Abstract
Purpose of Review
Air pollution is established as an independent risk factor for cardiovascular diseases (CVDs). Ambient particulate matter (PM), a principal component of air pollutant, has been considered as a main culprit of the adverse effects of air pollution on human health.
Recent Findings
Extensive epidemiological and toxicological studies have demonstrated particulate air pollution is positively associated with the development of CVDs. Short-term PM exposure can trigger acute cardiovascular events while long-term exposure over years augments cardiovascular risk to an even greater extent and can reduce life expectancy by a few years. Inhalation of PM affects heart rate variability, blood pressure, vascular tone, blood coagulability, and the progression of atherosclerosis. The potential molecular mechanisms of PM-caused CVDs include direct toxicity to the cardiovascular system or indirect injury by inducing systemic inflammation and oxidative stress in circulation.
Summary
This review mainly focuses on the acute and chronic effects of ambient PM exposure on the development of cardiovascular diseases and the possible mechanisms for PM-induced increases in cardiovascular morbidity and mortality. Additionally, we summarized some appropriate interventions to attenuate PM air pollution-induced cardiovascular adverse effects, which may promote great benefits to public health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ambient air pollution is the largest single environmental risk factor that causes more than 3.9 million premature deaths worldwide, particularly in developing countries with rapid industrialization and urbanization. Among diverse air pollutants, particulate matter (PM) is an important component and a complex mixture of microscopic solid and liquid droplets suspended in the air. Inhalation of PM can lead to multiple adverse health effects in human being, depending on their original source of emission or formation, chemical components, varying size, surface areas, and concentrations [1, 2]. Based on aerodynamic size, PM is generally classified into inhalable (≤ 10 μm; PM10), coarse (2.5–10 μm; PM2.5–10), fine (≤ 2.5 μm; PM2.5), and ultrafine (≤ 0.1 μm; PM0.1) particles. The smaller particles could be more pathogenic [3], as a result of their greater propensity to induce systemic pro-oxidant and pro-inflammatory effects [4].
Cardiovascular diseases (CVDs) are the leading cause of mortality and a major health burden in developed countries and have the similar profile in many Asian countries. In fact, clinical and epidemiological studies have unequivocally indicated positive correlation between the exposure to ambient PM and increased morbidity and mortality of CVDs [5, 6], even the levels of PM at or below existing air quality standards [7]. In particularly, the smaller particles (e.g., PM2.5 and PM0.1) may pose a higher risk for cardiovascular disorders [3, 8]. This review mainly focuses on the acute and chronic effects of ambient PM exposure on the development of CVDs and the possible mechanisms for PM-induced increases in cardiovascular morbidity and mortality. Additionally, we summarized some appropriate interventions to attenuate PM air pollution-induced cardiovascular adverse effects, which may promote great benefits to public health.
PM Inhalation Induces Cardiovascular Effects
Numerous epidemiologic studies have demonstrated the associations between short- and long-term exposures to ambient air pollution and increased risk for cardiovascular events, including myocardial infarction (MI), congestive heart failure (CHF), and stroke.
Short-Term Effects
Observational studies have documented that short-term exposure to increased PM is directly linked to increased morbidity of CVDs. Zanobetti and colleagues found an increase of 1.89% in CVDs, 2.25% in MI, and 1.85% in CHF admissions for a 10 μg/m3 increase in 2-day averaged PM2.5 concentration in 26 US communities [9]. In Shanghai, China, an 8-year period of observation study revealed that a 10 μg/m3 increase in the concentration of 2-day PM10 and PM2.5 resulted in increased hospital admissions for coronary heart disease (CHD) (0.23 and 0.74%, respectively) [10]. PM2.5 exposure in the USA was generally lower than exposures observed in China. The differential effect magnitudes between the USA and China may be due to pollution levels, individual activities, and population susceptibility in different locations. In addition, existing studies indicate that ambient PM is strongly associated with increased cardiovascular morbidity even at very low levels of exposure.
