Abstract
Food allergies are adverse immune responses to the ingestion of specific foods. This contrasts with food sensitivities, which refer to non-immune reactions. Food allergies are categorized based on their pathophysiology into IgE-mediated, non-IgE-mediated, and mixed immune reactions. Symptoms can range in severity from mild to severe, life-threatening anaphylaxis. Careful history-taking with the aid of a food diary can identify culprit allergens. Additional serum and skin diagnostic testing can further help elucidate etiology. The mainstay of therapy is allergen avoidance; however, pharmacotherapy can suppress inflammatory response and modulate symptoms. Management of food sensitivities similarly includes identifying culprit foods and avoiding them, though testing modalities are less well-defined, and the diagnosis and management differ based on the type of reaction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Food allergy
- Gastrointestinal intolerance
- Abdominal cramping
- Diarrhea
- Irritable bowel syndrome
- Nausea
- Vomiting
Introduction
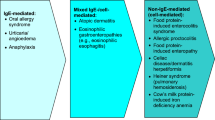
A food allergy is defined as an adverse immune response after ingestion of a specific food. Food allergies are usually characterized as IgE-mediated or non-IgE mediated immune reactions. This is distinct from food intolerance, which refers to a non-immune reaction. Intolerances are categorized as metabolic, toxic, pharmacologic, or other mechanisms (Fig. 10.1) [1].
Classification of adverse reactions to food (Reproduced from the Australasian Society of Clinical Immunology and Allergy [2]). Food allergies are characterized into immune-mediated and non-immune-mediated reactions. Immune-mediated food reactions are further categorized based on the pathophysiology into IgE-mediated, non-IgE-mediated, and mixed IgE- and non-IgE mediated etiologies
Extensive published literature estimates the prevalence of food allergies in the United States to be 8% and 10% in children and adults, respectively [3]. The prevalence of food allergies along with hospitalizations related to allergies has continued to rise over the past decade. These allergies are common in the early years of life and decrease over the first decade. There seems to be a geographic predisposition with more people affected in industrialized and western regions. Eight food categories, including peanuts (1.4%); tree nuts (1%); fish, shellfish, and eggs (1.5%); milk (2.5%); wheat (~0.4%); and soy (~0.4%), comprise a vast majority of allergic disease burden and account for over 90% of food allergens [3].
Food Allergies and Sensitivities
Individual physiologic and immunologic tolerance to ingested foods forms the foundation of food allergies and sensitivities that can be grouped into four categories: IgE-mediated, non-IgE-mediated, mixed, and non-immune. The mucosal immune system interacts with food antigens and is responsible for alterations and modulation of this immune reactivity. The gastrointestinal tract is composed of a single cell layer of the columnar epithelium joined by tight junctions and protected by trefoil factors (protease-resistant proteins that restore barrier), brush border enzymes, bile salts, and mucus. These factors work in combination to destroy pathogens and render antigens non-immunogenic. However, 2% of ingested food antigens are absorbed and transported into the body. These immunologically intact proteins do not usually provoke an immune response because of oral tolerance [4].
Oral tolerance normally occurs when a food antigen crosses an intact mucosal barrier and is delivered to antigen presenting cells (APCs), especially dendritic cells (DCs). Antigen-bound DCs in combination with suppressive cytokines, like interleukin 10, differentiate naïve T cells into regulatory T cells rather than food antigen-specific T-helper type 2 (TH2) cells. Upregulation of food-specific IgA and IgG antibodies with a compensatory decrease of IgE antibodies coupled with immune suppression of effector cells (mast cells and basophils) maintains tolerance and prevents these antigens from causing allergies (Fig. 10.2) [5].
Immunopathogenesis of food allergies (Reproduced from Anvari et al. 2018). Tolerance (left): Food allergens are exposed to macrophages in the intestinal lumen, which transfer antigens to dendritic cells in the gut lamina propria, which in turn present food peptides to T-cell receptors on naïve T cells. These T cells differentiate into T regulatory cells. Food-specific T cells with the help of cytokines TGF-beta and IL-10 encourage tolerance by suppressing mediator cells. Allergy (right): In the setting of immune barrier dysfunction, proinflammatory cytokines IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) are released and activate dendritic cells, which in turn present food peptides to T-cell receptors on naïve T cells to T-helper type 2 (Th2) cells. Food-specific Th2 cells secrete inflammatory cytokines such as IL-4, IL-5, and IL-13, promoting effector cell (eosinophils and basophils) recruitment. IL-4 also allows for B cells to produce food-specific IgE production
IgE-Mediated Reactions
Pathophysiology
Food allergies occur because of dysfunction of the immune system that normally maintains oral tolerance. An allergic response occurs in two steps: sensitization, which is defined as the development of food-specific IgE, and subsequent exposure. Sensitization occurs when food antigens cross a disrupted intestinal epithelial barrier in genetically predisposed individuals. This compromise in the integrity of the gut membrane results in the release of inflammatory cytokines, such as interleukin 25 (IL-25), IL-33, and thymic stromal lymphopoietin (TSLP), and allows antigens to freely pass through the barrier. When antigens are taken up by DCs in the presence of these inflammatory cytokines, the benign antigen is seen as a “threat.”
