Abstract
Food allergy is defined as an IgE-mediated hypersensitivity response to ingested food with allergic symptoms ranging from urticaria to life-threatening anaphylaxis. Food allergy is thought to develop because of (1) failed induction of tolerance upon initial exposure to food antigen or (2) breakdown of established tolerance to food antigen. We review current understanding of the pathogenesis, epidemiology, and natural history of food allergy, including the unconventional IgE-mediated food allergy to mammalian meat known as alpha-gal food allergy. We highlight emerging data on food allergy treatment and prevention, emphasizing the growing appeal of manipulating the gut microenvironment using probiotics and helminth products to blunt systemic allergic responses to food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Food allergy is defined as an IgE-mediated hypersensitivity response to ingested food with allergic symptoms ranging from urticaria to life-threatening anaphylaxis. IgE-mediated hypersensitivity reactions to food are typically acute in onset, developing less than 2 h after the ingestion and usually within minutes. Symptoms can involve any organ system, but commonly involve cutaneous, oropharyngeal, gastrointestinal, and respiratory systems [1]. The symptoms that result from food allergies can negatively impact the lives of patients and their families with physical, social, and financial consequences that diminish quality of life [2, 3]. Not surprisingly, there is great interest in treating food allergy and preventing it, particularly in high-risk, genetically susceptible individuals. We review current understanding of the pathogenesis, epidemiology and natural history of food allergy. We highlight emerging data on food allergy treatment and prevention, emphasizing the growing appeal of manipulating the gut microenvironment to blunt systemic allergic responses to food.
Pathogenesis of Food Allergy
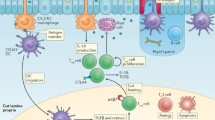
Food allergy is thought to develop because of (1) failed induction of tolerance upon initial exposure to food antigen or (2) breakdown of established tolerance to food antigen. Ordinarily, different components of the intestinal immune system integrate signals from intestinal luminal antigens derived from food and resident gut flora and communicate with the systemic immune system to generate tolerance to dietary antigens (Fig. 1) [4]. Food-allergic patients may have a defect in one or multiple components of the intestinal immune system, including the intestinal epithelial barrier, phagocytic innate immune cells, tolerogenic antigen presenting cells (APCs) and regulatory cells of the adaptive immune system. They develop a CD4+ T helper 2 (Th2) skewed immune response to orally delivered food antigens, triggering production of cytokines IL-4, IL-5, and IL-13. This prompts B cell activation and class switching, generating food antigen-specific IgE. Secreted IgE binds the surface of basophils and tissue resident mast cells. When the host is re-exposed to food antigen, it binds food-specific IgE attached to FcEpsilon receptors on mast cell and basophil cell surfaces. Crosslinking these receptors triggers degranulation of these innate immune cells, releasing mediators like histamine that generate the symptoms characteristic of immediate allergic reactions [5].
Commensal gut microflora unite innate and adaptive immune compartments to help generate an immune mileu favoring development of tolerance to orally delivered food antigens. Gut microflora interact with macrophages (Mac), dendritic cells (DC) and innate lymphoid cells (ILC) stimulating production of IL-22 that promotes intestinal epithelial barrier function. Direct crosstalk between gut microflora and intestinal epithelial cells triggers production of defensins which also shore up the epithelial barrier. Tolerogenic DC that take up both gut microflora and food antigen interact with naïve CD4+ T cells from the adaptive immune compartment favoring regulatory T cell (Treg) development. Treg development is also supported by butyrate generated from the breakdown of ingested carbohydrates by commensal flora. Tregs and other tolerogenic cells secrete regulatory cytokines like IL-10 and TGF-beta that favor B cell production and secretion of mucosal IgA further fortifying the intestinal epithelial barrier. Tregs suppress formation of food antigen-specific Th2 effector cells (Th2 cell) decreasing Th2 cytokine production. This prevents food antigen-specific IgE production and promotes food antigen-specific IgG4 production leading to tolerance to food antigen. Probiotics may function like endogenous gut microflora to promote tolerance to dietary antigen. Helminth products may further stimulate production of Tregs and other regulatory cells and cytokines to promote tolerance to foods
Epidemiology
The most common allergenic foods in the USA are milk, eggs, peanuts, tree nuts, soy, wheat, fish, and shellfish [6•]. Estimating the prevalence of food allergy is challenging because the gold standard for confirming the diagnosis of food allergy is the double-blind, placebo-controlled food challenge (DBPCFC), a time-consuming and expensive process that can trigger adverse reactions [1]. Thus, most food allergy prevalence estimates rely on self-report and vary in food allergy definitions and study methodology [7]. The prevalence of food allergy in children in the USA based on self-report ranges from 2–10 % [7]. One internet-based study estimated 8 % of children have food allergy with just under one third of these having multiple food allergies [8]. An NHANES study showed a prevalence of 6.53 % from 2007–2010 [6]. Prevalence of self-reported food allergy is similar in other industrialized regions including Canada (6.6 % adults, 7.1 % children) [9] and Europe (5.9 % from 2000–2012) [10].
