Abstract
Purpose of Review
Although genetic factors clearly play a role in the development of atopic dermatitis (AD), the recent dramatic increase in the prevalence of AD in low- and middle-income countries is not consistent with only a role of genetic factors. These findings strongly suggest that environmental factors may play an important role in the pathogenesis of AD.
Recent Findings
We reviewed the role of gene-environment studies; in utero exposures including tobacco smoke, alcohol, maternal stress, various digestive supplements, and gestational diabetes; early-life exposures including diet, gut microbiota, antibiotics, and breastfeeding; climate including temperature, ultraviolet radiation exposure, and air pollution; and household products, indoor allergens, water hardness, pH, and skin microbiota and their effects on AD.
Summary
Environmental factors definitely play a role in the pathogenesis of AD. However, identifying definitive factors continues to be difficult in the setting of conflicting evidence and the complex interactions between genotypes and the environment resulting in a multitude of AD phenotypes. All of the different environmental interactions discussed highlight the importance of intervening on multiple levels in a patient’s environment to improve or even prevent AD symptoms. Further, the importance of modifying environmental factors early on in a person’s life is demonstrated. When possible, all of these environmental factors should be considered in treating a patient with AD and the appropriate modifications should be made at population and individual levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
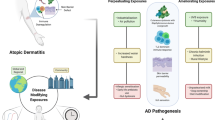
The recent dramatic global increase in the prevalence of atopic dermatitis (AD) [1] suggests that shifting environmental factors play a role in the development of AD [2, 3]. Numerous environmental factors, such as climate and stress, can be triggers of itch in AD [4]. This article is a non-systematic review of the current evidence for environmental risk factors in AD (Table 1). In general, we address systematic reviews and level of evidence where available. Otherwise, we address key individual studies of exposures.
AD Pathogenesis
AD is caused by a complex overlap of genetic, immunologic, and environmental factors.
Parental history of AD is associated with a 2–3-fold increased risk of childhood AD [5]. Monozygotic twins have ~ 3 times higher concordance rate than dizygotic twins [6]. The strongest genetic risk factor for AD is loss-of-function mutations of the filaggrin (FLG) gene, contributing to skin-barrier dysruption, [7] though many AD patients do not have FLG mutations, and ~ 40% of individuals with FLG null mutations do not have AD [8]. Another gene implicated is the serine protease inhibitor Kazal-type 5, which is important in skin-barrier homeostasis, including stratum corneum desquamation, lipid barrier construction, and development of the cornified cell envelope (CE) [9].
Keratinocytes produce antimicrobial peptides (AMPs) (e.g., human β-defensin 1) [9], and pattern recognition receptors recognize molecules found on pathogens and activate microbial and pro-inflammatory responses required to eliminate the infectious agents [10]. S. aureus colonizes skin of 30–100% of AD patients, but only 20% of healthy people [11]. S. aureus colonization may precede AD flares and facilitate inflammation in AD [12]. Consequently, mutations in pattern recognition receptors (e.g., toll-like receptor 2 (TLR2), TLR6, TLR9) and AMPs may play a role in the initiation and exacerbation of AD [9]. However, it is unclear if microbial change is a cause of or effect from barrier dysfunction and cutaneous inflammation [11].
Th2 effector cells producing interleukins (IL)-4, 5, 13, and 31 and Th22 cells producing IL-22 and S100A proteins predominate in the acute and chronic phases of disease, with increased IL-17 production shown in a subset of patients [13, 14]. These mediators are implicated in downregulation of terminal differentiation genes and tight junction products (e.g., claudins), which contribute to barrier defects in AD [13]. Keratinocytes differentiated in the presence of IL-4 and IL-13 exhibited reduced FLG gene expression, even in patients without FLG null mutations, and also downregulated loricrin and involucrin, important proteins that facilitate the terminal differentiation of the epidermis and formation of the CE. While Th2 cells facilitate Staphylococcus aureus binding and colonization, IL-4 and IL-13 inhibit skin production of AMPs, predisposing AD skin to staphylococcus infections. IL-31 is implicated in itch and skin-homing of T cells [13, 15]. Chronic AD lesions are also associated with enhanced IL-5 and IL-12 production, and increased Th1 cells and related cytokines (e.g., interferon gamma) [14, 15].
Gene-Environment Interactions
Individual responses to environmental exposures are driven by gene-environment interactions (GEIs). A systematic review (SR) of GEI in AD found that FLG null mutations were most widely replicated across studies and had the strongest effect size. GEIs were demonstrated between FLG genotype and multiple risk factors of AD, such that older siblings, phthalate exposure in household dust and urine phthalate metabolite levels, early-life exposure to cats, and water hardness were associated with increased AD risk. Breastfeeding duration was inversely associated with AD risk. There were no interactions between FLG and day-care attendance, gender, maternal parity, maternal AD, maternal smoking, early-life environmental tobacco smoke exposure, birth year, serum vitamin D levels, or maternal IgE sensitization. Evidence for GEI in AD is relatively limited, with few studies, small sample sizes, and study results not being replicated [16].
In Utero Exposures
Smoking
A SR and meta-analysis found that a diagnosis of AD is associated with higher odds of active smoking and exposure to passive smoke in both adults and children. In comparison, no association between AD and maternal smoking during pregnancy overall; however, in sensitivity analyses, childhood AD was associated with maternal smoking during pregnancy in Asian cohort studies, and inversely associated with maternal smoking in studies with a sample size ≥ 5000. No differences were observed between AD prevalence and amount of smoking (n = 5) [17]. A Japanese cross-sectional study found that fetal exposure to tobacco smoke after 28 weeks of gestation was associated with higher cumulative incidence of AD. Smoke exposure during the first 6 months of life was not associated with cumulative incidence of AD [18].
The mechanism of maternal smoking increasing risk of childhood AD is unclear. In one study, maternal smoking after 24 weeks of gestation altered DNA methylation of the aryl hydrocarbon receptor (AhR) in newborns with smoking-induced epigenetic changes persisting 18 months post-birth [19]. Polychlorinated biphenyls (PCBs) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are environmental pollutants present in cigarette smoke, whose biological effects are mediated by AhR [20]. However, the association of in utero smoking and childhood AD may be confounded by other AD risk factors, such as race/ethnicity, education, and socioeconomic status that account for this association. The regional differences for the association between childhood AD and maternal smoking during pregnancy may be related to public health policy or cultural differences such as prohibition against smoking indoors versus climate differences (e.g., humidity) [17]. Further large-scale studies are needed to determine the contribution of smoking during pregnancy toward the development of childhood AD while controlling for the various confounding factors mentioned above to elucidate regional differences.
Alcohol
Analysis of 2010–2012 National Health Interview Survey Data found that adult AD was associated with greater odds of ever drinking ≥ 12 alcoholic beverages annually, including current moderate and heavier intake [21]. However, a SR and meta-analysis of 12 studies found mixed results for the association between alcohol consumption and AD. There was an association between alcohol use during pregnancy and development of AD in offspring. However, there was no consistent association between alcohol use and AD in adults and adolescents [22].