The activated DCs convert naïve T cells into food antigen-specific Th2 cells, which results in the release of proinflammatory cytokines such as IL-4. This induces class switching of food antigen-specific B cells from IgA and IgG antibody production to IgE antibody production promoting a state of sensitization and allergy. Antibodies remain bound on effector cells (e.g., mast cells and basophils). Upon repeat exposure to the food antigen, cross-linking of IgE and the IgE receptors occurs on the surface of the effector cells resulting in the release of preformed mediators involved in anaphylaxis including histamine, tryptase, platelet -activating factor, prostaglandins, and leukotrienes (Fig. 10.2) [5].
Clinical Manifestations
Typical symptoms occur rapidly within minutes to hours after ingestion of causative food and can involve almost every organ system including the following: cutaneous (i.e., erythema, pruritis), ocular (i.e., tearing, conjunctival erythema), airway (i.e., cough, chest tightness, wheezing), gastrointestinal (i.e., nausea, emesis, diarrhea), and cardiac (i.e., tachycardia, hypotension) [5]. Clinical presentation and organ systems involved depend on certain factors such as underlying comorbidities (e.g., asthma), health status, activities performed during ingestion (e.g., exercise or alcohol consumption), dose ingested, route of exposure, and method of preparation of causative food [6]. Additionally, risk factors for reported fatal and near-fatal reactions include age, underlying respiratory diseases (e.g., asthma), concomitant use of β-blocker medications, reactions that do not involve the skin, and delay in treatment [6].
Most food reactions involve cutaneous manifestations, such as pruritic rash, urticaria, and angioedema. IgE-mediated respiratory symptoms can involve the upper and lower airway. Upper airway symptoms present with nasal congestion, rhinorrhea, and nasal pruritus, and lower airway symptoms include wheezing, shortness of breath, cough, and use of accessory muscles. The most severe airway symptom is stridor resulting from airway obstruction.
Gastrointestinal manifestations include itching of the mouth or throat, nausea, vomiting, abdominal pain, and diarrhea. Bloody diarrhea, delayed diarrhea (>4 hours after ingestion of allergen), constipation, weight loss, and/or malabsorption are not typically the result of IgE-mediated disease, and other etiologies should be investigated [5]. The cardiovascular system can be affected resulting in dizziness, lightheadedness, and syncope with resultant tachycardia, hypotension, cardiovascular collapse, or even death [5].
Diagnostic Evaluation
A detailed food diary can supplement a medical history in unveiling the responsible food as well as in describing the interval between ingestion and symptom presentation. Immediate food-induced allergic reactions begin within minutes to a few hours following ingestion of a causative food and typically are IgE-mediated. Delayed food reactions typically occur several hours to days following ingestion and involve cellular mechanisms.
When the history and food diary are unrevealing, allergy testing with IgE skin or blood tests can be performed. A skin test, performed by either prinking or intradermal injection of allergens, is positive if local pruritis, erythema, and swelling occurs, a manifestation of activated mast cells primed by allergen-specific IgE.
IgE-specific skin or serum tests alone cannot be used for diagnosis given high rates of false positives; however, they can support the patient’s clinical history. Specifically, patients can show evidence of sensitization to an allergen in both tests without having a clinical allergic reaction to that allergen. High titer-specific IgE measurements and strongly positive wheal diameters (greater than 8 mm) on skin prick testing, however, are highly predictive of clinical allergy.
Total IgE measurement has been found to have little clinical utility, low positive predictive values, and inability to exclude culprit food allergens [5, 6]. IgG food-specific antibodies, total IgG antibodies, basophil activation, leukotriene release assays, and atopy patch test are similarly not recommended [1, 6].
The gold standard for determining and confirming the responsible antigen is a double-blind, placebo-controlled food challenge. In this setting, the patient receives doses of suspected food allergen or placebo that neither the patient nor allergist is aware of. However, given the expense and inconvenience of this test, single-blind food challenge and open-food challenge are more commonly in clinical settings [1].
Management
The mainstay of therapy is to identify and avoid specific food allergens that incite symptoms. Severe IgE-mediated reactions, such as anaphylaxis, require emergent management. After identifying symptoms as part of an anaphylactic reaction, intramuscular (IM) epinephrine remains the first-line treatment. Delay in epinephrine injection is associated with increased mortality. Epinephrine acts by vasoconstricting blood vessels to maintain blood pressure and dilating airways to decrease airway edema and improve respiration.
Intramuscular epinephrine can be administered via an autoinjector placed into the mid-outer thigh (vastus lateralis muscle). IM route is preferred over intravenous and subcutaneous routes, and autoinjectors can be used in many individuals, except infants weighing under 10 kg and adults weighing over 50 kg (who require weight-based dosing of 0.01 mg/kg).