The positive relationship between CVD mortality and PM exposure has been demonstrated in several large time-series and case-cross-over studies. Kan and colleagues reported an increase in PM10 level (2-day moving average concentrations) by 10 μg/m3 was significantly associated with increases of 0.54% [11] and 0.36% [12] in daily stroke and CHD over the eight Chinese cities, respectively. This is the first multicity study in China, or even in other developing countries, to report the acute effect of PM air pollution on stroke and CHD mortality and similarly, a recent study conducted by the same group to evaluate short-term associations between PM2.5 and daily cause-specific mortality in 272 representative Chinese cities. Each 10 μg/m3 increase in 2-day moving average of PM2.5 concentrations was associated with an increment of 0.22% in mortality from total non-accidental causes, 0.27% from cardiovascular diseases, 0.39% from hypertension, 0.30% from CHD, and 0.23% from stroke [13]. In addition, several time-series studies have been conducted worldwide in recent years to address the daily PM-related CVDs mortality [14,15,16,17].
Long-Term Effects
Chronic ambient PM exposure is known to impair cardiovascular function, exacerbate disease, and increase cardiovascular mortality and morbidity. There is persuasive evidence on the negative impact of PM air pollution on cardiovascular events and outcomes, including electrocardiographic changes (e.g., reduced heart rate variability), endothelial dysfunction, atherosclerosis, and thrombosis [18,19,20,21].
Long-term effects of air pollution on mortality are investigated through cohort studies. Previous two landmark cohort studies of the Harvard Six Cities Study [22, 23] and American Cancer Society Study [24, 25] have repeatedly demonstrated the close positive relationship between levels of PM2.5 and cardiopulmonary mortality. Large cohort studies have shown that long-term exposure to relatively low levels of PM is associated with cardiovascular mortality in North America and Europe [26,27,28]. Nevertheless, few studies have assessed the association of CVDs with high-level air pollutants. A retrospective cohort study, containing 39,054 subjects from four cities in northern China, was conducted for mortality of all-cause and specific cardiovascular diseases from 1998 to 2009. For each 10 μg/m3 increase in PM10, mortality relative risk ratios (RRs) of all-causes, CVDs, ischemic heart disease, heart failure, and cerebrovascular disease were increased by 1.24, 1.23, 1.37, 1.11, and 1.23%, respectively [29]. These findings were strengthened by a Hong Kong cohort study of 66,820 participants (≥ 65 years of age), which demonstrated mortality hazard ratios (HRs) per 10 μg/m3 increase in PM2.5 were associated with an increase of 1.14% for all natural causes, 1.22% for CVDs, 1.42 for ischemic heart disease, and 1.24% for cerebrovascular disease [30]. However, a meta-analysis of 22 European cohort studies did not find any association between PM and cardiovascular mortality [31]. This discrepancy could be due to the changes in cardiovascular risk profile (e.g., reduced smoking, increased medication, and medical treatment). Compared with short-term studies, long-term cohort studies suggest consistently higher relative risk. The most likely explanation is that chronic studies can capture cumulative health effects due to long-term air pollutant exposure [19].
Possible Mechanisms of PM-Induced Cardiovascular Effects
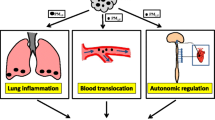
A number of PM-related mechanisms have been proposed to play a role in pathways leading to CVDs; however, these mechanisms may be associated with direct and indirect toxic effects of PM in the cardiovascular system [32, 33]. The main mechanisms involve, but not limited to, direct translocation of PM, in particular ultrafine particles (UFPs), into the blood stream; systemic inflammation and oxidative stress; abnormal coagulation function and vascular dysfunction; and disturbance of the autonomic nervous system induced by PM.
Direct and Acute Effects of UFPs
Among diverse air pollutants, UFPs are extremely small in size, large in quantity and surface area, and most importantly, capable of passing through the air-blood barrier. UFPs, even at low concentration, were shown to translocate into the blood circulation and can reach the heart and other remote organs [34, 35]. These results suggest that UFPs could induce direct cardiovascular toxic effects independent of their passage through the lungs. After deposit on vascular endothelium, the UFPs can promote oxidative stress and inflammation, resulting in atherosclerotic plaque instability, and finally the formation of thrombus [36]. In an animal study, increased left ventricle ejection fraction and premature ventricular beats were observed in rats intravenously injected with UFPs [37]. The finding from this study implied that inotropic effect of UFPs may be harmful to patients with pre-existing coronary artery disease, by increasing the oxygen demand and aggravating the ischemic symptom. The in vitro study of UFPs on cardiac performance demonstrated that the direct effects of UFPs most likely could be explained by their ability to generate reactive oxygen species (ROS), which can cause myocardial stunning and endothelial dysfunction [38, 39]. Taken together, UFPs can directly and acutely affect cardiac contractility and coronary flow. Nonetheless, more researches on human are needed to examine the capability of UFPs to induce adverse cardiovascular effects and directly, cardiac performance.