One study tracking change in prevalence of self-reported food allergy in US adults using the U.S. Food and Drug Administration (FDA) Food Safety Survey found that self-reported food allergy prevalence increased 1.5-fold from 9.1 % in 2001 to 13 % in 2010. In this same study, physician-diagnosed food allergy in adults was 5.3 % in 2001 and only 6.5 % by 2010 [11]. A meta-analysis suggests that the prevalence of self-reported food allergy in the US increased 1.2 % points per decade from 1988–2011 [12]. These findings suggest, but do not confirm, the increasing prevalence of food allergy. Reasons for this are not fully understood, although a popular hypothesis pinpoints alterations in gut microflora as a possible contributor (see below, The Gut Microbiome in Food Allergy).
Natural History of Food Allergy
Roughly 70–80 % of children allergic to egg, milk, wheat, and soy will outgrow these allergies by adolescence [13–18]. Approximately 50 % of children outgrow egg and cow’s milk allergy (CMA) by age 6 [19, 20]. In contrast, about 20 % of children with peanut allergy and 10 % with tree nut allergy outgrow these allergies [21, 22]. Recent studies examining the natural history of peanut allergy continue to support this. Arshad et al. observed natural resolution of peanut allergy in 17 % of subjects ages 4 to 10 and 25 % ages 10 to 18 in a birth cohort observed through age 18 [23]. A longitudinal study of children diagnosed with peanut allergy at 1 year of age showed that peanut allergy resolved by age 4 in 22 % of children [24•]. Taken together, these studies show that the majority of peanut-allergic patients remain peanut-allergic through adulthood, providing impetus for research into prevention and treatments for peanut allergy in particular.
Alpha-gal Mammalian Meat Allergy
There is increasing awareness of an unusual IgE-mediated food allergy to mammalian meat known as alpha-gal food allergy. First described in 2009 [25••], alpha-gal food allergy challenges the current paradigm for food allergy. In contrast to conventional food allergies which require IgE antibodies specific to a food protein, alpha-gal allergy is associated with the presence of IgE antibodies against a sugar moiety galactose-alpha-1,3-galactose (also known as “alpha-gal”) that lines the surface of non-primate mammalian tissue. Antibodies directed against alpha-gal moieties have a well-established role in acute organ rejection [26]. Preexisting alpha-gal specific IgE antibodies have been implicated in anaphylactic reactions to cetuximab, a chimeric mouse-human IgG1 monoclonal antibody that has the oligosaccharide galactose-alpha-1,3-galactose on the Fab portion of the cetuximab heavy chain [27].
Reactions to alpha-gal are intermittent and may not occur with every allergen exposure. Variability in adverse reactions to alpha-gal depends on how much allergen is ingested which may depend on fat content of the cut of mammalian meat consumed. Additional factors include how much alpha-gal is digested, processed, packaged and presented to the immune system and the underlying state of the immune system at the time—i.e., whether the immune system is activated in the setting of acute or chronic infection, autoimmune disease, malignancy, etc. Unlike other IgE-mediated food allergies, IgE-mediated allergic reactions to alpha-gal in ingested mammalian meat are typically delayed in onset, frequently developing 3–6 h after ingestion, although alcohol and exercise may decrease time frame to reaction [26]. Commins et al. demonstrated this delayed onset of symptoms through open food challenges of patients with a history of severe cutaneous reactions 3 to 6 h after eating beef, pork, or lamb and positive alpha-gal specific serum IgE. By sampling blood hourly during each oral food challenge to check for basophil expression of the activation marker CD63 following in vitro allergen re-stimulation, they showed that basophil activation correlated with the appearance of clinical symptoms [28]. Their findings suggest that, like protein allergen in conventional food allergy, alpha-gal can crosslink alpha-gal-specific IgE bound to basophil FcEpsilon receptors and activate basophils.
Immunomodulatory properties of ticks play an important role in the development of alpha-gal mammalian meat allergy. Several case reports describe patients who previously tolerated mammalian meat developing meat allergy after tick bites [29]. Commins et al. demonstrated that serum IgE antibodies to alpha-gal rise after tick bites from the lone star tick, Amblyomma americanum [30••]. Tick bites from Ixodes ricinus and Ixodes holocyclus have also been reported to generate alpha-gal specific IgE [31]. Reasons for this are not fully understood. Tick bites may drive proliferation of a plasmablast population that produces alpha-gal specific IgE antibodies and repeated tick bites could promote persistence of this population of antibody-producing cells (Commins, personal communication). Over 1000 cases of delayed urticarial or anaphylactic reactions to mammalian meat have been described in the USA, primarily in the southeastern states within the known geographic distribution of Amblyomma americanum, including Tennessee, Virginia, North Carolina, Arkansas, and southern Missouri [30••]. Cases described in Australia, Western Europe, Japan, and Korea also link tick bites with sensitization to alpha-gal and subsequent mammalian meat allergy [31]. Additional studies on the prevalence of alpha-gal specific serum IgE in the general population, the prevalence of alpha-gal mammalian meat allergy itself, and continued studies on the immunomodulatory role of tick bites in triggering this allergy will improve understanding of this intriguing food allergy.