In the case of anaphylaxis, massive fluid shifts can occur. These patients should receive large volume of fluid resuscitation with normal saline. Following epinephrine and hydration, adjunctive therapies can be used in the treatment of continued reactions including antihistamines, bronchodilators, and glucocorticoids. These medications should not be first-line therapies in anaphylaxis as they do not improve respiratory obstruction or cardiovascular compromise. However, they are the mainstay in managing symptoms of less severe food-induced IgE-mediated allergic reactions.
First- and second-generation antihistamines relive pruritis and hives but can produce side effects (e.g., sedation). In the case of anaphylaxis, IV formulations are preferred, whereas less severe allergic reactions can be treated with oral formulations. H1 antihistamines like diphenhydramine, given with an H2 antihistamine, like ranitidine and famotidine, provide additional relief of hives. Inhaled bronchodilators administered by a mouthpiece and nebulizer can improve bronchospasm not responsive to epinephrine. Glucocorticoids have an onset of action over several hours and are thought to prevent biphasic or protracted reactions. There is an overall lack of evidence supporting the benefit of glucocorticoids, though they are commonly used.
The main long-term management strategy of IgE-mediated food allergies is strict food allergen avoidance. To reduce the risk of recurrence, patients should follow up with an allergist, who may aid in allergen identification, and a registered dietitian, who may counsel on recipes, meal plans, and analysis of food labels. Patients should also be given a prescription for epinephrine with instructions outlining proper use.
Oral immunotherapy, an emerging modality, is accomplished by using a small, increasing amount of culprit allergens or cross-reactive allergens to desensitize the patient and possibly induce tolerance. Allergen-specific immunotherapy improves clinical symptoms of FA while on therapy; however, long-term clinical benefit and safety data of immunotherapy is unknown. Other modes of immunotherapy including epicutaneous and sublingual are also being studied and may become useful in the future.
Non-IgE-Mediated Reactions
Non-IgE-mediated food allergies encompass a wide spectrum of disorders including food protein-induced enterocolitis syndrome (FPIES), allergic proctocolitis (AP), food protein-induced enteropathy (FPE), and gluten-related disorders (Fig. 10.1). The pathophysiology of non-IgE-mediated food allergies is poorly defined but likely T-cell-mediated. Unlike IgE-mediated FA, symptom onset is delayed from hours to weeks after ingestion of causative food. Given the lack of temporal association between ingestion and symptoms as well as paucity of noninvasive confirmatory testing, diagnosis of non-IgE-mediated food hypersensitivity can be challenging.
Food Protein-Induced Enterocolitis Syndrome (FPIES)
FPIES represents the more severe end of the non-IgE-mediated food hypersensitivity spectrum that occurs almost exclusively in infants and young children. Although the pathophysiology is not well understood, it is thought to be a T-cell-mediated disorder. It is hypothesized food allergens promote T-cell activation and release of proinflammatory cytokines resulting in local intestinal inflammation and subsequent increased intestinal permeability and fluid shifts. The local inflammation may be mediated by activated peripheral mononuclear cells, increased TNF-α, and decreased expression of TGF-β receptors in the intestinal mucosa. Humoral responses are also poorly understood, but studies reveal an increased number of IgM- and IgA-containing plasma cells [7]. More studies are required to understand the underlying mechanism of FPIES.
The most common inciting allergens are cow’s milk and soy proteins, but proteins in rice, oat, egg, wheat, and fish have also been implicated. The suggested incidence of cow’s milk-induced FPIES is 0.34% [8]. Age of onset is generally within the first year of life, and the inciting allergen correlates with early introduction of this food. FPIES to cow’s milk and soy usually starts within the first 3–6 months of life, while FPIES to solid foods starts later at 4–8 months of age.
Acute FPIES presents with severe, projectile emesis, diarrhea, dehydration, and possibly shock within 1–6 hours after ingestion of causative food protein. Stools contain occult blood and inflammatory cells including neutrophils and eosinophils. Chronic FPIES is less prevalent and characterized by intermittent but progressive emesis, watery diarrhea, and failure to thrive. Unlike acute FPIES, there does not appear to be a clear temporal association between trigger food antigen and onset of symptoms.
Allergic Proctocolitis (AP)
Allergic proctocolitis (AP) represents a milder end of the non-IgE-mediated food hypersensitivity spectrum. The pathophysiology is not well identified but also thought to be a T-cell-mediated.
This disease is exclusively identified in young infants within months after birth, with a prevalence of 1–2% [9]. Cow’s milk, found in either formula or breast milk, remains the most common offending antigen with an incidence of 76% [9]. Other dietary triggers include egg, soy, and corn, with some infants having multiple offenders.