System Inflammation and Oxidative Stress
It has been proposed that inhaled PM may trigger inflammation-related cascade in the respiratory tract, in particular, PM2.5 and UFPs can lead to systemic inflammation, increasing the risk of CVDs. Indeed, following exposure to concentrated ambient PM [40] and diesel exhaust particles (DEP) [41], local inflammation and oxidative stress can release from lung cells into bronchial fluid and the blood stream of several representative inflammatory markers, such as the cytokines interleukin (IL)-1β, IL-6, interferon-γ, granulocyte macrophage colony-stimulating factor (GM-CSF) [42], and tumor necrosis factor-α (TNF-α) [43]. Brook et al. showed that exposure to concentrated PM for 2 h induced increases in total white blood cell and neutrophil counts immediately [44]. This was strengthened by the panel studies that showed associations between ambient PM exposure and the acute phase response, as evidenced by increases in C-reactive protein (CRP) [45], fibrinogen [46], plasma viscosity [47], and altered leucocyte expression of adhesion molecules [48]. In addition, a ROS-mediated mechanism was involved in PM-triggered pro-inflammatory pathway [49], which linked with atherosclerosis, vascular dysfunction, cardiac arrhythmias, and myocardial injury.
Abnormal Coagulation Function
Another potential mechanism for ambient PM-induced cardiovascular detrimental effect is the abnormal activation of the hemostatic system. In response to vessel injury, the human body relies on the coagulation factors to strengthen and complete thrombus formation initiated by platelet activity, with fibrinogen being central to this process. A previous study has shown that PM exposure was linked to prothrombin time (PT), endogenous thrombin potentials (ETPs), and fibrinogen, which provided the evidence of long-term effects on coagulation [50]. Similarly, a recent study has demonstrated that the increase in fibrinogen levels was associated with increased black carbon (BC), PM2.5, and UFP at multiple lag times [51]. Toxicological studies have demonstrated that instillation of DEP reduced the time to thrombotic occlusion in vivo and increased platelet-monocyte aggregates via inhibiting tissue plasminogen activator (t-PA) and plasminogen activator inhibitor (PAI)-1 release from human umbilical vein endothelial cells (HUVECs) [52].
Vascular Dysfunction
The endothelium plays a critical role in regulating blood pressure, atherogenesis, and thrombosis; thus, endothelial dysfunction could contribute to cardiovascular dysfunction associated with PM exposure. A large multicity cohort study has shown long-term PM exposure was significantly associated with reduced endothelial function by decreasing flow-mediated dilation (FMD) and vasoconstriction [53]. In support of this observation, recent studies have shown that PM exposure could induce endothelial cell apoptosis via various inflammatory signaling pathways [54,55,56].
Disturbance of the Autonomic Nervous System
PM air pollution has been shown to disrupt the autonomic nervous system [57]. Heart rate variability (HRV) refers to the cyclical changes of sinus rhythm, and is a well-defined indicator of cardiac autonomic function. Epidemiological studies have demonstrated that PM air pollution is associated with decreased HRV such as SDNN and rMSSD [58,59,60]. Moreover, blood pressure (BP), heart rate (HR), and rMSSD among the elderly were affected by the total mass of PM2.5 [61]. Recently, a randomized cross-over study has reported that acute exposure to PM affects parasympathetic control of heart function and increases methylation of a pro-inflammatory gene in healthy individuals. This study suggested PM exposure was sufficient to trigger parasympathetic dysautonomia, independently from changes in sympathetic control, and inflammation [62].
Dietary and Pharmacological Intervention
Inflammation and oxidative stress pathways have been proposed to mediate the adverse cardiovascular effects of PM air pollution [4]. It is therefore reasonable to propose the use of anti-inflammatory and antioxidant dietary supplements or medications as an intervention approach to blunt these pathways and reduce the CVD risk of PM exposure [63]. Controlled human and animal studies have demonstrated that antioxidant vitamins, polyunsaturated fatty acids (PUFAs), or medications with anti-inflammatory or antioxidant properties can alleviate PM-induced cardiopulmonary effects.
Pharmacological Intervention
Statins are wildly recognized as one of the most effective therapeutic strategies in the treatment and prevention of CVDs. A panel study has found that statins eliminated the effects of PM2.5 on HRV mediated by oxidative stress in subjects lacking of glutathione S-transferase M1 (GSTM1) allele [64]. In another panel study, stain was shown to mitigate vascular inflammatory/endothelial response induced by BC [65]. Moreover, a strong association between long-term PM2.5 exposure and levels of the inflammatory marker CRP among certain susceptible subgroups, and CRP levels was lower in those individuals taking statins [66].