Fish Oil and Omega-3 Fatty Acids
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are long-chain omega-3 polyunsaturated fatty acids (n-3 PUFA), found in oily fish, that are thought to inhibit the formation of prostaglandin E2 and leukotriene B4 and might protect against allergic diseases [23]. Conflicting results were found regarding n-3 PUFA supplementation during pregnancy and risk of infantile AD [24]. Three RCTs examined the development of food allergy and eczema after n-3 PUFA supplementation during pregnancy [25]. One study demonstrated a reduction in incidence of IgE-associated eczema in infants with a family history of allergic disease after very high doses of DHA and EPA [26]. In a prospective birth cohort study, high maternal intake of margarine and vegetable oils during the last 4 weeks of pregnancy was associated with AD during the first 2 years of the offspring’s life, whereas high maternal fish intake was inversely associated with AD [27]. Similar results were observed in a longitudinal birth cohort study that followed children until age 5 years [28].
Pooled results from a SR and meta-analysis (10 prospective cohort studies, 5 RCTs) showed reduced AD in the first 12 months of life with maternal intake of n-3 PUFAs, albeit with considerable heterogeneity of results [29].
Hygiene Hypothesis
The hygiene hypothesis states that the decreasing incidence of infections in western countries (and more recently developing countries) is leading to increasing incidence of autoimmune and allergic diseases [30]. Conversely, early-life exposures to microbes and their products may help steer the immature immune system away from AD and allergic disease. In several studies, maternal exposure to farm animals in pregnancy was associated with reduced risk of childhood AD, and increased immune responses that may protect against asthma and allergies [31]. Further, early-life exposure to older siblings or children in day-care nurseries may protect against atopy [32]. A SR of seven observational studies showed that prenatal antibiotic use was associated with increased eczema in infancy, and antibiotic exposure in utero was likely associated with eczema even after 1 year of age [33].
Proposed mechanisms for the hygiene hypothesis include decreased antigenic stimulation from fewer childhood infections leading to decreased levels of regulatory cytokines (e.g., IL-10 and TGF-β). A matched case-control study found that incident AD was lower for children who had ≥ 4 infections vs. no infections. Daily contact with dogs was also inversely associated with AD risk [32].
Immune stimulation by endotoxin may also be important for maturation of the immune system [34]. A cross-sectional study of 812 children ages 6–13 years from farming and nonfarming households in rural Europe found that higher endotoxin levels in mattress dust correlated with decreased frequency of hay fever, allergic asthma, and allergic sensitization [35]. However, not all microbial pathogens protect against AD. Respiratory syncytial virus appears to increase the risk of AD [15]. Thus, each pathogen may have distinct effects on AD and allergic risk.
Prebiotics
The effects of prebiotics and probiotics in AD and allergic diseases may be related to the hygiene hypothesis [36]. Prebiotics are non-digestible fibers (e.g., oligosaccharides) that stimulate the growth and/or activity of gut microbiota (probiotics) for beneficial effects [37]. The effect of prebiotic supplementation on the prevention and treatment of AD has been less studied than the effect of probiotic supplementation. A 2013 Cochrane review of RCTs or quasi-RCTs found that prebiotics given to infants reduced AD risk [38]. A double-blind, placebo-controlled RCT fed a prebiotic-supplemented (0.8 g/100 ml short-chain galactofructoside [scGOS]/long-chain fructo-oligosaccharide [lcFOS]) or placebo-supplemented (0.8 g/100 ml maltodextrin) hypoallergenic formula to infants during the first 6 month of life and found lower 5-year cumulative incidence of AD in the scGOS/lcFOS vs. placebo group; persistent AD was numerically less common [39]. Further interventional studies are needed to determine definitive risk reduction.
Probiotics
Probiotics may inhibit Th2 and stimulate Th1 cytokines, e.g., interferon gamma [36]. In murine studies, probiotics reduced AD symptoms, IgE levels, infiltration of lymphocytes and granulocytes, and levels of IL-4, 5, 10, and 13 [40]. A meta-analysis of 22 RCTs showed that probiotic supplementation during pregnancy and/or infancy reduced AD incidence. Intervention with Lactobacillus and Bifidobacterium had the largest effect. Probiotic supplementation during pregnancy and/or infancy was more effective at preventing AD in children age ≤ 2 vs. > 2 years [41].
Postbiotics
Postbiotics (e.g., microbial cells, cell constituents, and metabolites) are bioactive compounds produced by food-grade microorganisms during fermentation [42]. Little evidence is available regarding the use of postbiotics during the prenatal or postnatal periods for AD prevention. A pilot study found that ingesting fermented rice flour containing Lactobacillus paracasei CBA-L74 for 12 weeks was associated with lower SCORAD scores in children ages 6 months to 6 years; all had a SCORAD < 35 and reduced or suspended topical corticosteroid application [43]. Further large-scale RCTs are needed to draw definitive conclusions.
Gestational Diabetes
A study conducted in 680 children, with mother-child dyads recruited at birth and followed prospectively until age 3.2 ± 2.3 years, showed that gestational diabetes mellitus (GDM) was associated with childhood AD and early allergen sensitization independent of maternal pre-pregnancy BMI and fetal growth. These associations were not observed in preterm births [44]. One study showed that at 6-month post-delivery, 30.8% of offspring from mothers who had GDM developed AD, food allergy, or allergic proctocolitis compared to none from the healthy controls group. At the third trimester of pregnancy, blood samples from the GDM group had a higher proportion of Th2, Th17, and Treg cells compared to the control group. Higher circulating c-reactive protein and total IgE levels were noted in the GDM group [45].
The mechanism by which GDM affects AD and allergen sensitization remains unclear. Placental microbiota may contribute to fetal homeostasis and healthy barrier function. Placentas in women with GDM showed lower amounts of bacteria belonging to the Pseudomonadales order and Acinetobacter genus compared to healthy controls. Lower levels of Acinetobacter were associated with lower placental expression of IL-10, a key anti-inflammatory cytokine, and TIMP3. Hence, GDM may modify the placental microbiome, leading to lower IL-10 levels, pushing toward allergen sensitization and AD development [46].
Maternal Stress
Data from 13,782 pediatric subjects (8,091 mothers) < 18 years in the 2007–2014 Korea National Health and Nutrition Examination Surveys showed that maternal depression was associated with children’s AD [47]. Data from the Fragile Families and Child Wellbeing Study found that maternal depression in the past year was associated with higher odds of AD at age 5, 9, and/or 15 years in US children. Postpartum depression was also associated with more persistent AD in children, [48] whereas a Danish study of 8,062 children with AD found no associations between paternal or prenatal maternal psychiatric disease with AD in the offspring [49].
In a SR of 11 studies, maternal stress was associated with eczema risk in their offspring [50]. Stress factors included the following: depression, postpartum depression, prenatal anxiety, maternal stress during pregnancy, prenatal adverse life events, job strain during pregnancy, prenatal distress, and perceived stress. The limited number and heterogeneity of studies precluded determining the time period in pregnancy most impacted by maternal stress [50].
Similar results were found in a study of two general population-based cohorts (Cohort for Childhood Origin of Asthma and Allergic Diseases [COCOA] and the Panel Study on Korean Children [PSKC]). Prenatal maternal distress increased AD risk in offspring in COCOA and PSKC. In COCOA, prenatal maternal depression and anxiety scores were related to the predicted probability of AD. Prenatal distress decreased placental glutathione to glutathione disulfide ratios and 11β-hydroxysteroid dehydrogenase type 2 levels, especially in those who developed AD and increased IgE levels at age 1 year [51].