Symptoms can begin as early as the first week of life. While some infants can be fussy and irritable, others can develop altered stool patterns varying from multiple daily stools with visible blood and mucus streaks to infrequent stools with occasional bleeding. Most infants are healthy appearing and thriving . This can result in delayed diagnosis.
Food Protein-Induced Enteropathy (FPE)
Food protein-induced enteropathy (FPE) is rare with unknown prevalence. Cow’s milk is the most common food allergen causing FPE; however, it has also been associated with soy, egg, wheat, rice, chicken, and fish protein allergens. Eosinophils, cow’s milk-specific TH2 lymphocytes, and localized production of IgE in mucosa of the small intestine have been implicated in the pathophysiology of FPE.
FPE manifests in infancy with the most prominent symptoms being watery diarrhea and failure to thrive accompanied by vomiting and abdominal distention and fullness. Malabsorption and steatorrhea distinguish this entity from FPIES and AP.
Laboratory work-up and endoscopy with biopsies are necessary to confirm the diagnosis and to differentiate this condition from other disorders that cause failure to thrive and diarrhea. Laboratory findings may suggest malabsorption with anemia (20–70%), hypoalbuminemia, and fat-soluble vitamin deficiency. Serologically, milk IgA and IgG antibodies are present. Endoscopic assessment can show villous effacement with histology showing elevation and predominance of intraepithelial lymphocytes, mast cells, and eosinophils. Despite cessation, endoscopic remission may require 6 to 18 months of allergen avoidance.
Gluten-Related Disorders
Gluten is the main structural protein complex found in wheat, rye, and barley. The immunogenic protein fractions of gluten include prolamins (gliadin) and glutenins. Three main forms of gluten reactions exist: (1) allergic (wheat allergy), (2) autoimmune (celiac disease, gluten ataxia, and dermatitis herpetiformis), and (3) possible immune-mediated (gluten sensitivity). Wheat allergy occurs via an IgE-mediated immune response with gluten peptides triggering a classic food allergy affecting the skin, gastrointestinal tract, and/or respiratory tract as described above in the IgE-mediated section.
Celiac Disease (CD)
CD is an immune-mediated enteropathy triggered by ingestion of gluten in genetically susceptible individuals that occurs in up to 1% of the population (see details in Chap. 6). Here we will discuss the immunogenic process of CD. Genetic predisposition plays a role in CD with all patients expressing a gene that encodes for the major histocompatibility complex (MHC) human leukocyte antigen (HLA) class II proteins HLA DQ2 (approximately 95%) and HLD-DQ8, located on chromosome 6p21. While the presence of these HLA class II proteins alone does not ensure CD, their presence is necessary for disease development.
The development of CD relies on exposure to gliadin, one of the soluble protein components of gluten. Gliadin fragments gain entry through the epithelial barrier into the lamina propria and are deaminated by tissue transglutaminase (TTG). Gliadin is then deaminated by TTG activating both the adaptive and innate immune systems. In the adaptive immune response, APCs, including macrophages, DCs, and B cells, express HLA class II DQ2 and/or DQ8 molecules on their surface which then uptake and display gliadin peptides. These APCs bind with gliadin-specific CD4 Th1 cells, producing proinflammatory cytokines. The resultant effect is crypt hyperplasia and villous blunting in the small intestine. Similarly, the innate immune response increases inflammatory mediators like IL-15 and interferon alpha with subsequent recruitment of intraepithelial lymphocytes to the intestinal epithelium.
Classic CD is characterized by diarrhea or signs and symptoms of malabsorption with steatorrhea, weight loss, or vitamin deficiency; however, patients often present with minor gastrointestinal complaints with extraintestinal manifestations including anemia, osteoporosis, arthritis, increased transaminases, neurological symptoms, and/or infertility.
Non-celiac Gluten Sensitivity
Non-celiac gluten sensitivity (NCGS) is a term used to describe individuals who do not have CD or wheat allergy but develop intestinal and/or extraintestinal signs and symptoms induced by gluten ingestion that improve when gluten-containing grains are removed from the diet [10]. The true prevalence is unknown due to the lack of definitive diagnostic testing. It is thought, however, to be more prevalent than celiac disease.
The pathophysiology of NCGS remains largely undetermined. While gliadin plays a prominent role in the pathogenesis of gluten sensitivity, it is hypothesized that other components, like α-amylase/trypsin inhibitors, may also contribute. Gliadin fragments bind the CXCR3 chemokine receptor allowing the release of zonulin, a modulator of intracellular tight junctions which regulates gut permeability. This reaction occurs in all individuals who ingest gluten, usually without any consequences [11]. However, these events can cause an inflammatory process in genetically predisposed individuals when gluten is mistaken as a pathogen by the immunologic surveillance system. Increased permeability of the epithelial barrier facilitates gliadin fragments trafficking from the gut lumen to lamina propria resulting in the activation of the intestinal innate immune system. Unlike in celiac disease, there is no subsequent activation in the adaptive immune system which explains the lack of enteropathy and villous blunting in this condition [11].