Dietary Intervention
Besides pharmacological interventions, intake of essential nutrients from dietary supplement such as antioxidant vitamins C and E and PUFAs is relatively safe. The results from animal and in vitro studies have suggested that vitamin C and vitamin E could prevent PM-induced cardiovascular injury through suppression of oxidative stress [67,68,69,70]. However, in humans, vitamins C and E in cardiovascular disease prevention seem to be less effective [71,72,73,74], or even increasing all-cause mortality when supplemented with high-dose vitamin E (≥ 400 IU/d) [75]. Therefore, more studies are needed to examine the efficacy of antioxidant vitamins C and E or the combination on ambient PM-induced adverse cardiovascular effects.
Another dietary supplement PUFA has shown promising protection against air pollution-induced detrimental effects on the cardiovascular system. Romieu and colleague found that fish oil supplementation appeared to significantly diminish the time- and frequency-domain HRV associated with PM2.5 exposure [76]. Similarly, Tong and colleague in a controlled human study also observed that omega-3 PUFAs could antagonize PM-induced alterations of HRV, cardiac repolarization indices, and lipids changes in middle-aged healthy adults [77]. In addition, epidemiological and clinical studies have reported that PM air pollution exposure might increase QT intervals in coronary artery disease patients. Supplementation of fish or omega-3 PUFAs could decrease the QT interval prolongation and susceptibility of the heart to atrial and ventricular arrhythmia [78,79,80]. Olive oil is a rich source of the omega-9 PUFAs oleic acid, which can decrease blood lipid and platelet aggregation along with improving endothelial function [81,82,83]. A recent study showed that intake of olive oil could blunt endothelial dysfunction induced by exposure to concentrated ambient PM, accompanied with an increase in tissue plasminogen activator (t-PA) in blood [84].
Conclusions
Epidemiologic and toxicological studies have demonstrated that particulate air pollution is directly associated with adverse cardiovascular events. The underlying mechanisms of PM-caused CVDs may include direct damage by PM2.5 and UFPs translocating from the lung to the circulation and remote localization to the heart, or indirect injury by inducing systemic inflammation and oxidative stress, abnormal coagulation function, vascular dysfunction and disturbance of the autonomic nervous system, and ultimately leading to heart and vascular damage. A better understanding of the mechanisms underlying the adverse effects of particulate air pollution on CVDs is key in helping to improve environmental health policy and also in providing advices for individuals at risk. In addition, some studies are continuously exploring the new and effective therapeutic approaches, which may promote great benefits to public health by reducing the risk of CVDs.
Abbreviations
- CVDs:
-
Cardiovascular diseases
- PM:
-
Particulate matter
- PM10 :
-
PM with an aerodynamic diameter less than 10 μm
- PM2.5–10 :
-
PM with an aerodynamic diameter 2.5–10 μm
- PM2.5 :
-
PM with an aerodynamic diameter less than 2.5 μm
- UFPs/PM0.1 :
-
PM with an aerodynamic diameter less than 0.1 μm
- BC:
-
Black carbon
- DEP:
-
Diesel exhaust particles
- HRV:
-
Heart rate variability
- CHF:
-
Congestive heart failure
- CHD:
-
Coronary heart disease
- RRs:
-
Relative risk ratios
- HRs:
-
Hazard ratios
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- CRP:
-
C-reactive protein
- ROS:
-
Reactive oxygen species
- t-PA:
-
Tissue plasminogen activator
- PAI-1:
-
Plasminogen activator inhibitor
- FMD:
-
Flow-mediated dilation
- PUFAs:
-
Polyunsaturated fatty acids
- GSTM1:
-
Glutathione S-transferase M1
References
Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:339–62.
Harrison RM, Yin J. Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ. 2000;249:85–101.
Franck U, Odeh S, Wiedensohler A, et al. The effect of particle size on cardiovascular disorders--the smaller the worse. Sci Total Environ. 2011;409:4217–21.
Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78.
Lawal AO. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: the role of Nrf2 and AhR-mediated pathways. Toxicol Lett. 2017;270:88–95.
Langrish JP, Bosson J, Unosson J, et al. Cardiovascular effects of particulate air pollution exposure: time course and underlying mechanisms. J Intern Med. 2012;272:224–39.