A recent SR and meta-analysis (n = 6 studies) showed the prevalence of parental depression to be higher in children with AD versus without AD or healthy controls [52]. Caring for children with AD may lead to parental stress that may increase the incidence rates of AD in future offspring.
Early-Life Exposures
Maternal Diet
A 2014 Cochrane review of five trials involving 952 participants did not observe a protective effect of maternal dietary antigen avoidance during pregnancy on the incidence of AD in the first 18 months of life, but did note an association with lower mean gestational weight [53]. However, higher concentrations of vitamin C in breast milk were associated with reduced risk of atopy in the infant [54]. Similarly, low vitamin D levels in breastmilk might be a risk factor for infantile AD [55].
Birth Weight
A SR of 42 studies showed that a birth weight increase of 1 kg was associated with a 17% greater risk of AD in children, and a 34% greater risk of ever or current AD in infants up to 2 years of age [56]. A study of Japanese children found that AD occurred at a lower prevalence at age 18 months but not at 3 years among those who had low (< 2500 g) vs. normal (> 2500 g) birthweight [57]. Similarly, a SR and meta-analysis (n = 10 studies) found that low birth weight was a protective factor against the occurrence of AD. Moreover, increased birth weight was a risk factor for AD [58].
Vaginal vs. Cesarean Section
In a study examining the relationship between mode of delivery and the structure of the initial microbiota body habitats in newborns, vaginally delivered infants acquired bacterial communities (in all body habitats—skin, oral, nasopharyngeal, and gut) resembling their own mother’s vaginal microbiota, dominated by Lactobacillus, Prevotella, or Sneathia spp., and cesarean section (C-section) infants harbored bacterial communities (in all body habitats—skin, oral, nasopharyngeal, and gut) similar to those found on the skin surface, dominated by Staphylococcus, Corynebacterium, and Propionibacterium species [59]. Children born by C-section, especially those with assisted birth, were at greater risk for developing asthma, flexural eczema, and sensitization [60]. In contrast, a large cohort study found an association between C-section and AD, but this effect was attenuated after adjustment. In stratified analyses, there was some evidence that C-section may increase AD risk among certain subgroups such as firstborns or overweight/obese pre-pregnancy BMI, but the associations were weak [61].
Breastfeeding
Breast milk is rich in PUFAs. As mentioned previously, PUFAs can be categorized as n-3 or n-6 PUFAs; while n-3 PUFAs have anti-inflammatory properties and stabilize T cell membranes, n-6 PUFAs can enhance inflammatory responses [62]. In a recent SR and meta-analysis of 27 prospective cohort studies, the pooled estimate for the effect of exclusive breastfeeding on AD was not significant. Heterogeneity was substantial across studies. There was weak evidence for a protective effect of breastfeeding against AD in cohorts with a history of family atopy. In cohorts without atopic heredity, there was actually an increased risk of AD in exclusively breast-fed infants [63].
An observational study of eighty-seven exclusively breast-fed infants with AD found that discontinuation of breastfeeding and shifting to partially hydrolyzed whey formula might actually improve symptoms and shorten AD duration, regardless of sex, age, and parental atopy history [64]. A 2015 SR including 42 studies found low- to very-low-grade quality evidence, from pooling six cohort studies, that exclusive breastfeeding for greater than 3–4 months vs other feeding types was associated with a reduced risk of eczema up to 2 years of age [65]. More well-designed interventional studies are needed to conclusively determine the role of breastfeeding and its effects on AD.
A recent SR and meta-analysis of 45 studies from 20 different countries showed that no associations were observed between early introduction of cow’s milk or cow’s milk formula and the development of eczema or AD. Little high-quality evidence was available [66]. A meta-analysis of 17 studies showed that timing of solid food introduction was not associated with eczema. One controlled trial provided weak evidence for early introduction of allergenic foods around 4 months with reduced risk of eczema [67].
Childhood and Adolescent Diet
Diet may play a role in the development of AD, especially in children and adolescents [68]. Of note, many studies examined the relationship of different aspects of diet and nutrition on AD. However, each aspect was only assessed in one or two studies, limiting evidence-based conclusions about their role in AD.
A cross-sectional analysis from the 2017 Korean Youth Risk Behavior Web-based survey found that adolescents with a 1-year vs. past history of diagnosed AD were more likely to consume fast foods, energy drinks, or convenience food, especially in high school students [69]. Findings from the International Study of Asthma and Allergies in Childhood Phase III found increased eczema prevalence and severity in children and adolescents who consumed fast food ≥ 3 times per week. In particular, consumption of butter, eggs, margarine, nuts, pasta, potato, rice, and seafood ≥ 3 times per week was associated with increased eczema prevalence [70]. Consumption of hamburgers was associated with eczema severity [71].
A survey of 169 patients found that dietary modification best improved skin in AD when white flour products, gluten, and nightshades were removed, and vegetables, organic foods, and fish oil were added [72].
Short-chain fatty acids (SCFA) exert anti-inflammatory responses and modulate itch through neuroendocrine mechanisms mediated by the gut. A diet rich in fat and low in fiber was shown to alter the gut microbiome by impairing SCFA production and shifting immune homeostasis toward a pathogenic Th2 phenotype [73]. A murine study showed that western diet for 10 months led to increased incidence of dermatitis compared to a control diet. Bile acid receptors, e.g., Takeda G protein receptor 5 and sphinosine-1-phosphate receptor 2, IL-17A, IL-6, TNFα, IL-23, IL-4, and IL-31, were elevated in lesional skin [74]. These may regulate itch, keratinocyte proliferation, metabolism, and/or inflammation.
Future large-scaled prospective and interventional studies are needed to fully confirm the association between AD and diet and the possibility that diet and weight control in childhood may help to mitigate or even prevent AD or symptom development.
Micronutrients
A SR of ten studies found no consistent associations between maternal vitamin D levels in pregnancy or at birth and risk of developing eczema in infants (≤ 1 year old) [75]. Another SR and meta-analysis found that lower maternal vitamin D during pregnancy was associated with increased risk of childhood eczema [76]. A meta-analysis of three vitamin D supplementation trials found clinical meaningful improvements of SCORAD scores, with greatest improvement in trials lasting 3 months. AD patients, especially children, may have decreased vitamin D levels, and benefit from supplementation, though more studies are needed to support definitive recommendations [77].
There is limited and inconsistent evidence to support the relationship between AD and other micronutrient deficiencies, or whether micronutrient supplementation improves AD. A SR including 49 studies examined the relationship between oral micronutrients and AD. One study of 17 young adults (ages 20–42 years) found that AD severity, as judged by SCORAD, increased as plasma vitamin C levels and levels of epidermal ceramides decreased [78]. A prospective follow-up study from birth to 48 months of 159 children with a family history of allergic disease found that increased intake of retinol, calcium, and zinc with perinatal administration of probiotics reduced the risk of AD while an increase in vitamin C intake increased the likelihood of AD [79].