Food Allergy Prevention
There is considerable interest in preventing the development of food allergies, and peanut allergy in particular, since approximately 80 % of peanut-allergic individuals remain peanut-allergic into adulthood. Twenty years ago, expert guidelines for infants with family histories of allergy suggested delaying the introduction of allergenic foods like eggs, peanuts, and tree nuts until ages 2–3 years [32]. However, additional epidemiologic studies summarized in the American Academy of Pediatrics 2008 clinical report on the role for early nutritional interventions on development of atopic disease showed no strong evidence to support delayed introduction of any complementary solid foods, including commonly allergenic foods, beyond 4-6 months of age to prevent development of allergies to these foods [33••]. In addition, there is no strong evidence that breastfeeding exclusively beyond 4-6 months of age or diet alteration in breastfeeding mothers reduces the risk of allergic sensitization or prevents the development of food allergy in high-risk infants [2, 34•].
The ideal time to introduce commonly allergenic foods like milk, egg, or peanut into a child’s diet is still unknown, but a retrospective study involving over 3000 infants suggested that introducing cooked egg into the infant’s diet between 4–6 months of age was associated with a lower risk of egg allergy than introducing egg at 10–12 months or older [35]. Similarly, authors of a large scale prospective study involving over 13,000 infants found that infants with IgE-mediated CMA were more likely to have had supplemental cow’s milk protein (CMP)-based formulas introduced between 3.5–6.5 months of age compared to non-allergic infants, who received supplemental CMP formula within the first 2 weeks of life. This suggests that early exposure to CMP as a supplement to breastfeeding may prevent development of CMA [36].
Studies also indicate that early peanut ingestion may protect against development of peanut allergy in high-risk children. A 2008 study found that prevalence of peanut allergy in Jewish primary schoolchildren in the UK was nearly tenfold higher than peanut allergy prevalence in a similar population in Israel, even after controlling for differences in atopy, socioeconomic status and genetic background. Israeli infants eat much larger quantities of peanut than British infants with a median monthly peanut consumption of 7.1 g of peanut protein in Israeli infants ages 8–14 months compared to 0 g of peanut protein in this age group in the UK [37]. These findings suggested that early introduction of peanut during infancy might prevent development of peanut allergy. This led to a randomized trial of early peanut consumption in individuals at risk for peanut allergy known as the Learning Early about Peanut Allergy (LEAP) study. The LEAP trial demonstrated that early peanut introduction can be successfully carried out in a population at high risk for developing peanut allergy. In this trial, 640 infants, ages 4 to 11 months, with preexisting egg allergy, severe eczema, or both and with skin prick test sizes to peanut of 4 mm or less were randomly assigned to consume or avoid peanuts until 60 months of age. There was an 11 to 25 % absolute reduction in risk of peanut allergy in this population when peanut was introduced by ages 4–11 months [38••]. Subjects assigned to consume peanut consumed peanuts at least three times weekly with at least 6 g of peanut protein incorporated into the diet each week. In a follow-up study, the LEAP-On study, the investigators explored the persistence of oral tolerance to peanut in subjects randomized to peanut consumption within the first year of life through age 60 months, by determining peanut allergy prevalence in this population at age 72 months, after a 12-month period of peanut avoidance. The prevalence of peanut allergy in this group stayed low, 3.6 % at 60 months in the primary LEAP trial and 4.8 % at 72 months in the follow-up LEAP-On study, in the intention-to-treat populations for both studies. Peanut allergy prevalence was four to fivefold lower in the peanut-consumption group compared to the peanut-avoidance group at the end of both the primary LEAP trial and the follow-up LEAP-On study [39•].
In the follow-up LEAP-On study, adherence to peanut avoidance still allowed for cumulative consumption of up to 18 g of peanut protein in a 12-month period, which might be expected with accidental exposure. While overall adherence to peanut-avoidance was high in the original peanut-avoidance group at 90.4 %, the adherence rate in the original peanut-consumption group was only 69.3 %. The follow-up study was still adequately powered to demonstrate its primary outcome. Moreover, despite variation in overall adherence to peanut-avoidance during the 12-month peanut-avoidance period in the original prolonged peanut-consumption group, the prevalence of peanut allergy remained low in this population, suggesting that subsequent intermittent low-dose consumption of peanut after prolonged consumption does not trigger new-onset peanut allergy [39•].
The striking findings from the LEAP and LEAP-On studies demonstrating benefit with early peanut introduction for the majority of study subjects at high-risk for peanut allergy encouraged experts from several different professional societies to recommend early peanut introduction in high-risk infants ages 4–11 months under an allergist’s supervision [40]. Whether alternative doses of peanut besides the 6–9 g of peanut consumed per week in the LEAP study can induce tolerance to peanut remains unclear, as does the treatment duration time required to ensure sustained tolerance to peanut. It is also unknown if high-risk individuals have an increased risk of developing peanut allergy if peanut consumption is stopped before 60 months or with more sporadic peanut feeding. Moreover, recent consensus guidelines on early peanut introduction do not apply to children at low risk for peanut allergy. Development of more extensive guidelines on early introduction of peanut-containing foods into the diets of more general pediatric populations, not just “high-risk” as defined by the LEAP study, is ongoing [40].