Clinical symptoms are like CD and include abdominal pain, bloating, and altered bowel habits (diarrhea, constipation, or both). Extraintestinal manifestations include mental fog, defined as slowed thinking, headache, joint and muscle pain, fatigue, depression, leg or arm numbness, dermatitis, and anemia.
Unlike CD , NCGS has no validated serum biomarkers for diagnosis. Given the overlap in symptoms between CD, NCGS, and wheat allergy, it becomes important to diagnose an underlying disease with serologic and histologic evaluation.
Management
The cornerstone of the management of FPIES, FPE, AP, and gluten-related disorders is the avoidance of offending foods. For acute management of FPIES, intravenous or oral rehydration may be required based on the ability to tolerate oral intake. Anti-emetics, such as ondansetron, may be considered to control emesis. With rehydration and food avoidance, acute FPIES resolves in a few hours, and chronic FPIES resolves in days to weeks. Similarly, FPE symptoms resolve within 1–4 weeks with avoidance; however, resolution of biopsy findings can take up to 18 months.
In breast-fed infants with AP, eliminating the offending agent in mom’s diet, usually cow’s milk, is key to resolution, with bleeding improving in 72 to 96 hours. Unremitting symptoms can require change from breastfeeding to a casein hydrolysate formula or amino acid-based formula. In formula-fed infants with AP, transition to extensively hydrolyzed formula is considered first-line therapy especially in infants less than 6 months with failure to thrive.
For both AP and FPE, food avoidance is not permanent. Foods can be reintroduced gradually if skin prick test and food-specific IgE antibody levels are negative. In FPIES, food can be reintroduced under medical supervision given the risk of hypotension.
In gluten-related disorders, the mainstay of management is avoidance of gluten-containing foods. Unlike CD and wheat allergy, NCGS may be transient. Current recommendations are to follow a gluten-free diet (GFD). In instances of NCGS, gluten may be introduced after a finite amount of time to determine tolerance. Based on severity of symptoms, some patients with NCGS may choose to follow a GFD indefinitely. These patients, along with patients diagnosed with CD and wheat allergy, should be monitored closely by a gastroenterologist and registered dietician to confirm they are avoiding inadvertent exposures and meeting daily fiber and micronutrient goals.
Mixed IgE- and Non-IgE-Mediated Food Allergy
Eosinophilic Gastrointestinal Disorders
Some food allergy disorders result from both IgE- and non-IgE-mediated immune processes. A common example is allergic eosinophilic gastrointestinal disorders (EGIDs), which are characterized by pathologic eosinophilic infiltration of the esophagus, stomach, small intestine, and/or colon leading to organ dysfunction and clinical symptoms. EGIDs include eosinophilic esophagitis (EoE), eosinophilic gastritis (EG), eosinophilic enteritis (EE), eosinophilic colitis (EC), and eosinophilic gastroenteritis (EGE). The most common of the disorders is eosinophilic esophagitis (EoE), with an estimated prevalence of 1/1000. (refer to Chap. 5 for an in-depth discussion of EoE). For this chapter, we will focus on the other subtypes of EGIDs.
The estimated prevalence of EG, EGE, and EC are 6.3/100,000, 8.4/100,000, and 3.3/100,000, respectively [12]. Overall, the prevalence of non-EoE EGIDs remains rare in the United States with less than 50,000 total people affected [12]. Genetic predisposition in combination with environmental factors and host’s immune system plays a role in pathogenesis. Familial clustering has been reported with 10% of patients having an immediate family member with an eosinophilic gastrointestinal disorder [12].
Pathophysiology
Eosinophils are normally present in all regions of the gastrointestinal tract except for the esophagus and participate in immune homeostasis. However, a large number of mucosal eosinophils reflect a pathologic process driven by exposure to food antigen. A T-helper type 2 (Th2) cell immune response and increased levels of mucosal permeability are the primary abnormalities found in EGIDs. TH2 immune response increases production of cytokines, such as IL-5, which promotes eosinophil development, activation, survival, and recruitment to sites of inflammation, and IL-13, which induces gene expression necessary to accumulate eosinophils in the mucosa [13].
Once densely infiltrated, eosinophils become activated, releasing granules of proinflammatory mediators, leukotrienes, and prostaglandins. The resultant effect is increased epithelial infiltration and alteration of sensory and motor activities of the mucosa. Augmented intestinal permeability makes entry of food and environmental allergens into subepithelial tissues easier. This stimulates a Th2 cell-mediated immune response which leads to eosinophilic inflammation and eventual tissue remodeling and fibrosis [14].
Clinical Manifestations
The clinical presentation depends on the location, extent, and layer(s) of the gastrointestinal tract involved. The most common symptoms are abdominal pain, nausea, vomiting, early satiety, and diarrhea with only 33% of patients developing weight loss. Those diagnosed with EG primarily present with nausea, vomiting, abdominal pain, and early satiety. Diffuse small bowel involvement in EE and EGE disrupts the intestinal barrier resulting in malabsorption, protein-losing enteropathy, and failure to thrive. Those with EC can present with diarrhea, abdominal pain, and hematochezia.