Vitamin E impedes synthesis and release of prostaglandins [80]. In a double-blind, placebo-controlled RCT of 70 patients with mild-to-moderate AD, vitamin E (400 IU/day) treatment for 4 months was associated with improvement in itching, extent of lesion, and SCORAD index [81]. In a food-questionnaire study of 180 children with AD and 242 without AD, dietary vitamin E levels were inversely associated with AD, and reduced AD risk was found with increased serum alpha-tocopherol [82].
Zinc serves as a cofactor in cellular growth, proliferation, and regeneration and is thought to possess anti-inflammatory properties [80]. Serum zinc levels were measured in 65 children with AD and were lower than in healthy controls [83]. An 8-week double-blind placebo-controlled RCT of oral zinc sulfate 185.4 mg per day in 50 children with AD, ages 1–16 years, did not show improvement in disease severity [84]. In contrast, in 58 children with AD (2–14 years of age), low hair zinc levels were found at baseline. After 8 weeks of zinc supplementation, eczema assessment severity index (EASI) scores, TEWL, visual analogue scales for pruritus, and hair zinc levels improved in comparison to children with AD [85]. While individual studies showed some link between AD and micronutrient deficiency or improvement of AD with micronutrient supplementation, the level of evidence to support clinical recommendations is very weak.
Climate
A SR explored the mechanisms for worsening AD in the wintertime and proposed that low humidity and temperatures can lead to an overall decrease in skin-barrier function making it more susceptible to mechanical stress and more reactive toward skin irritants and allergens [86].
A US population-based ecological study of 91,642 children ages 0–17 years showed that the 1-year prevalence of AD was associated with decreased outdoor temperature, low humidity, and low ultraviolet B radiation index, and the use of increased indoor heating [87]. Study data from ISAAC, including children ages 6–7 and adolescents ages 13–14 years, suggests that extremely low or high UVR levels might increase the risk of AD [88]. Another study found that higher temperatures, humidity, and increased sun exposure were associated with poorly controlled eczema. However, higher humidity lost statistical significance in multivariate analysis [89].
A Danish study found that lower monthly temperature was associated with more AD clinic visits and hospitalizations and more topical corticosteroid and calcineurin inhibitor prescriptions [90]. More hours of cloud cover were associated with increased healthcare utilization for AD, while more hours of bright sunlight were inversely associated with healthcare utilization [90]. Denmark has a relatively homogeneous and colder climate, whereas a study of ambulatory visits for AD to all physicians in the USA, which has one of the most diverse climates in the world, did not find increased outpatient healthcare utilization for AD during winter time in any region [91]. The greatest number of visits occurred in May and June, with smaller peaks in January and October [91].
Hospitalizations for AD in the USA were highest in the spring and summer for adults and children. Hospitalization rates for AD were highest in the northeast (colder) and lowest in the south (warmer) during the winter, but highest in the south (warmer) and lowest in the northeast (colder) during the summer [92]. Extreme cold temperature may increase risk of hospitalization for AD in the winter, whereas extreme hot temperature may worsen AD in the spring and summer.
Air Pollution
Multiple studies found associations of air pollution with AD prevalence and AD-related outcomes. In a retrospective study performed in Hubei, China, exposure to high levels of air pollutants during the gestational period and first year of life was associated with increased incidence of AD in preschool children [93]. A time-stratified case-crossover study in Beijing, China, found that short-term increases in small particle air pollution (PM2.5, PM10) overall, and nitrogen dioxide (NO2) and sulfur dioxide (SO2) in particular, were associated with increased outpatient visits for eczema/dermatitis [94]. Associations of air pollutants with outpatient visits for eczema/dermatitis were stronger in older patients and females, and when high concentrations of air pollution occurred for prolonged consecutive days [94]. The associations of outpatient visits for eczema/dermatitis with PM2.5, PM10, and NO2 were stronger during the warm season (May–October) [94]. Similar outcomes were observed in Chengdu, China, where the frequency of eczema visits to the hospital correlated with air pollutants and barometric pressure in univariate analyses, but negatively correlated with relative humidity [95].
Mechanisms of air pollutants exacerbating AD include penetrating skin through larger pores and hair follicles [96], generating free radicals, inducing inflammatory responses and disrupting skin barrier, activating AhR that regulates cell proliferation, inflammation, and melanogenesis, and/or altering skin flora [97]. Particulate matter leads to secretion of pro-inflammatory cytokines, e.g., tumor necrosis factor (TNF)-α, IL-1α, IL-8, and upregulation of matrix metalloproteinases 1, 2, and 9 [96].
Household Products
An online survey of 1,500 South Korean households evaluated the relationship between use of 23 different types of household products in the past year and AD in adults [98]. Highest-quartile use of household products was associated with ever being diagnosed with AD, symptoms, and treatment of AD in the past year [98].
Pet Dander
A 2012 meta-analysis of 26 publications from 21 birth cohort studies found that exposure to dogs or pets overall was associated with lower risk of developing AD, suggesting that early-life pet exposure protects against AD [99].
In contrast, a Chilean study of children ages 0–17 years with active AD found no association of tobacco exposure, pet ownership, aerosol use, visible dust, or home carpets/rugs with AD severity. While dust samples from all homes had dog and cat dander, children with AD living in homes with higher concentrations of dust mites and animal dander had more severe AD compared to those who lived in homes with lower concentrations [100]. In the pediatric population, exposure to unfamiliar pets was associated with increased AD severity [101]. Persistent AD lesions occurred more often in patients with sensitization to animal dander, and IgE sensitization to animal dander may increase risk of developing asthma or rhinitis in AD patients [102]. Pet exposures may differentially affect AD, with early-life exposures potentially protecting against AD, and exposures after AD onset potentially worsening disease.
Dust Mites
Homes of patients with AD vs. healthy controls found that patients with moderate-severe eczema had increased concentration of house dust mites (HDM) vs. healthy controls (median 85 vs. 8 mites/0.1 g mattress dust) [103]. Hypersensitivity to HDM was found in up to 90% of atopic patients with AD or allergic bronchial asthma [104]. However, a SR and meta-analysis of 7 RCTs found that HDM avoidance provided no benefit in preventing AD [105]. Further, patch testing to dust mites allergens had no clinical utility in determining the diagnosis or etiology of dermatitis; there was no association observed between “positive” HDM patch testing with a personal history of AD, asthma, or hay fever compared with a nonatopic clinical population of patients with dermatitis [106]. These studies suggest that while HDM may be associated with AD, causation still cannot be established; further, no adequate interventions currently exist to eradicate HDM.
Water Hardness
UK infants exposed to above-average levels of water hardness had increased risk of having visible AD at age 3 months. Hard water may affect skin-barrier integrity via CaCO3, leading to dryness and inflammation, and increase skin pH leading to enhanced protease activity in the stratum corneum, accelerating breakdown of corneodesmosomes, and reducing lipid lamellae synthesis [107]. High CaCO3 levels increased TEWL loss in children with and without FLG mutations [107]. Similar results were observed in other large studies [108, 109]. A study of 46 AD patients with and without FLG mutations and 34 healthy control subjects found that sites washed with hard water had increased sodium lauryl sulfate deposits that increased TEWL and caused irritation, particularly in AD patients carrying FLG mutations [110]. However, in an observer-blind RCT involving 336 children with moderate/severe AD, use of water softeners provided no additional benefit to usual care [111, 112]. There is inadequate evidence to support clinical recommendations regarding use of water softeners.
pH
The acid mantle refers to the slightly acidic pH (4–6) of normal skin that contains amino acids, lactic acid, fatty acids, and other components such as ceramide which play an important role in skin-barrier integrity. An acidic pH is required to synthesize ceramides [113], and inhibit the catalytic activity of kallikrein 5 and 7 skin proteases [114]. Elevated pH can decrease expression of LEKT1, a kallikrein inhibitor, leading to enhanced desquamation in AD-affected skin [115].