The Gut Microbiome in Food Allergy
An estimated 1014 microbes inhabit the human intestinal lumen performing numerous functions critical for human health [41•]. Multiple experimental models indicate that maintaining tolerance to these commensal gut flora helps establish and sustain host tolerance to food [4]. Some hypothesize that environmental, dietary, and medical practices of industrialized nations have altered the gut commensal microbiota leading to an upswing in food allergy prevalence. Industrialized populations are exposed to antibiotics through their extensive use in agriculture to promote livestock growth and through medically prescribed antibiotics [42]. Mouse models have shown that neonatal antibiotic treatment can alter gut microbial diversity in fecal and ileal samples [43, 44••, 45]. Stefka et al. demonstrated that the altered gut microenvironment enhances food allergen sensitization in a murine model of peanut allergy [44••]. Human studies looking at 16S ribosomal RNA profiling of the gut microbiome in pediatric populations have also found associations between altered gut microbial populations and prevalence of food allergen sensitization [46, 47]. Researchers have noted differences in gut microbiota of children with food allergy and their food allergy-free counterparts [48, 49]. Hua et al. used data on self-reported food and environmental allergies and fecal 16S ribosomal RNA sequence data from the American Gut Project to demonstrate that adult allergic participants in the study had fecal microbial communities significantly different than those in non-allergic participants. Subjects with nut and seasonal allergies had more Bacteriodales and a reduction in Clostridiales taxa [50], recently shown in animal models to be integral to intestinal barrier integrity [44••].
Probiotics, defined as ingested microbes that provide health benefits to the host, may play a role in preventing food allergy. Mouse studies have shown that germ-free mice, which lack normal commensal microbes, have significantly underdeveloped regulatory T cell compartments and humoral IgA compartments, immune components considered critical for establishing tolerance to dietary antigens [42]. Stefka and colleagues demonstrated that sensitization to peanut is enhanced in both germ-free mice and mice that have received antibiotics in the neonatal period dramatically altering their normal gut commensal flora. The researchers also established that microbiota from the Clostridia class had allergy-protective properties in this peanut allergy model. When microbes from this class were reintroduced to antibiotic-treated mice, they prevented sensitization to peanut. Clostridia colonization triggered production of the cytokine IL-22, known to modulate intestinal barrier permeability [51, 52]. These researchers showed that treating mice who received antibiotics as neonates with either oral Clostridia or exogenous parenteral IL-22 was sufficient to reduce serum concentrations of peanut allergens Ara h6 and Ara h2 [44••]. This study suggests that populating the gut with appropriate microflora may reinforce intestinal barrier epithelial function, possibly preventing the development of food allergy.
Human studies using probiotics to try to prevent or treat food allergy have shown variable results. Probiotics studied so far in clinical trials for allergy prevention and treatment include the early gut colonizers and facultative anaerobes Lactobacillus and Streptococcus and later gut colonizer and obligate anaerobe Bifidobacterium [41•]. Bernini Canini and colleagues in a prospective clinical trial showed that supplementing extensively hydrolyzed casein formula (EHCF, a formula typically given to children with CMA) with Lactobacillus GG (LGG) increased the rate at which infants with CMA were tolerized to CMP when compared to infants fed with EHCF alone. These infants were able to tolerate cow’s milk for at least 6 months after a double-blind placebo-controlled food challenge confirmed initial tolerance to cow’s milk [53]. Subsequent studies looking at the composition of fecal microbiota in LGG-supplemented EHCF-fed infants showed increases in the number of genera producing the short chain fatty acid butyrate, an energy source for colonocytes that has also been linked to maintaining regulatory T cell populations and epithelial barrier function [42, 49]. By contrast, in a double-blind placebo-controlled trial with 119 infants with CMA fed either extensively hydrolyzed formula (EHF) supplemented with Lactobacillus casei and Bifidobacterium lactis or EHF alone, CMA infants fed EHF plus probiotics acquired tolerance to CMP at the same rate as CMA infants given EHF alone [54]. Discrepancies in these studies’ findings may be due to differences in probiotic strains used, dose administered, study population and study design.
In another study, children considered high-risk for developing atopic disease were fed standard infant formula or a nonhydrolyzed fermented infant formula containing heat-killed Bifidobacterium breve C50 and Streptococcus thermophilus 065. Researchers found no statistically significant difference in the incidence of CMA between the two groups based on history and oral challenge even though infants fed the fermented formula with heat-killed probiotics were more likely to have negative skin prick tests to CMP [55]. Allen and colleagues also found reduced skin prick test sensitivity to CMP or hen’s egg protein at age 6 months in children fed with Lactobacillus and Bifidobacterium daily from birth through 6 months born to women given the same probiotics daily from 36 weeks gestation to delivery when compared to mothers and infants fed with placebo [56]. These studies suggest that probiotics modulate development of allergic sensitization to foods as measured by allergy skin prick testing, but this does not automatically translate into food allergy prevention. Probiotic preparation, for example, live vs. heat-killed, may impact whether probiotic supplementation prevents food allergy.