Simkhovich BZ, Kleinman MT, Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol. 2008;52:719–26.
Schulz H, Harder V, Ibald-Mulli A, et al. Cardiovascular effects of fine and ultrafine particles. J Aerosol Med. 2005;18:1–22.
Zanobetti A, Franklin M, Koutrakis P, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58.
Ye X, Peng L, Kan H, et al. Acute effects of particulate air pollution on the incidence of coronary heart disease in Shanghai. China PLoS One. 2016;11:e0151119.
Chen R, Zhang Y, Yang C, et al. Acute effect of ambient air pollution on stroke mortality in the China air pollution and health effects study. Stroke. 2013;44:954–60.
Li H, Chen R, Meng X, et al. Short-term exposure to ambient air pollution and coronary heart disease mortality in 8 Chinese cities. Int J Cardiol. 2015;197:265–70.
Chen R, Yin P, Meng X, et al. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med. 2017;196:73–81.
Samoli E, Touloumi G, Zanobetti A, et al. Investigating the dose-response relation between air pollution and total mortality in the APHEA-2 multicity project. Occup Environ Med. 2003;60:977–82.
Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005;113:88–95.
Roberts S, Martin MA. Applying a moving total mortality count to the cities in the NMMAPS database to estimate the mortality effects of particulate matter air pollution. Occup Environ Med. 2006;63:193–7.
Stylianou M, Nicolich MJ. Cumulative effects and threshold levels in air pollution mortality: data analysis of nine large US cities using the NMMAPS dataset. Environ Pollut. 2009;157:2216–23.
Kunzli N, Jerrett M, Mack WJ, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–6.
Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–42.
Puett RC, Hart JE, Suh H, et al. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect. 2011;119:1130–5.
Robertson S, Miller MR. Ambient air pollution and thrombosis. Part Fibre Toxicol. 2018;15:1.
Dockery DW, Pope CA 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9.
Laden F, Schwartz J, Speizer FE, et al. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–72.
Pope CA 3rd, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–74.
Pope CA 3rd, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41.
Cesaroni G, Badaloni C, Gariazzo C, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324–31.
Hart JE, Garshick E, Dockery DW, et al. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–8.
Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58.
Zhang LW, Chen X, Xue XD, et al. Long-term exposure to high particulate matter pollution and cardiovascular mortality: a 12-year cohort study in four cities in northern China. Environ Int. 2014;62:41–7.
Wong CM, Lai HK, Tsang H, et al. Satellite-based estimates of long-term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect. 2015;123:1167–72.
Beelen R, Stafoggia M, Raaschou-Nielsen O, et al. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 2014;25:368–78.
Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705.
Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond). 2008;115:175–87.
Nemmar A, Hoet PH, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–4.
Furuyama A, Kanno S, Kobayashi T, et al. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch Toxicol. 2009;83:429–37.
Kilinc E, Van Oerle R, Borissoff JI, et al. Factor XII activation is essential to sustain the procoagulant effects of particulate matter. J Thromb Haemost. 2011;9:1359–67.
Wold LE, Simkhovich BZ, Kleinman MT, et al. In vivo and in vitro models to test the hypothesis of particle-induced effects on cardiac function and arrhythmias. Cardiovasc Toxicol. 2006;6:69–78.
Bolli R, Zughaib M, Li XY, et al. Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect on contractile function. A pathophysiological basis for chronic myocardial “stunning”. J Clin Invest. 1995;96:1066–84.
Ikeda M, Watarai K, Suzuki M, et al. Mechanism of pathophysiological effects of diesel exhaust particles on endothelial cells. Environ Toxicol Pharmacol. 1998;6:117–23.
Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–8.
Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–9.
van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164:826–30.
Tornqvist H, Mills NL, Gonzalez M, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400.
Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–67.
Peters A, Frohlich M, Doring A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg study. Eur Heart J. 2001;22:1198–204.
Pekkanen J, Brunner EJ, Anderson HR, et al. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–22.
Peters A, Doring A, Wichmann HE, et al. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349:1582–7.
Frampton MW, Stewart JC, Oberdorster G, et al. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect. 2006;114:51–8.
Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–55.
Bonzini M, Tripodi A, Artoni A, et al. Effects of inhalable particulate matter on blood coagulation. J Thromb Haemost. 2010;8:662–8.
Croft DP, Cameron SJ, Morrell CN, et al. Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation and thrombosis in cardiac patients. Environ Res. 2017;154:352–61.
