media suited to maximize the durability of the cells in vivo following injection [45, 47].
In both reports, female patients with SUI due to ISD refractory to conventional therapy were injected with MDSCs under transurethral ultrasound guidance. The myoblast suspension was injected directly into the rhabdosphincter, while the fibroblast/collagen suspension was injected circumferentially into the submucosa. Patients were instructed to perform pelvic floor exercises for 12 weeks after injection, followed by transvaginal electric stimulation for 4 additional weeks [45, 47–49]. In one of the reports 79% of 123 women were fully continent and did not require the use of pads during their daily lives 1 year after injection. Incontinence and quality-of-life scores as well as rhabdosphincter function were notably improved after injection [48].
MDSC injections have yielded promising results in North America as well. Following MDSC injection under a local anesthetic, 62.5% of women (n = 8) had achieved complete continence at the end of follow-up (mean, 16.5 months; range, 3–24 months). No serious adverse events were reported [50].
Patient Selection
Early studies suggested that bulking agents were only ideally suited for older female patients with pure ISD, with multiple comorbidities precluding them from tolerating more invasive surgical approaches. Recent data, however, have supported the use of bulking agents in additional SUI patient populations.
In a retrospective study, Lee et al. [51] evaluated the efficacy of bulking agents for the treatment of recurrent or persistent SUI among 23 women who failed midurethral sling procedures. Patients were treated with Macroplastique or Durasphere. The median interval between midurethral sling placement and urethral bulking agent injection procedure was 12 months (range, 3–65 months). The cure rate at a median follow-up of 10 months was 34.8%; however, 92% of patients stated that they had benefited from the treatment. Meanwhile, 77% of patients reported that they were satisfied with the treatment. The authors concluded that bulking agent injection therapy for failed midurethral sling procedures demonstrated a low cure rate but high patient satisfaction with no significant complications [51]. As such, bulking agents should be considered as an alternative to more invasive techniques in this patient population.
Several studies conducted in patients with ISD and concomitant hypermobility have recently shown success rates similar to those in patients with ISD alone [36–38]. Herschorn et al. [38, 52] showed that patients with hypermobility could benefit from the injection of urethral bulking agents. No significant differences were noted in the patients with or without hypermobility. In fact, patients with hypermobility required less collagen to achieve a favorable outcome [1, 38, 52].
Most recently, ter Meulen et al. [53] in 2009 presented the results of a prospective, randomized controlled trial to evaluate the efficacy of Macroplastique injection in women with SUI and hypermobility without a history of previous incontinence surgery. Twenty-four women received injection therapy after an unsuccessful conservative treatment, while 21 controls underwent home-based pelvic floor muscle therapy. Patients were followed up at 3 and 12 months. It was noted that pad usage decreased significantly more in the Macroplastique group than in the control group (P = 0.015) at 3 months. According to physician and patient self-assessment, respectively, 71% and 63% of women in the Macroplastique group were considered cured or markedly improved, and these improvements were sustained at 12 months. In addition, quality-of-life scores were significantly higher among patients in the injection groups compared with those among controls (P = 0.017). Adverse events were mild and transient [53].
Urethral bulking agents also have been applied to male patients with SUI, particularly after RP. In men, circumferential endoscopic injection of GAX-collagen in the submucosa just distal to the urethral sphincter has been described. In men, postinjection results have been variable. GAX-collagen injection has been associated with a 58% improved or good result at a mean follow-up of 10.3 months [54]. Overall success rates have ranged from 17% to 38% [2]. At the most recent International Consultation on Incontinence, urethral bulking agents were considered as showing only modest success rates, with low cure rates for male SUI.
Conclusions
SUI is associated with substantial health care costs and a significant negative impact on the quality of life of patients living with the condition. The use of urethral bulking agents is a well-established therapeutic approach available for use in the management of SUI among certain populations of patients. Various materials are available for use; however, no one bulking agent to date has been shown to be consistently superior to another. This is mainly secondary to the wide variability in success rate definitions coupled with the lack of large randomized controlled trials. For clinically available bulking agents, early outcomes have ranged from 22% to 78% for cure. Despite historical trends toward using biocompatible material, recent shifts toward the use of synthetic materials have occurred as a result of their theoretical decreased immunogenicity and improved durability. Although GAX-collagen has been the most widely used and examined urethral bulking agent, it is no longer being manufactured. Silicone in small patient series has shown promising durability.
Although originally indicated for older women with SUI secondary to ISD without hypermobility, recent studies have shown favorable outcomes in all patients with SUI independent of urethral mobility. In addition, urethral bulking agents should be considered early on in men with SUI who fail conservative therapy after RP or female patients who have failed prior midurethral sling placement.