Corneodesmosomes function in holding the corneocytes together and contain natural moisturizing factor (NMF). The conversion of filaggrin into NMF can help restore the acidic pH and functions as a buffer in skin. When less filaggrin is present, as in AD, serine proteases are triggered leading to enhanced breakdown of corneodesmosomes and epidermal-barrier dysruption [115,116,117].
Skin pH is elevated in AD [115]. Frequent bathing and personal care products, e.g., soaps, surfactants, and detergents, can remove NMF and skin lipids and increase skin pH beyond optimal physiologic levels [118,119,120]. Frequent washing with alkaline soaps can reduce buffering capacity, which increases risk for irritation and AD flares [114,115,116, 119, 121, 122]. Vehicles with a slightly acidic pH, milder surfactants, and free of fragrances or other common irritants may improve skin-barrier function in AD. In some studies, cleansing and moisturizing helped maintain skin pH levels by enabling sufficient water retention and improving atopic skin [121,122,123,124].
Skin Microbiome
Staphylococcus aureus typically colonizes lesions, and to a lesser extent, non-lesional skin and noses of AD patients. Staphylococcus colonization rates and abundance in lesional skin increases with AD severity [12]. AD patients have higher prevalences of IgE against staphylococcal enterotoxin (SE) A and B compared with healthy controls [125]. Moreover, Staphylococcus colonization in AD lesional and non-lesional skin is accompanied by diminished microbial diversity, particularly with decreased Streptococcus, Corynebacterium, and Prophionibacterium [126].
It remains unclear whether the increased levels of Staphylococcus aureus is a byproduct or cause of AD. Dysbiosis might trigger barrier disruption in AD. SE B can act as a superantigen that activates lymphocytes and macrophages. Phenol-soluble modulin (PSM)-α from S. aureus can stimulate the production of IL-36α and IL-1α in keratinocytes, leading to production of IL-17 in γδT cells, innate lymphoid cell type 3, and CD4+ T cells, and enhanced neutrophil recruitment. S. aureus also triggers Th2 skewing by initiating production of thymic stromal lymphopoietin and stimulating mast cell degranulation through TLR2-dependent mechanisms. Finally, S. aureus can enhance the production of serine proteases by keratinocytes and metalloproteases in dermal fibroblasts which can further disrupt the skin barrier [127]. In contrast, skin colonization by S. aureus was attributed to the inadequate induction of cathelicidin and β-defensins, which directly inhibit the growth of bacteria. Relative expression of these AMPs was lower in AD skin than in other skin inflammatory conditions, e.g., psoriasis, rosacea, and wounds, and the amount of AMPs is not enough to suppress the growth of S. aureus [127].
Conventional AD treatments, including emollient use, water baths, bleach baths, and topical steroids, may restore bacterial diversity [128, 129].
Conclusion
Myriad environmental exposures may impact AD. These environmental factors should be considered when assessing and treating AD patients. Multi-level interventions are warranted, particularly early in life, to address these environmental factors and potentially prevent and/or improve AD. Currently, the strongest albeit far from complete evidence is available for the associations between probiotic supplementation during pregnancy, active smoking in children and adults, climate, air pollution, pH, and cutaneous Staphylococcus aureus and AD. However, a lot more evidence is required in order to make definitive conclusions about the benefits of specific interventions.
Abbreviations
- AD:
-
Atopic dermatitis
- GEI:
-
Gene-environment interactions
- UVR:
-
Ultraviolet radiation
- FLG:
-
Filaggrin
- GDM:
-
Gestational diabetes mellitus
- SCFA:
-
Short-chain fatty acids
- AD-E:
-
Atopic dermatitis or eczema
- ISAAC:
-
International Study of Asthma and Allergies in Childhood
- TRAP:
-
Traffic-related air pollution
- SNP:
-
Single-nucleotide polymorphisms
- MMP:
-
Matrix metalloproteinase
- TNF:
-
Tumor necrosis factor
- IL:
-
Interleukin
- HPs:
-
Household products
- NMF:
-
Natural moisturizing factor
- SR:
-
Systematic review
- PRR:
-
Pattern recognition receptor
- AMP:
-
Antimicrobial peptide
- RCT:
-
Randomized controlled trial
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- PUFA:
-
Polyunsaturated fatty acids
- scGOS:
-
Short-chain galactofructoside
- lcFOS:
-
Long-chain fructo-oligosaccharide
- BMI:
-
Body mass index
- COCOA:
-
Cohort for Childhood Origin of Asthma and Allergic Diseases
- PSKC:
-
Panel Study on Korean Children
- ISAAC:
-
International Study of Asthma and Allergies in Childhood Phase III
- C-section:
-
Cesarean section
- PM2.5, PM10 :
-
atmospheric particulate matter that have a diameter of less than 2.5 micrometers and 10 micrometers
- NO2 :
-
Nitrogen dioxide
- SO2 :
-
Sulfur dioxide
- AhR:
-
Aryl hydrocarbon receptor
- TEWL:
-
Transepidermal water loss
- HDM:
-
House dust mites
- SE:
-
Staphylococcal enterotoxin
- CE:
-
Cornified cell envelope
References
Deckers IA, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7(7):e39803.
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124(6):1251–1258.e1223.
Sandilands A, O'Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, et al. Prevalent and rare mutations in the gene encoding filaggrin cause Ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Investig Dermatol. 2006;126(8):1770–5.
Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, Chavda R, Gabriel S, Patel KR, Singam V, Kantor R, Hsu DY, Cella D. Association of itch triggers with atopic dermatitis severity and course in adults. Ann Allergy Asthma Immunol. 2020 Jun 13;S1081-1206(20)30409-9. https://doi.org/10.1016/j.anai.2020.06.014. Epub ahead of print. PMID: 32544530.
Wadonda-Kabondo N, Sterne JAC, Golding J, Kennedy CTC, Archer CB, Dunnill MGS. Association of parental eczema, hayfever, and asthma with atopic dermatitis in infancy: birth cohort study. Arch Dis Child. 2004;89(10):917–21.
Elmose C, Thomsen SF. Twin studies of atopic dermatitis: interpretations and applications in the filaggrin era. J Allergy. 2015;2015:902359.
Løset M, Brown SJ, Saunes M, Hveem K. Genetics of atopic dermatitis: from DNA sequence to clinical relevance. Dermatology. 2019;235(5):355–64.
Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy, Asthma Immunol Res. 2018;10(3):207–15.
Al-Shobaili HA, Ahmed AA, Alnomair N, Alobead ZA, Rasheed Z. Molecular genetic of atopic dermatitis: an update. Int J Health Sci (Qassim). 2016;10(1):96–120.
Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front Immunol. 2018 Oct 16;9:2379. https://doi.org/10.3389/fimmu.2018.02379. PMID: 30459758; PMCID: PMC6232773.
Kim JE, Kim HS. Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. 2019;8(4):444.
Totté JEE, van der Feltz WT, Hennekam M, van Belkum A, van Zuuren EJ, Pasmans SGMA. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2016;175(4):687–95.
Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4s):S65–s76.
Egawa G, Weninger W. Pathogenesis of atopic dermatitis: a short review. Cogent Biol. 2015;1(1):1103459.
Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13(1):15–26.
Blakeway H, Van-de-Velde V, Allen VB, Kravvas G, Palla L, Page MJ, Flohr C, Weller RB, Irvine AD, McPherson T, Roberts A, Williams HC, Reynolds N, Brown SJ, Paternoster L, Langan SM; (on behalf of UK TREND Eczema Network). What is the evidence for interactions between filaggrin null mutations and environmental exposures in the aetiology of atopic dermatitis? A systematic review. Br J Dermatol. 2020 Sep;183(3):443-451. https://doi.org/10.1111/bjd.18778. Epub 2020 Feb 11. PMID: 31794059.
Kantor R, Kim A, Thyssen JP, Silverberg JI. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(6):1119–1125.e1111.
Shinohara M, Matsumoto K. Fetal Tobacco Smoke Exposure in the Third Trimester of Pregnancy Is Associated with Atopic Eczema/Dermatitis Syndrome in Infancy. Pediatr Allergy Immunol Pulmonol. 2017 Sep 1;30(3):155-162. https://doi.org/10.1089/ped.2017.0758. PMID: 29062585; PMCID: PMC5649395.
Novakovic B, Ryan J, Pereira N, Boughton B, Craig JM, Saffery R. Postnatal stability, tissue, and time specific effects of AHRR methylation change in response to maternal smoking in pregnancy. Epigenetics. 2014;9(3):377–86.
Brembilla NC, Ramirez J-M, Chicheportiche R, Sorg O, Saurat J-H, Chizzolini C. In vivo dioxin favors interleukin-22 production by human CD4+ T cells in an aryl hydrocarbon receptor (AhR)-dependent manner. PLoS One. 2011;6(4):e18741.
Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135(3):721–728.e726.
Halling-Overgaard AS, Hamann CR, Holm RP, Linneberg A, Silverberg JI, Egeberg A, et al. Atopic dermatitis and alcohol use - a meta-analysis and systematic review. J Eur Acad Dermatol Venereol: JEADV. 2018;32(8):1238–45.
Kunisawa J, Arita M, Hayasaka T, Harada T, Iwamoto R, Nagasawa R, et al. Dietary omega3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci Rep. 2015;5:9750.
Kang CM, Chiang BL, Wang LC. Maternal Nutritional Status and Development of Atopic Dermatitis in Their Offspring. Clin Rev Allergy Immunol. 2020 Mar 10. https://doi.org/10.1007/s12016-020-08780-y. Epub ahead of print. PMID: 32157654.
Ciaccio CE, Girdhar M. Effect of maternal ω3 fatty acid supplementation on infant allergy. Ann Allergy Asthma Immunol. 2014 Mar;112(3):191-4. https://doi.org/10.1016/j.anai.2014.01.009. PMID: 24565593; PMCID: PMC3961846.
Furuhjelm C, Warstedt K, Larsson J, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr (Oslo, Norway : 1992). 2009;98(9):1461–7.
Group LS. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85(2):530–7.
Willers SM, Devereux G, Craig LCA, McNeill G, Wijga AH, Abou el-Magd W, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62(9):773–9.
Best KP, Gold M, Kennedy D, Martin J, Makrides M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am J Clin Nutr. 2016;103(1):128–43.
Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9.
von Mutius E. Maternal farm exposure/ingestion of unpasteurized cow’s milk and allergic disease. Curr Opin Gastroenterol. 2012;28(6):570–6.
Chatenoud L, Bertuccio P, Turati F, Galeone C, Naldi L, Chatenoud L, et al. Markers of microbial exposure lower the incidence of atopic dermatitis. Allergy. 2020;75(1):104–15.
Huang FQ, Lu CY, Wu SP, Gong SZ, Zhao Y. Maternal exposure to antibiotics increases the risk of infant eczema before one year of life: a meta-analysis of observational studies. World J Pediatr: WJP. 2020;16(2):143–51.
Weiss ST. Eat dirt--the hygiene hypothesis and allergic diseases. N Engl J Med. 2002;347(12):930–1.
Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77.
Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71(4):814–21.
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–75.
Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD006474. https://doi.org/10.1002/14651858.CD006474.pub3. PMID: 23543544.
Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138(6):1091–5.
Kwon H-K, Lee C-G, So J-S, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4<sup>+</sup>Foxp3<sup>+</sup> T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci. 2010;107(5):2159–64.
Yin DG, He Z, Duan XY, Fan FX, Liao XB, Wang QC. Effect of probiotic supplementation during pregnancy and infancy in preventing atopic dermatitis in children: a meta analysis. Zhongguo dang dai er ke za zhi = Chin J Contemp Pediatr. 2019;21(1):82–8.
D'Auria E, Pilloni S, Beretta S, Paradiso L, Zuccotti GV. "Probiotics, Prebiotics and Postbiotics in Atopic Dermatitis." Biomed J Scie Tech Res. Biomedical Research Network+, 2019;LLC, vol. 22(5), pages 16930-16933, November.
Beretta S, Fabiano V, Petruzzi M, Budelli A, Zuccotti GV. Fermented rice flour in pediatric atopic dermatitis. Dermatitis. 2015;26(2):104–6.
Kumar R, Ouyang F, Story RE, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. 2009;124(5):1031–1038.e1034.
Sifnaios E, Mastorakos G, Psarra K, et al. Gestational diabetes and T-cell (Th1/Th2/Th17/Treg) immune profile. In Vivo. 2019;33(1):31–40.
Bassols J, Serino M, Carreras-Badosa G, Burcelin R, Blasco-Baque V, Lopez-Bermejo A, et al. Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatr Res. 2016;80(6):777–84.
Kim CH, Kim SH, Lee JS. Association of maternal depression and allergic diseases in Korean children. Allergy Asthma Proc. 2017;38(4):300–8.
McKenzie, Costner BA∗; Silverberg, Jonathan I. MD, PhD, MPH† Maternal Depression and Atopic Dermatitis in American Children and Adolescents, Dermatitis: 1/2 2020 - Volume 31 - Issue 1 - p 75-80. https://doi.org/10.1097/DER.0000000000000548
Hamann CR, Egeberg A, Silverberg JI, Gislason G, Skov L, Thyssen JP. Exploring the association between parental psychiatric disease and childhood atopic dermatitis: a matched case–control study. J Eur Acad Dermatol Venereol. 2019;33(4):725–34.
Chan CWH, Law BMH, Liu YH, Ambrocio ARB, Au N, Jiang M, Chow KM. The Association between Maternal Stress and Childhood Eczema: A Systematic Review. Int J Environ Res Public Health. 2018 Feb 25;15(3):395. https://doi.org/10.3390/ijerph15030395. PMID: 29495329; PMCID: PMC5876940.