Clearly, more studies are warranted to identify the appropriate probiotic strains, preparations, and doses to use to try to prevent or treat food allergy. Several studies, however, have shown no harm associated with probiotic supplementation [57, 58]. Despite conflicting findings on the benefits of probiotics in allergic disease, emerging data in the field is promising enough for the World Allergy Organization to support the use of probiotics in (1) pregnant women at high risk for delivering an allergic child (2) breastfeeding mothers with infants at high risk for allergy, and (3) infants at high risk for developing allergy with the caveat that evidence backing these recommendation is of low quality [59].
Emerging Therapies to Treat Food Allergy: Probiotics and Helminth Products
There are no FDA-approved cures or disease-modifying therapies for food allergy. Management of food allergy involves counseling patients to strictly avoid culprit food allergens and carry antihistamines and an epinephrine autoinjector in case of accidental exposure [1, 5]. Given the considerable anxiety for patients and families surrounding food allergy and the challenge of strict avoidance, there is strong interest in long-term therapeutic solutions for food allergy. Currently, all therapies for food allergy are investigational.
By increasing the amounts of target allergen delivered to the host until a maintenance dose is reached, allergy immunotherapy seeks to reprogram the immune system, halting Th2-skewed immune responses to allergens. Mechanisms behind allergy immunotherapy are not completely understood but seem to involve generation of regulatory T cells that inhibit or modify allergen-specific responses by pathogenic B and T cells through secretion of IL-10, TGF-beta, and other regulatory cytokines and by promoting generation and release of allergen-specific IgG4 antibodies over IgE [60•].
For many patients and families, having access to therapies promoting desensitization would be satisfactory. Food-allergic individuals are desensitized when the amount of food required to generate an adverse allergic response is increased from baseline. Desensitization requires continuous administration of food allergy immunotherapy. Creating therapies that promote desensitization and ultimately generate tolerance (or sustained unresponsiveness to food) is the goal for many in food allergy immunotherapy development. Tolerance is achieved when an individual can eat the food without developing symptoms even after prolonged avoidance of the food [1, 5].
Emerging therapeutic techniques to treat food allergy including subcutaneous immunotherapy (SCIT), oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT), recombinant vaccines, immunobiologics, and herbal therapeutics have been reviewed extensively elsewhere [3, 5, 60•, 61, 62]. Here, we highlight possible roles for probiotics and helminths as food allergy OIT adjuvants since they impact both the gut microenvironment and the systemic immune system.
OIT involves ingesting gradually increasing doses of allergenic food and is the most effective experimental therapy available to induce desensitization to foods, including milk, egg, and peanut [63–66]. Whether OIT can induce sustained unresponsiveness is controversial. Published OIT trials do not follow patients long enough and/or do not have all the appropriate controls to distinguish between immunotherapy-induced sustained unresponsiveness and subjects naturally outgrowing their allergy during the treatment course [67]. Adverse reactions, though typically mild and limited to the oropharynx, occur in virtually all patients, contributing to poor OIT adherence [61, 62, 68]. Animal and human studies assessing the ability of probiotics to enhance OIT efficacy and safety are scant. In a mouse egg allergy model, treating egg-allergic mice with egg-specific OIT plus the probiotic Clostridium butyricum was more effective at alleviating intestinal allergic inflammation in egg-allergic mice than egg-specific immunotherapy alone or probiotic alone [69]. The one published human study exploring the efficacy of probiotic-supplemented OIT was done in peanut-allergic children. Subjects received either placebo or peanut OIT and Lactobacillus Rhamnosus CMCC 1.3724 for 18 months. Nearly 90 % of subjects receiving peanut OIT and probiotics were desensitized to peanut as compared to only 7 % of subjects receiving placebo. 82 % of patients treated with peanut OIT and probiotics who then strictly avoided peanuts for 2–5 weeks after completing 18 months of therapy showed sustained unresponsiveness to peanut upon repeat oral challenge compared to only 3.6 % of patients on placebo [70•]. There was no peanut OIT-only arm in this study, so the relative contributions of probiotics vs. OIT in generating desensitization and sustained unresponsiveness to peanut are not clear. It is also unclear if the addition of probiotic-supplemented OIT decreased the number of adverse reactions or improved adherence to therapy compared to OIT alone. Additional studies exploring the ability of probiotics to improve safety and efficacy of OIT are needed.
Helminth infection, like probiotics, can alter gut microenvironment and systemic host immune responses. While acute helminth infection generates Th2-mediated immune responses, helminths also trigger host production of immunoregulatory lymphocytes and cytokines that assist in helminth immune evasion and promote chronic helminth infection [71]. Epidemiologic studies demonstrate that chronic helminth infection can protect against allergic disease [72]. Bashir et al. showed that mice infected with the helminth Heligmosomoides polygyrus were not sensitized to peanut in a mouse model of peanut allergy. Anaphylactic signs and peanut-specific IgE were significantly muted in helminth-infected mice while helminth-free mice developed anaphylaxis and elevated serum peanut-specific IgE. The immunoregulatory effect of helminth infection depended on IL-10 [73]. Jouvin and Kinet performed a small study in six adults with peanut or tree nut allergy, feeding subjects ova from the pig helminth Trichuris suis. Subjects did experience mild spontaneously resolving gastrointestinal side effects and transient eosinophilia following eight oral doses of 2500 ova every other week. However, no subject dropped out or was removed from the study due to adverse events. This small study had no placebo control group and was not designed to assess whether T. suis ova treatment cured food allergy. One subject lost skin prick test reactivity to peanut following T. suis ova treatment, but the remaining five subjects had no change in skin prick test reactivity or allergen-specific IgE levels [74•]. No studies have explored OIT plus helminth products to treat food allergy. These studies would be intriguing because the immunoregulatory environment triggered by helminths might enhance inhibition of pathogenic allergen-specific immune responses. Future studies are needed to address possible therapeutic roles for helminths in treatment of food allergy.