EGIDs can affect the mucosal, muscular, and serosal layers, with mucosal involvement most common. Involvement of the muscular layer results in wall thickening and impaired motility. Patients may present with gastric or intestinal obstruction reporting nausea, vomiting, abdominal distention, and rarely perforation. Sub-serosal disease is the rarest form and can present with isolated ascites or ascites in combination with symptoms seen in the other subtypes.
Diagnosis
EGIDs are suspected in patients with concerning clinical manifestations associated with peripheral eosinophilia, which is seen in 80% of patients. Eosinophil counts can range from 5% to 35% of total white blood cells with an average absolute eosinophil count of greater than 500 cells/μL. Mucosal and sub-serosal diseases are characterized by higher eosinophil count compared with the disease that involves the muscular layer. Those with malabsorption can have hypoalbuminemia, iron deficiency anemia from occult bleeding and erosions/ulcerations, increased fecal fat excretion, and prolonged prothrombin time due to vitamin deficiencies. Serum IgE levels are markedly elevated. In 25% of the cases, elevated ESR is seen. Evaluation of patients suspected to have an EGID should exclude alternate causes of eosinophilia.
Imaging is not necessary for diagnosis; however, barium studies and cross-sectional imaging may reveal thickening or nodularity in the antrum and thickening or “saw-tooth” mucosa in the small intestine. Despite abnormalities being present, these findings are not sensitive or specific for diagnosis.
Diagnosis is made during upper endoscopy with biopsies. Because eosinophilia can be patchy in patients, multiple biopsies of both normal and abnormal mucosa must be taken to increase sensitivity. It is important to remember that biopsies are normal in sub-serosal and muscular disease. It is important to notify the pathologist for clinical suspicion of this diagnosis. Because the stomach and duodenum are the most affected sites, initial endoscopic evaluation is limited to the upper gastrointestinal tract. Diarrhea-prominent disease should be investigated with a colonoscopy and subsequent examination of the terminal ileum, which can show erythema, nodularity, and thickened folds.
Management
Like EOE, elimination diet and corticosteroids are the mainstay of therapy of EGIDs. Administration of prednisone, a systemic glucocorticoid, at 30–40 mg/day is the most widely used treatment for EGE. The use of swallowed topical administration is also an option; however, effectiveness of this approach has not been elucidated in the literature. Histamine H1 receptor antagonists, leukotriene receptor antagonists, mast cell stabilizers, and immunosuppressive agents have been reported for use in both patients who respond to steroids and those who do not. The effectiveness of these drugs is unknown because randomized controlled trials are limited. A six-food elimination diet, cutting out wheat, milk, egg, soy, nuts and tree nuts, and seafood and reintroducing eliminated components, may allow for improvement in symptoms.
Non-immune-Mediated GI Adverse Reactions to Food
Food intolerance or sensitivity refers to a non-immunologic reaction to food and can result from a wide range of etiologies. It affects up to 15–20% of the population [15]. Intolerances are categorized into metabolic, pharmacologic, and other etiologies based on their pathophysiology. In this section, we will focus on enzymatic defects (e.g., disaccharidase deficiencies), pharmacologic food intolerances, toxic reactions to food, and other food intolerances including those related to specific ingestions (i.e., fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs), food additives, and food pathogens).
Enzymatic Defects: Disaccharide Intolerance
Disaccharides are sugar molecules composed of a combination of two monosaccharides (glucose, fructose, galactose) and include lactose (glucose-galactose), sucrose (glucose-fructose), maltose (glucose-glucose), and trehalose (glucose-glucose). Disaccharides are broken down into their single sugar components by enzymes found in the intestinal brush border known as disaccharidases. Disaccharide intolerance occurs in the setting of disaccharidase ingestion out of proportion of available enzyme and/or activity. Adult-onset lactose intolerance is by far the most common type, affecting up to 67% of the global population [16].
Lactose intolerance is especially prevalent among Asian, African, Native-American, and Mediterranean populations. Lactase activity peaks at birth and is reduced during childhood to facilitate breastfeeding weaning. Lactose intolerance is less common in Caucasians due to a gain of function mutation leading to lactase persistence [17]. Lactose intolerance occurs when there is inadequate lactase activity resulting in unabsorbed lactose after ingestion. Gut bacteria metabolize unabsorbed sugars resulting in the production of hydrogen, methane, and short-chain fatty acids, which lead to the GI symptoms of abdominal pain and cramping, bloating, diarrhea, and borborygmi. Symptom development depends on the mismatch of lactose ingestion with enzyme activity and can be worsened by visceral hypersensitivity associated with anxiety or IBS [17]. Lactose content is higher in milk, ice cream, and butter products compared to yogurt and cheese because bacteria used to produce the latter break-down lactose resulting in lower total lactose levels.