Tabor CM, Shaw CA, Robertson S, et al. Platelet activation independent of pulmonary inflammation contributes to diesel exhaust particulate-induced promotion of arterial thrombosis. Part Fibre Toxicol. 2016;13:6.
Krishnan RM, Adar SD, Szpiro AA, et al. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol. 2012;60:2158–66.
Wan Q, Yang YP, Liu ZY. Puerarin attenuates PM2.5-induced vascular endothelial cells injury via ERK1/2 signaling pathway. Zhongguo Zhong Yao Za Zhi. 2016;41:2309–14.
Pope CA 3rd, Bhatnagar A, McCracken JP, et al. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119:1204–14.
Zhang Y, Ji X, Ku T, et al. Inflammatory response and endothelial dysfunction in the hearts of mice co-exposed to SO2, NO2, and PM2.5. Environ Toxicol. 2016;31:1996–2005.
Magari SR, Schwartz J, Williams PL, et al. The association between personal measurements of environmental exposure to particulates and heart rate variability. Epidemiology. 2002;13:305–10.
Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–73.
Devlin RB, Ghio AJ, Kehrl H, et al. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 2003;40:76s–80s.
Pope CA 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–45.
Lim YH, Bae HJ, Yi SM, et al. Vascular and cardiac autonomic function and PM2.5 constituents among the elderly: a longitudinal study. Sci Total Environ. 2017;607-608:847–54.
Tobaldini E, Bollati V, Prado M, et al. Acute particulate matter affects cardiovascular autonomic modulation and IFN-gamma methylation in healthy volunteers. Environ Res. 2018;161:97–103.
Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 1860;2016:2891–8.
Schwartz J, Park SK, O'Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–33.
Alexeeff SE, Coull BA, Gryparis A, et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–6.
Ostro B, Malig B, Broadwin R, et al. Chronic PM2.5 exposure and inflammation: determining sensitive subgroups in mid-life women. Environ Res. 2014;132:168–75.
Frikke-Schmidt H, Roursgaard M, Lykkesfeldt J, et al. Effect of vitamin C and iron chelation on diesel exhaust particle and carbon black induced oxidative damage and cell adhesion molecule expression in human endothelial cells. Toxicol Lett. 2011;203:181–9.
Bo L, Jiang S, Xie Y, et al. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS One. 2016;11:e0152216.
Du X, Jiang S, Bo L, et al. Combined effects of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced cardiovascular injury in rats. Chemosphere. 2017;173:14–21.
Lin H, Liu T, Xiao J, et al. Mortality burden of ambient fine particulate air pollution in six Chinese cities: results from the Pearl River Delta study. Environ Int. 2016;96:91–7.
Shekelle PG, Morton SC, Jungvig LK, et al. Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease. J Gen Intern Med. 2004;19:380–9.
Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8.
Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e56803.
Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33.
Miller ER 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46.
Romieu I, Tellez-Rojo MM, Lazo M, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172:1534–40.
Tong H, Rappold AG, Diaz-Sanchez D, et al. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect. 2012;120:952–7.
Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8.
Mozaffarian D, Prineas RJ, Stein PK, et al. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48:478–84.
Samet JM, Rappold A, Graff D, et al. Concentrated ambient ultrafine particle exposure induces cardiac changes in young healthy volunteers. Am J Respir Crit Care Med. 2009;179:1034–42.
Perona JS, Cabello-Moruno R, Ruiz-Gutierrez V. The role of virgin olive oil components in the modulation of endothelial function. J Nutr Biochem. 2006;17:429–45.
Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, et al. Olive oil and haemostasis: platelet function, thrombogenesis and fibrinolysis. Curr Pharm Des. 2011;17:778–85.
Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90.
Tong H, Rappold AG, Caughey M, et al. Dietary supplementation with olive oil or fish oil and vascular effects of concentrated ambient particulate matter exposure in human volunteers. Environ Health Perspect. 2015;123:1173–9.
Funding
This work is supported by the grants from the National Natural Science Foundation of China (81573112, 81373030, 81703182). Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergies and the Environment
Rights and permissions
About this article
Cite this article
An, Z., Jin, Y., Li, J. et al. Impact of Particulate Air Pollution on Cardiovascular Health. Curr Allergy Asthma Rep 18, 15 (2018). https://doi.org/10.1007/s11882-018-0768-8
Published:
DOI: https://doi.org/10.1007/s11882-018-0768-8