Conclusion
Advances made in our understanding of the pathogenesis, epidemiology, and natural history of food allergy have fueled development of strategies to prevent and treat food allergy. From early introduction of commonly allergenic foods to the diets of children at high-risk for developing food allergy to modulating the gut microenvironment and systemic immune response with probiotics and helminth (Fig. 1), discovering new ways to prevent and treat food allergy will expand knowledge of the immunology behind maintaining tolerance to dietary antigens.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kulis M, Wright BL, Jones SM, Burks AW. Diagnosis, management, and investigational therapies for food allergies. Gastroenterology. 2015;148(6):1132–42. doi:10.1053/j.gastro.2015.01.034.
de Silva D, Geromi M, Halken S, Host A, Panesar SS, Muraro A, et al. Primary prevention of food allergy in children and adults: systematic review. Allergy. 2014;69(5):581–9. doi:10.1111/all.12334.
Kobernick AK, Chambliss J, Burks AW. Pharmacologic options for the treatment and management of food allergy. Expert Rev Clin Pharmacol. 2015;8(5):623–33. doi:10.1586/17512433.2015.1074038.
Iweala OI, Nagler CR. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol Rev. 2006;213:82–100. doi:10.1111/j.1600-065X.2006.00431.x.
Burbank AJ, Burks W. Food specific oral immunotherapy: a potential treatment for food allergy. Expert Rev Gastroenterol Hepatol. 2015;9(9):1147–59. doi:10.1586/17474124.2015.1065177.
McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013;132(5):1216–9. doi:10.1016/j.jaci.2013.07.018. The first report of the overall prevalence of self-reported food allergy and the prevalence of specific food allergies using NHANES survey data from over 20,000 individuals from 2007-2010.
Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin N Am. 2015;35(1):45–59. doi:10.1016/j.iac.2014.09.004.
Dyer AA, Gupta R. Epidemiology of childhood food allergy. Pediatr Ann. 2013;42(6):91–5. doi:10.3928/00904481-20130522-08.
Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St Pierre Y, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130(4):986–8. doi:10.1016/j.jaci.2012.06.029.
Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. doi:10.1111/all.12423.
Verrill L, Bruns R, Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc. 2015;36(6):458–67. doi:10.2500/aap.2015.36.3895.
Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. 2014;112(3):222–9. doi:10.1016/j.anai.2013.12.007. e3.
Ohtani K, Sato S, Syukuya A, Asaumi T, Ogura K, Koike Y, et al. Natural history of immediate-type hen's egg allergy in Japanese children. Allergol Int. 2015. doi:10.1016/j.alit.2015.10.005.
Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120(6):1413–7. doi:10.1016/j.jaci.2007.09.040.
Czaja-Bulsa G, Bulsa M. The natural history of IgE mediated wheat allergy in children with dominant gastrointestinal symptoms. Allergy Asthma Clin Immunol. 2014;10(1):12. doi:10.1186/1710-1492-10-12.
Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009;102(5):410–5. doi:10.1016/S1081-1206(10)60513-3.
Kattan JD, Cocco RR, Jarvinen KM. Milk and soy allergy. Pediatric clinics of North America. 2011;58(2):407–26. doi:10.1016/j.pcl.2011.02.005.
Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol. 2010;125(3):683–6. doi:10.1016/j.jaci.2009.12.994.
Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol. 2014;133(2):492–9. doi:10.1016/j.jaci.2013.12.1041.
Spergel JM. Current options for the treatment of food allergy. Immunol Allergy Clin N Am. 2015;62(6):1531–49. doi:10.1016/j.iac.2015.08.008.
Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107(2):367–74. doi:10.1067/mai.2001.112129.
Fleischer DM. The natural history of peanut and tree nut allergy. Curr Allergy Asthma Rep. 2007;7(3):175–81.
Arshad SH, Venter C, Roberts G, Dean T, Kurukulaaratchy R. The natural history of peanut sensitization and allergy in a birth cohort. J Allergy Clin Immunol. 2014;134(6):1462–3. doi:10.1016/j.jaci.2014.09.026. e6.
Peters RL, Allen KJ, Dharmage SC, Koplin JJ, Dang T, Tilbrook KP, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population-based assessment. J Allergy Clin Immunol. 2015;135(5):1257–66 e-12. doi:10.1016/j.jaci.2015.01.002. Prospectively collected data on the natural history of peanut allergy in early childhood. In an attempt to avoid bias toward declaring persistent peanut allergy solely using skin tests and serum peanut-IgE levels, study subjects underwent oral food challenge both at diagnosis at 1 year old and at follow up at 4 years of age.
Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33. doi:10.1016/j.jaci.2008.10.052. First report characterizing delayed anaphylaxis to non-primate mammalian meat mediated by IgE antibodies to the carbohydrate epitope alpha-gal.
Commins SP, Jerath MR, Cox K, Erickson LD, Platts-Mills T. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65(1):16–20. doi:10.1016/j.alit.2015.10.001.
Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. doi:10.1056/NEJMoa074943.
Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108–15. doi:10.1016/j.jaci.2014.01.024.
Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190(9):510–1.
Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93e6. doi:10.1016/j.jaci.2011.02.019. Prospective study demonstrating that alpha-gal specific IgE rises following tick bites, providing the first example of response to an ectoparasite leading to a food allergy.
Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13(4):354–9. doi:10.1097/ACI.0b013e3283624560.
American Academy of Pediatrics. Committee on nutrition, hypoallergenic infant formulas. Pediatrics. 2000;106(2 Pt 1):346–9.
Greer FR, Sicherer SH, Burks AW, American Academy of Pediatrics Committee on N, American Academy of Pediatrics Section on A Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–91. doi:10.1542/peds.2007-3022. From the Allergy and Immunology Section of the American Academy of Pediatrics, this article reviews nutritional options during pregnancy, lactation, and the first year of life that may affect the development of atopic disease, including food allergy, in early life. Emphasizes that there is little evidence that delaying the timing of the introduction of complementary foods beyond 4 to 6 months of age prevents atopic disease.
Jelding-Dannemand E, Malby Schoos AM, Bisgaard H. Breast-feeding does not protect against allergic sensitization in early childhood and allergy-associated disease at age 7 years. J Allergy Clin Immunol. 2015;136(5):1302–8 e13. doi:10.1016/j.jaci.2015.02.023. Prospective study involving 335 children that showed no significant association between the duration of breastfeeding the development of sensitization by skin prick testing and serum specific IgE to 10 different foods and 12 different aeroallergens.
Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126(4):807–13. doi:10.1016/j.jaci.2010.07.028.
Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow's milk protein is protective against IgE-mediated cow's milk protein allergy. J Allergy Clin Immunol. 2010;126(1):77–82. doi:10.1016/j.jaci.2010.04.020. e1.
Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–91. doi:10.1016/j.jaci.2008.08.039.
Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–13. doi:10.1056/NEJMoa1414850. The LEAP trial showed that infants at higher risk of developing peanut allergy than the general population benefited from early introduction of peanut between ages 4-11 months old and regular peanut consumption over 5 years, experiencing an 11 to 25% absolute reduction in risk of peanut allergy compared to those avoiding peanut.
Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med. 2016. doi:10.1056/NEJMoa1514209. The LEAP-On follow-up study to the LEAP trial demonstrated that in children at high-risk for developing peanut allergy who consumed peanut within the first year of life through age 5, avoiding peanuts for a 12-month period was not assoicated with an increased rate of peanut-allergy in this population.
Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan ES, Muraro A, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Allergy, Asthma Clin Immunol. 2015;11(1):23. doi:10.1186/s13223-015-0087-8.
Bridgman SL, Kozyrskyj AL, Scott JA, Becker AB, Azad MB. Gut microbiota and allergic disease in children. Ann Allergy Asthma Immunol. 2016;116(2):99–105. doi:10.1016/j.anai.2015.10.001. A nice review of the development of the human gut microbiome, characteristics of the microbiome in allergic disease, and evidence and recommendations regarding probiotics and prebiotics to prevent and treat allergic disease.
Berni Canani R, Gilbert JA, Nagler CR. The role of the commensal microbiota in the regulation of tolerance to dietary allergens. Curr Opin Allergy Clin Immunol. 2015;15(3):243–9. doi:10.1097/ACI.0000000000000157.
Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172(11):6978–87.
Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111(36):13145–50. doi:10.1073/pnas.1412008111. Using a mouse peanut allergy model, this group demonstrated that sensitization to food allergen is increased in antibiotic-treated mice or mice lacking commensal microflora. Protection from food allergy can be conferred by Clostridia-containing microbiota that induces host production of the cytokine IL-22, restoring intestinal epithelial barrier function, preventing allergic sensitization to food.
Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–93. doi:10.1126/science.1219328.
Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–43. doi:10.1111/cea.12487.
Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. 2015. doi:10.1111/pai.12522.
Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. 2014;80(8):2546–54. doi:10.1128/AEM.00003-14.
Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2015. doi:10.1038/ismej.2015.151.
Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: analysis of the American Gut Project. EBioMedicine. 2016;3:172–9. doi:10.1016/j.ebiom.2015.11.038.
Li LJ, Gong C, Zhao MH, Feng BS. Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol. 2014;20(48):18177–88. doi:10.3748/wjg.v20.i48.18177.
Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39(1):11–8. doi:10.1097/SHK.0b013e3182749f96.
Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580-2–2 e-15. doi:10.1016/j.jaci.2011.10.004.