Sucrose intolerance occurs due to inadequate sucrase-isomaltase and can be congenital or acquired. New evidence estimates that 2–9% of Americans of European descent may be affected by sucrose intolerance [18]. Maltase and trehalase deficiencies are rarer types of disaccharidase deficiencies with unknown prevalence. Maltose is a disaccharide formed from two units of glucose with an alpha (1–4) bond, compared to the alpha (1–6) bond of isomaltose. The pathophysiology of these disaccharide intolerances is similar to lactose intolerance in that undigested sugars accumulate in the intestinal lumen leading to osmotic diarrhea and bacterial fermentation that induces additional changes in bowel habits, bloating, and abdominal pain.
Disaccharidase intolerances, such as lactose deficiency, can be assessed by disaccharide breath tests, but clinical aptitude of these tests is questionable, and an elimination diet may be more effective in diagnosing the condition and recommending sugar avoidance. Additionally, saliva tests are available for evaluation of sucrase activity, though these have similar clinical limitations.
Pharmacologic Food Intolerance
Pharmacologic food intolerance results from ingestion of vasoactive amines including dopamine, histamine, norepinephrine, phenylethylamine, serotonin, and tyramine. A common example is ingestion of histamine in the form of matured cheeses, alcoholic beverages, and fermented foods, which leads to systemic and gastrointestinal complaints. Histamine is metabolized extracellularly by diamine oxidase and intracellularly by histamine-N-methyltransferase [19]. Reduced activity of these enzymes leads to histamine toxicity and symptoms. Overall, histamine-rich food avoidance is crucial for diagnosis and management because there is limited utility in checking serum histamine levels.
Tyramine toxicity most frequently occurs in patients taking MAO inhibitors who ingest tyramine-rich foods such as cheese and wine but can also occur due to increased bacterial decarboxylation activity in poorly preserved foods [20]. Excess tyramine results in sympathetic stimulation with hypertensive crisis, headache, and flushing. Management of hypertensive crisis includes administration of phentolamine or nitroprusside. Beta-blockers should be avoided to prevent unopposed alpha receptor activation, which worsens elevated blood pressures .
Toxic Food Intolerance
Ingestion of toxic food components can also induce systemic and gastrointestinal complaints. Scombroid poisoning is the most common presentation, representing a histamine toxicity that occurs due to ingestion of spoiled dark meat fish such as tuna, mahi-mahi, or mackerel. During the spoilage period, bacterial histidine decarboxylase converts histidine to histamine. Symptoms occur 20–30 minutes after ingestion and are usually mild and self-limiting. These include facial flushing, burning sensation of the mouth, abdominal pain, diarrhea, headache, and palpitations [21]. Scombroid poisoning is frequently misdiagnosed as fish allergy, so history-taking and nuanced assessment of symptoms are critical. First-line immediate treatment is antihistamines. Epinephrine is rarely used, though may be necessary if the patient develops anaphylaxis with hypotension, angioedema, and bronchospasm.
Specific Food Component Intolerances
Fermentable Carbohydrates
Fermentable oligo-, di-, and monosaccharides and polyols (FODMAPs) are short-chain carbohydrates and sugar alcohols such as fructose, lactose, sorbitol, and mannitol that are fermented by intestinal bacteria. Examples of high-FODMAP foods include beans, wheat and rye, dairy products, dried fruit, artificial sweeteners, and alcohol.
These foods cause GI symptoms such as bloating, abdominal pain, nausea, and altered bowel habits (diarrhea and/or constipation) due to poor intestinal absorption, high osmotic activity, rapid fermentation, and increased gas production by intestinal bacteria. The combined effects of increased water delivery and gas in the lumen cause distention and lead to pain and discomfort in susceptible patients. Because IBS patients have increased visceral hypersensitivity, they are also more likely to experience functional GI symptoms from FODMAP ingestion.
Studies show that a low-FODMAP diet leads to clinical response and improvement in symptoms for 50–80% of patients with IBS [22]. Low-FODMAP diets have also helped mitigate IBS-like symptoms in IBD patients, but recommendations are still controversial due to the risk of undernutrition with dietary restriction in this population [23]. Low-FODMAP dietary education should be provided to avoid dietary over-restriction and nutritionally replete diet. This education consists of initially eliminating FODMAPs from the diet for 2 to 8 weeks and then, if symptom resolution occurs and patient is considered a responder, sequentially reintroducing foods high in fermentable carbohydrates to determine individual tolerance and define personalized dietary approach.
Food Additives
Food additives , such as preservatives, nutritional additives, coloring, flavoring, and texturing agents, are used during food production at allowed doses. However, these bioactive chemicals can cause physiological changes and have potentially harmful health effects. Specifically, sulphites, nitrites, nitrates, and monosodium glutamate have been implicated as causing asthma, rhinitis, urticaria, pruritus, and migraines. An ongoing area of research is the effect of food additives on the human gut microbiota, which can have pervasive effects on various metabolic processes and diseases such as obesity, diabetes, and cardiovascular disease. Future studies in humans will be critical to define safety.