Stem cell injection has shown a promising efficacy and safety profile among women with SUI in Europe and Canada. In the future, larger randomized studies should be performed to evaluate the short- and long-term effects of this therapy, particularly given the existence of such exciting, promising data from prior international studies.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance
Kerr LA. Bulking agents in the treatment of stress urinary incontinence: history, outcomes, patient populations, and reimbursement profile. Rev Urol. 2005;7 Suppl 1:S3–S11.
Sandhu JS. Treatment options for male stress urinary incontinence. Nat Rev Urol. 2010;7(4):222–8.
Fultz NH, Burgio K, Diokno AC, Kinchen KS, Obenchain R, Bump RC. Burden of stress urinary incontinence for community-dwelling women. Am J Obstet Gynecol. 2003;189(5):1275–82.
Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63(3):461–5.
van der Vaart CH, de Leeuw JR, Roovers JP, Heintz AP. The effect of urinary incontinence and overactive bladder symptoms on quality of life in young women. BJU Int. 2002;90(6):544–9.
Borgermann C, Kaufmann A, Sperling H, Stohrer M, Rubben H. The treatment of stress incontinence in men: part 2 of a series of articles on incontinence. Dtsch Arztebl Int. 2010;107(27):484–91.
Keegan PE, Atiemo K, Cody J, McClinton S, Pickard R. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2007;3:CD003881.
Dmochowski RR, Appell RA. Injectable agents in the treatment of stress urinary incontinence in women: where are we now? Urology. 2000;56(6 Suppl 1):32–40.
Kershen RT, Dmochowski RR, Appell RA. Beyond collagen: injectable therapies for the treatment of female stress urinary incontinence in the new millennium. Urol Clin North Am. 2002;29(3):559–74.
Appell RA WJ. Campbell-Walsh urology. In: Editor-in-chief, Wein AJ; Kavoussi LR, et al., editors. 3. Saunders, Philadelphia; 2007.
Schulz JA, Nager CW, Stanton SL, Baessler K. Bulking agents for stress urinary incontinence: short-term results and complications in a randomized comparison of periurethral and transurethral injections. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(4):261–5.
Gonzalez de Garibay AS, Castillo Jimeno JM, Villanueva Perez I, Figuerido Garmendía E, Vigata López MJ, Sebastían Borruel JL. Treatment of urinary stress incontinence using paraurethral injection of autologous fat. Arch Esp Urol. 1991;44(5):595–600.
Lee PE, Kung RC, Drutz HP. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol. 2001;165(1):153–8.
Bartynski J, Marion MS, Wang TD. Histopathologic evaluation of adipose autografts in a rabbit ear model. Otolaryngol Head Neck Surg. 1990;102(4):314–21.
Lightner DJ. Review of the available urethral bulking agents. Curr Opin Urol. 2002;12(4):333–8.
Remacle M, Marbaix E. Collagen implants in the human larynx. Pathological examinations of two cases. Arch Otorhinolaryngol. 1988;245(4):203–9.
Sharifi-Aghdas F. Surgical management of stress urinary incontinence. Urol J. 2005;2(4):175–82.
Walters MD, Daneshgari F. Surgical management of stress urinary incontinence. Clin Obstet Gynecol. 2004;47(1):93–103.
Faerber GJ. Endoscopic collagen injection therapy in elderly women with type I stress urinary incontinence. J Urol. 1996;155(2):512–4.
Bano F, Barrington JW, Dyer R. Comparison between porcine dermal implant (Permacol) and silicone injection (Macroplastique) for urodynamic stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(2):147–50. discussion 150.
Berg S. Polytef augmentation urethroplasty. Correction of surgically incurable urinary incontinence by injection technique. Arch Surg. 1973;107(3):379–81.
McKinney CD, Gaffey MJ, Gillenwater JY. Bladder outlet obstruction after multiple periurethral polytetrafluoroethylene injections. J Urol. 1995;153(1):149–51.
Lopez AE, Padron OF, Patsias G, Politano VA. Transurethral polytetrafluoroethylene injection in female patients with urinary continence. J Urol. 1993;150(3):856–8.
Buckley J, Lingham K, Meddings R, et al. Injectable silicone microparticles for female stress incontinence. Proceedings of the British Association of Urologic Surgeons Bristol, United Kingdom; 1995.
• Ghoniem G, Corcos J, Comiter C, Westney OL, Herschorn S. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol. 2010;183(4):1444–9. For urethral injection of Macroplastique (silicone), substantial durable results were sustained during 2 years, with 84% of patients maintaining significant Stamey grade improvement from the 12-month assessment.
Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;166(4):1350–3.
Ritts RE. Re: particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2002;167(4):1804–5.