Chang HY, Suh DI, Yang SI, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016;138(2):468–475.e465.
Patel KR, Immaneni S, Singam V, Rastogi S, Silverberg JI. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(2):402–10.
Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid Based Child Health. 2014 Jun;9(2):447-83. https://doi.org/10.1002/ebch.1972. PMID: 25404609.
Hoppu U, Rinne M, Salo-Väänänen P, Lampi AM, Piironen V, Isolauri E. Vitamin C in breast milk may reduce the risk of atopy in the infant. Eur J Clin Nutr. 2005;59(1):123–8.
Wang LC, Chiang BL, Huang YM, Shen PT, Huang HY, Lin BF. Lower vitamin D levels in the breast milk is associated with atopic dermatitis in early infancy. Pediatr Allergy Immunol. 2020 Apr;31(3):258-264. https://doi.org/10.1111/pai.13179. Epub 2019 Dec 11. PMID: 31758588.
Wooldridge AL, McMillan M, Kaur M, Giles LC, Marshall HS, Gatford KL. Relationship between birth weight or fetal growth rate and postnatal allergy: a systematic review. J Allergy Clin Immunol. 2019;144(6):1703–13.
Hikino S, Nakayama H, Yamamoto J, Kinukawa N, Sakamoto M, Hara T. Food allergy and atopic dermatitis in low birthweight infants during early childhood. Acta Paediatr. 2001;90(8):850–5.
Panduru M, Salavastru CM, Panduru NM, Tiplica GS. Birth weight and atopic dermatitis: systematic review and meta-analyis. Acta Dermatovenerol Croat : ADC. 2014;22(2):91–6.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5.
Gerlich J, Benecke N, Peters-Weist AS, Heinrich S, Roller D, Genuneit J, et al. Pregnancy and perinatal conditions and atopic disease prevalence in childhood and adulthood. Allergy. 2018;73(5):1064–74.
Richards M, Ferber J, Chen H, Swor E, Quesenberry CP, Li DK, et al. Caesarean delivery and the risk of atopic dermatitis in children. Clin Exp Allergy. 2020;50(7):805–14.
Waidyatillake NT, Dharmage SC, Allen KJ, Lodge CJ, Simpson JA, Bowatte G, et al. Association of breast milk fatty acids with allergic disease outcomes—a systematic review. Allergy. 2018;73(2):295–312.
Lin B, Dai R, Lu L, Fan X, Yu Y. Breastfeeding and atopic dermatitis risk: a systematic review and meta-analysis of prospective cohort studies. Dermatology. 2020;236(4):345–60.
Lin H-P, Chiang B-L, Yu H-H, Lee JH, Lin YT, Yang YH, et al. The influence of breastfeeding in breast-fed infants with atopic dermatitis. J Microbiol Immunol Infect. 2019;52(1):132–40.
Lodge CJ, Tan DJ, Lau MXZ, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104(S467):38–53.
Yuan M, Tan M, Moore D, Shen S, Qiu X, Thomas GN, et al. Timing of cow’s milk or cow’s milk formula introduction to the infant diet and atopic risk in children: a systematic review and meta-analysis. Clin Rev Allergy Immunol. 2020;59(1):46–60.
Waidyatillake NT, Dharmage SC, Allen KJ, Bowatte G, Boyle RJ, Burgess JA, Koplin JJ, Garcia-Larsen V, Lowe AJ, Lodge CJ. Association between the age of solid food introduction and eczema: A systematic review and a meta-analysis. Clin Exp Allergy. 2018 Aug;48(8):1000-1015. https://doi.org/10.1111/cea.13140. Epub 2018 Apr 23. PMID: 29570230.
Ellwood P, Asher MI, Garcia-Marcos L, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax. 2013;68(4):351–60.
Cho SI, Lee H, Lee DH, Kim KH. Association of frequent intake of fast foods, energy drinks, or convenience food with atopic dermatitis in adolescents. Eur J Nutr. 2020 Oct;59(7):3171-3182. https://doi.org/10.1007/s00394-019-02157-4. Epub 2019 Dec 10. PMID: 31822988.
Ellwood P, Asher MI, García-Marcos L, Williams H, Keil U, Robertson C, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax. 2013;68(4):351–60.
Wang CS, Wang J, Zhang X, Zhang L, Zhang HP, Wang L, et al. Is the consumption of fast foods associated with asthma or other allergic diseases? Respirology. 2018;23(10):901–13.
Nosrati A, Afifi L, Danesh MJ, Lee K, Yan D, Beroukhim K, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatol Treat. 2017;28(6):523–38.
Salem I, Ramser A, Isham N, Ghannoum MA. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
Jena PK, Sheng L, McNeil K, et al. Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J Dermatol Sci. 2019;95(1):13–20.
Mustapa Kamal Basha MA, Majid HA, Razali N, Yahya A. Risk of eczema, wheezing and respiratory tract infections in the first year of life: a systematic review of vitamin D concentrations during pregnancy and at birth. PLoS One. 2020;15(6):e0233890.
Wei Z, Zhang J, Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: A systematic review and meta-analysis. Pediatr Allergy Immunol. 2016 Sep;27(6):612-9. https://doi.org/10.1111/pai.12593. Epub 2016 Jul 20. PMID: 27145360.
Hattangdi-Haridas SR, Lanham-New SA, Wong WHS, Ho MHK, Darling AL. Vitamin D deficiency and effects of vitamin D supplementation on disease severity in patients with atopic dermatitis: a systematic review and meta-analysis in adults and children. Nutrients. 2019;11(8):1854.
Shin J, Kim YJ, Kwon O, Kim NI, Cho Y. Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis. Nutr Res Pract. 2016;10(4):398–403.
Laitinen K, Kalliomäki M, Poussa T, Lagström H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow-up study from birth to 4 years. Br J Nutr. 2007;94(4):565–74.
Vaughn AR, Foolad N, Maarouf M, Tran KA, Shi VY. Micronutrients in atopic dermatitis: a systematic review. J Altern Complement Med. 2019;25(6):567–77.
Jaffary F, Faghihi G, Mokhtarian A, Hosseini SM. Effects of oral vitamin E on treatment of atopic dermatitis: A randomized controlled trial. J Res Med Sci. 2015 Nov;20(11):1053-7. https://doi.org/10.4103/1735-1995.172815. PMID: 26941808; PMCID: PMC4755091.
Oh SY, Chung J, Kim MK, Kwon SO, Cho BH. Antioxidant nutrient intakes and corresponding biomarkers associated with the risk of atopic dermatitis in young children. Eur J Clin Nutr. 2010;64(3):245–52.
David TJ, Wells FE, Sharpe TC, Gibbs AC. Low serum zinc in children with atopic eczema. Br J Dermatol. 1984;111(5):597–601.
Ewing CI, Gibbs AC, Ashcroft C, David TJ. Failure of oral zinc supplementation in atopic eczema. Eur J Clin Nutr. 1991;45(10):507–10.
Kim JE, Yoo SR, Jeong MG, Ko JY, Ro YS. Hair zinc levels and the efficacy of oral zinc supplementation in patients with atopic dermatitis. Acta Derm Venereol. 2014;94(5):558–62.
Engebretsen KA, Johansen JD, Kezic S, Linneberg A, Thyssen JP. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30(2):223–49.
Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Investig Dermatol. 2013;133(7):1752–9.
Fuertes E, Flohr C, Silverberg JI, Standl M, Strachan DP. Global associations between UVR exposure and current eczema prevalence in children from ISAAC Phase Three. J Investig Dermatol. 2017;137(6):1248–56.
Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134(1):51–7.
Hamann CR, Andersen YMF, Engebretsen KA, Skov L, Silverberg JI, Egeberg A, et al. The effects of season and weather on healthcare utilization among patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2018;32(10):1745–53.
Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58(4):465–71.
Narla S, Hsu DY, Thyssen JP, Silverberg JI. Inpatient financial burden of atopic dermatitis in the United States. J Invest Dermatol. 2017;137(7):1461–7.
Deng S, Huang D, Wang W, Yan H, Li S, Xiang H. Associations of gestational and the first year of life exposure to ambient air pollution with childhood eczema in Hubei, China. Environ Sci Pollut Res. 2019;26(23):23842–9.
Guo Q, Liang F, Tian L, Schikowski T, Liu W, Pan X. Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing: a time-stratified case-crossover analysis. Environ Sci Process Impacts. 2019;21(1):163–73.
Li A, Fan L, Xie L, Ren Y, Li L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis. J Eur Acad Dermatol Venereol: JEADV. 2018;32(3):486–94.
Kim KE, Cho D, Park HJ. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–34.
Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol: JEADV. 2015;29(12):2326–32.
Choi J, Kim J, Kim K. Assessment of relationship between the use of household products and atopic dermatitis in Seoul: focused on products with associated risks. Environ Health Toxicol. 2019;34(2):e2019006.
Pelucchi C, Galeone C, Bach JF, La Vecchia C, Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: a meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2013;132(3):616–622.e617.
Cid BJ, Perez-Mateluna G, Iturriaga C, Zambrano MJ, Vives MI, Valenzuela PM, et al. Is there an association between indoor allergens and the severity of atopic dermatitis? Int J Dermatol. 2019;58(4):433–9.
Langan SM, Silcocks P, Williams HC. What causes flares of eczema in children? Br J Dermatol. 2009;161(3):640–6.
Čelakovská J, Ettlerová K, Ettler K, Vaněčková J, Bukač J. Sensitization to aeroallergens in atopic dermatitis patients: association with concomitant allergic diseases. J Eur Acad Dermatol Venereol : JEADV. 2015;29(8):1500–5.
Beck HI, Korsgaard J. Atopic dermatitis and house dust mites. Br J Dermatol. 1989;120(2):245–51.
Werfel T, Kapp A. Environmental and other major provocation factors in atopic dermatitis. Allergy. 1998;53(8):731–9.
Bremmer SF, Simpson EL. Dust mite avoidance for the primary prevention of atopic dermatitis: a systematic review and meta-analysis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2015;26(7):646–54.
Silverberg JI, Hanifin JM, Law S, White K, Storrs FJ. Lack of association between dust mite sensitivity and atopic dermatitis. Dermatitis. 2016;27(2):59–67.
Perkin MR, Craven J, Logan K, Strachan D, Marrs T, Radulovic S, et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: a population-based cross-sectional study. J Allergy Clin Immunol. 2016;138(2):509–16.
Engebretsen KA, Bager P, Wohlfahrt J, et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: cohort study. J Allergy Clin Immunol. 2017;139(5):1568–1574.e1561.
Jabbar-Lopez ZK, Craven J, Logan K, Greenblatt D, Marrs T, Radulovic S, et al. Longitudinal analysis of the effect of water hardness on atopic eczema: evidence for gene-environment interaction. Br J Dermatol. 2020;183(2):285–93.
Danby SG, Brown K, Wigley AM, Chittock J, Pyae PK, Flohr C, et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. J Investig Dermatol. 2018;138(1):68–77.
Thomas KS, Dean T, O'Leary C, Sach TH, Koller K, Frost A, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLoS Med. 2011;8(2):e1000395.
Thomas KS, Koller K, Dean T, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the Softened Water Eczema Trial (SWET). Health Technol Assess (Winchester, England). 2011;15(8):v-vi, 1–156.
Rawlings AV. Recent advances in skin ‘barrier’ research. J Pharm Pharmacol. 2010;62(6):671–7.
Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012;122(2):440–7.
Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129(8):1892–908.
Bandier J, Johansen JD, Petersen LJ, Carlsen BC. Skin pH, atopic dermatitis, and filaggrin mutations. Dermatitis. 2014;25(3):127–9.
Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–7.
Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergology international : official journal of the Japanese Society of Allergology. 2013;62(2):151–61.
Walters RM, Mao G, Gunn ET, Hornby S. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917. https://doi.org/10.1155/2012/495917. Epub 2012 Aug 13. PMID: 22927835; PMCID: PMC3425021.
Clark C. Atopic eczema: clinical features and diagnosis. Clin Pharm. 2010 Sept; 2:285–9.
Lynde CW, Andriessen A. A cohort study on a ceramide-containing cleanser and moisturizer used for atopic dermatitis. Cutis. 2014;93(4):207–13.
Kircik LH. Clinical insights about the role of skin pH in inflammatory dermatological conditions introduction. J Drugs Dermatol : JDD. 2019;18(12):212.
Guenther L, Lynde CW, Andriessen A, Barankin B, Goldstein E, Skotnicki SP, et al. Pathway to dry skin prevention and treatment. J Cutan Med Surg. 2012;16(1):23–31.
Vender RB, Andriessen A, Barankin B, Freiman A, Kyritsis D, Mistos LM, et al. Cohort using a ceramides containing cleanser and cream with salicylic acid for dry, flaking, and scaling skin conditions. J Drugs Dermatol : JDD. 2019;18(1):80–5.
de Wit J, Totté JEE, van Buchem FJM, Pasmans SGMA. The prevalence of antibody responses against Staphylococcus aureus antigens in patients with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2018;178(6):1263–71.
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–9.
Nakatsuji T, Gallo RL. The role of the skin microbiome in atopic dermatitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2019;122(3):263–9.
Seite S, Flores GE, Henley JB, Martin R, Zelenkova H, Aguilar L, et al. Microbiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatment. J Drugs Dermatol: JDD. 2014;13(11):1365–72.
Gonzalez ME, Schaffer JV, Orlow SJ, et al. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J Am Acad Dermatol. 2016;75(3):481–493.e488.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Allergies and the Environment
Rights and permissions
About this article
Cite this article
Narla, S., Silverberg, J.I. The Role of Environmental Exposures in Atopic Dermatitis. Curr Allergy Asthma Rep 20, 74 (2020). https://doi.org/10.1007/s11882-020-00971-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11882-020-00971-z
Keywords
- Atopic dermatitis
- Environment
- Prenatal
- Tobacco smoke
- Alcohol
- Maternal stress
- Gene-environment interactions
- Fatty acids
- Prebiotics
- Probiotics
- Postbiotics
- Gestational diabetes
- Antibiotics
- Breastfeeding
- Climate
- Temperature
- Ultraviolet radiation
- Air pollution
- Household products
- Indoor allergens
- Water hardness
- pH
- Skin microbiota
- Gut microbiota
- Cesarean section
- Hygiene hypothesis