Food Poisoning
Food poisoning is the ingestion of foods contaminated with bacteria, toxins, viruses, parasites, or chemicals. Common pathogens include staphylococcal enterotoxins, Bacillus cereus toxins, gram-negative enteric pathogens, and hepatitis A virus. The time course of developing symptoms varies depending on the pathogen ingested, within hours for toxins and days for bacteria or viruses. Treatment is supportive, and resolution of symptoms is also variable, ranging from hours to weeks depending on the offending agent. Bacterial and protozoal infections are more likely than viral infections to lead to prolonged post-infective irritable bowel syndrome (PI-IBS) [24].
References
Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. https://doi.org/10.1016/j.jaci.2010.10.007.
Food Intolerance. In: Information for patients, consumers and carers. Australian society of clinical immunology and allergy. 2019.
Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. 2019;2(1):e185630. https://doi.org/10.1001/jamanetworkopen.2018.5630.
Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013;23(9):R389–400. https://doi.org/10.1016/j.cub.2013.02.043.
Anvari S, Miller J, Yeh CY, Davis CM. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57(2):244–60. https://doi.org/10.1007/s12016-018-8710-3.
Atkins D, Bock SA. Fatal anaphylaxis to foods: epidemiology, recognition, and prevention. Curr Allergy Asthma Rep. 2009;9(3):179–85. https://doi.org/10.1007/s11882-009-0027-0.
Feuille E, Nowak-Węgrzyn A. Definition, etiology, and diagnosis of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol. 2014;14(3):222–8. https://doi.org/10.1097/ACI.0000000000000055.
Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large-scale, prospective population-based study. J Allergy Clin Immunol. 2011;127(3):647–53.e1-3. https://doi.org/10.1016/j.jaci.2010.12.1105.
Lake AM. Food-induced eosinophilic proctocolitis. J Pediatr Gastroenterol Nutr. 2000;30(Suppl):S58–60. https://doi.org/10.1097/00005176-200001001-00009.
Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148(6):1195–204. https://doi.org/10.1053/j.gastro.2014.12.049.
Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318(7):647–56. https://doi.org/10.1001/jama.2017.9730.
Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a National Administrative Database. J Pediatr Gastroenterol Nutr. 2016;62(1):36–42. https://doi.org/10.1097/MPG.0000000000000865.
Hill DA, Spergel JM. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep. 2016;16(2):9. https://doi.org/10.1007/s11882-015-0592-3.
Kinoshita Y, Oouchi S, Fujisawa T. Eosinophilic gastrointestinal diseases – pathogenesis, diagnosis, and treatment. Allergol Int. 2019;68(4):420–9. https://doi.org/10.1016/j.alit.2019.03.003.
Zopf Y, Baenkler HW, Silbermann A, Hahn EG, Raithel M. The differential diagnosis of food intolerance. Dtsch Arztebl Int. 2009;106(21):359–69; quiz 69–70; 4 p following 70. https://doi.org/10.3238/arztebl.2009.0359.
Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):738–46. https://doi.org/10.1016/S2468-1253(17)30154-1.
Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68(11):2080–91. https://doi.org/10.1136/gutjnl-2019-318404.
Cohen SA. The clinical consequences of sucrase-isomaltase deficiency. Mol Cell Pediatr. 2016;3(1):5. https://doi.org/10.1186/s40348-015-0028-0.
Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85(5):1185–96. https://doi.org/10.1093/ajcn/85.5.1185.
Marcobal A, De las Rivas B, Landete JM, Tabera L, Munoz R. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit Rev Food Sci Nutr. 2012;52(5):448–67. https://doi.org/10.1080/10408398.2010.500545.
Feng C, Teuber S, Gershwin ME. Histamine (Scombroid) fish poisoning: a comprehensive review. Clin Rev Allergy Immunol. 2016;50(1):64–9. https://doi.org/10.1007/s12016-015-8467-x.
Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8):1517–27. https://doi.org/10.1136/gutjnl-2017-313750.
Gibson PR. Use of the low-FODMAP diet in inflammatory bowel disease. J Gastroenterol Hepatol. 2017;32(Suppl 1):40–2. https://doi.org/10.1111/jgh.13695.
Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136(6):1979–88. https://doi.org/10.1053/j.gastro.2009.02.074.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nadpara, N., Matan, A., Kesavarapu, K. (2022). Food Allergies and Sensitivities. In: Newberry, C., Laster, J., Pickett-Blakely, O. (eds) Nutrition, Weight, and Digestive Health. Springer, Cham. https://doi.org/10.1007/978-3-030-94953-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-94953-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94952-5
Online ISBN: 978-3-030-94953-2
eBook Packages: MedicineMedicine (R0)