Lightner D, Calvosa C, Andersen R, Klimberg I, Brito CG, Snyder J, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of Durasphere. Urology. 2001;58(1):12–5.
Chrouser KL, Fick F, Goel A, Itano NB, Sweat SD, Lightner DJ. Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended follow-up. J Urol. 2004;171(3):1152–5.
Dmochowski R. Multicenter randomized controlled study to evaluate Uryx urethral bulking agent in treating female stress urinary incontinence. Proceedings of the International Continence Society, 32nd Annual Meeting. 2002.
Administration USFaD: Uryx? Urethral Bulking Agent-P030030: Summary of safety and effectiveness data. 2004.
Kuhn A, Stadlmayr W, Sohail A, Monga A. Long-term results and patients’ satisfaction after transurethral ethylene vinyl alcohol (Tegress) injections: a two-centre study. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(4):503–7.
Hurtado E, McCrery R, Appell R. The safety and efficacy of ethylene vinyl alcohol copolymer as an intra-urethral bulking agent in women with intrinsic urethral deficiency. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):86973.
Mayer RD, Dmochowski RR, Appell RA, Sand PK, Klimberg IW, Jacoby K, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69(5):876–80.
Lai HH, Hurtado EA, Appell RA. Large urethral prolapse formation after calcium hydroxylapatite (Coaptite) injection. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(9):1315–7.
Swami S, Batista JE, Abrams P. Collagen for female genuine stress incontinence after a minimum 2-year follow-up. Br J Urol. 1997;80(5):757–61.
Steele AC, Kohli N, Karram MM. Periurethral collagen injection for stress incontinence with and without urethral hypermobility. Obstet Gynecol. 2000;95(3):327–31.
Herschorn S, Radomski SB. Collagen injections for genuine stress urinary incontinence: patient selection and durability. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(1):18–24.
Lightner D, Rovner E, Corcos J, Payne C, Brubaker L, Drutz H, et al. Randomized controlled multisite trial of injected bulking agents for women with intrinsic sphincter deficiency: mid-urethral injection of Zuidex via the Implacer versus proximal urethral injection of Contigen cystoscopically. Urology. 2009;74(4):771–5.
• Lightner DJ, Fox J, Klingele C. Cystoscopic injections of dextranomer hyaluronic acid into proximal urethra for urethral incompetence: efficacy and adverse outcomes. Urology. 2010;75(6):1310–4. This study highlighted a high rate of complications associated with cystoscopically injected dextranomer hyaluronic acid at the bladder neck, suggesting that this approach should be abandoned.
Strasser H, Tiefenthaler M, Steinlechner M, Bartsch G, Konwalinka G. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet. 1999;354(9182):918–9.
Strasser H, Tiefenthaler M, Steinlechner M, Eder I, Bartsch G, Konwalinka G. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol. 2000;164(5):1781–5.
Digesu GA, Robinson D, Cardozo L, Khullar V. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2009;28(1):90–4.
Kershen RT, Fefer SD, Atala A. Tissue-engineered therapies for the treatment of urinary incontinence and vesicoureteral reflux. World J Urol. 2000;18(1):51–5.
Proano AR, Medrano A, Garrido G, Mazza O. Muscle-derived stem cell therapy for stress urinary incontinence. Actas Urol Esp. 2010;34(1):15–23.
Bent AE. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20(2):157–65.
Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Frauscher F, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet. 2007;369(9580):2179–86.
Mitterberger M, Marksteiner R, Margreiter E, Pinggera GM, Colleselli D, Frauscher F, et al. Autologous myoblasts and fibroblasts for female stress incontinence: a 1-year follow-up in 123 patients. BJU Int. 2007;100(5):1081–5.
Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, et al. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008;53(1):169–75.
Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(6):881–3.
Lee HN, Lee YS, Han JY, Jeong JY, Choo MS, Lee KS. Transurethral injection of bulking agent for treatment of failed mid-urethral sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21(12):1479–83.
Herschorn S, Radomski SB, Steele DJ. Early experience with intraurethral collagen injections for urinary incontinence. J Urol. 1992;148(6):1797–800.
ter Meulen PH, Berghmans LC, Nieman FH, van Kerrebroeck PE. Effects of Macroplastique Implantation System for stress urinary incontinence and urethral hypermobility in women. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(2):177–83.
Cummings JM, Boullier JA, Parra RO. Transurethral collagen injections in the therapy of post-radical prostatectomy stress incontinence. J Urol. 1996;155(3):1011–3.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taylor, A.K., Dielubanza, E. & Hairston, J. Use of Injectable Urethral Bulking Agents in the Management of Stress Urinary Incontinence. Curr Bladder Dysfunct Rep 6, 159–166 (2011). https://doi.org/10.1007/s11884-011-0104-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11884-011-0104-9