Hol J, van Leer EH, Elink Schuurman BE, de Ruiter LF, Samsom JN, Hop W, et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol. 2008;121(6):1448–54. doi:10.1016/j.jaci.2008.03.018.
Morisset M, Aubert-Jacquin C, Soulaines P, Moneret-Vautrin DA, Dupont C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur J Clin Nutr. 2011;65(2):175–83. doi:10.1038/ejcn.2010.250.
Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor MB, Garaiova I, et al. Probiotics in the prevention of eczema: a randomised controlled trial. Arch Dis Child. 2014;99(11):1014–9. doi:10.1136/archdischild-2013-305799.
van den Nieuwboer M, Brummer RJ, Guarner F, Morelli L, Cabana M, Claassen E. Safety of probiotics and synbiotics in children under 18 years of age. Benefic Microbes. 2015;6(5):615–30. doi:10.3920/BM2014.0157.
van den Nieuwboer M, Claassen E, Morelli L, Guarner F, Brummer RJ. Probiotic and synbiotic safety in infants under two years of age. Benefic Microbes. 2014;5(1):45–60. doi:10.3920/BM2013.0046.
Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization–McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8(1):4. doi:10.1186/s40413-015-0055-2.
Jongejan L, van Ree R, Poulsen LK. Hypoallergenic molecules for subcutaneous immunotherapy. Expert Rev Clin Immunol. 2016;12(1):5–7. doi:10.1586/1744666X.2016.1103182. Reviews how advances in chemical modification of allergens to be used in SCIT for food allergy is reviving research interest in developing food allergy SCIT with a better safety and efficacy profile than food allergy SCIT using standard unmodified food allergens.
Nowak-Wegrzyn A, Albin S. Oral immunotherapy for food allergy: mechanisms and role in management. Clin Exp Allergy. 2015;45(2):368–83. doi:10.1111/cea.12382.
Vazquez-Ortiz M, Turner PJ. Improving the safety of oral immunotherapy for food allergy. Pediatr Allergy Immunol. 2015. doi:10.1111/pai.12510.
Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. doi:10.1016/j.jaci.2009.05.022. e1-97.
Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131(1):128–34 e-13. doi:10.1016/j.jaci.2012.10.048.
Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135(5):1283–92. doi:10.1016/j.jaci.2014.11.010.
Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–43. doi:10.1056/NEJMoa1200435.
Vickery BP. Does clinical protection persist after food allergen oral immunotherapy? Immunotherapy. 2015;7(8):851–3. doi:10.2217/imt.15.59.
Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127(3):558–73. doi:10.1016/j.jaci.2010.12.1098. quiz 74-5.
Shi Y, Xu LZ, Peng K, Wu W, Wu R, Liu ZQ, et al. Specific immunotherapy in combination with Clostridium butyricum inhibits allergic inflammation in the mouse intestine. Sci Rep. 2015;5:17651. doi:10.1038/srep17651.
Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 2015;135(3):737–44e8. doi:10.1016/j.jaci.2014.11.034. First randomized placebo-controlled trial in peanut-allergic children evaluating the co-administration of probiotics with peanut OIT and assessing sustained unresponsiveness in children with peanut allergy. No OIT only arm, making it unclear the relative contributions of probiotics versus OIT.
Nutman TB. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol. 2015;37(6):304–13. doi:10.1111/pim.12194.
Hamid F, Amoah AS, van Ree R, Yazdanbakhsh M. Helminth-induced IgE and protection against allergic disorders. Curr Top Microbiol Immunol. 2015;388:91–108. doi:10.1007/978-3-319-13725-4_5.
Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169(6):3284–92.
Jouvin MH, Kinet JP. Trichuris suis ova: testing a helminth-based therapy as an extension of the hygiene hypothesis. J Allergy Clin Immunol. 2012;130(1):3–10. doi:10.1016/j.jaci.2012.05.028. Small proof of concept study in 6 subjects demonstrating safety of administering helminth products, in this case pig whipworm eggs ( Trichuris suis ova ), to individuals with food allergy. Not powered to show effect of treatment on persistence of food allergy.
Acknowledgments
We thank Andrew Spector and Pamela Steele for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Iweala declares no conflicts of interest. Dr. Burks has the following disclosures: Advisory Board: Stallergenes; NIH AITC; Competing Relationships: Allergen Research Corporation - Grantee; National Institutes of Health - Grantee; FARE - Chair, Research Advisory Board; Hycor Biomedical - Grantee; Consultant: GLG Research; Adept Field Solutions; Genentech; First Manhattan Co; Insys Therapeutics; ActoGeniX; SRA International; Sanofi US Services; Valeant Pharmaceuticals North America LLC; Stocks: Alltertein; Mastcell Pharaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Allergy and Immunology
Rights and permissions
About this article
Cite this article
Iweala, O.I., Burks, A.W. Food Allergy: Our Evolving Understanding of Its Pathogenesis, Prevention, and Treatment. Curr Allergy Asthma Rep 16, 37 (2016). https://doi.org/10.1007/s11882-016-0616-7
Published:
DOI: https://doi.org/10.1007/s11882-016-0616-7