Abstract
Allergic asthma is on the rise in developed countries, and cockroach exposure is a major risk factor for the development of asthma. In recent years, a number of studies have investigated the importance of allergen-associated proteases in modulating allergic airway inflammation. Many of the studies have suggested the importance of allergen-associated proteases as having a direct role on airway epithelial cells and dendritic cells. In most cases, activation of the protease activated receptor (PAR)-2 has been implicated as a mechanism behind the potent allergenicity associated with cockroaches. In this review, we focus on recent evidence linking cockroach proteases to activation of a variety of cells important in allergic airway inflammation and the role of PAR-2 in this process. We will highlight recent data exploring the potential mechanisms involved in the biological effects of the allergen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence and severity of asthma has risen dramatically over the past two decades. Cockroach allergy was first reported by Bernton and Brown, two medical students who noticed skin rashes immediately after a cockroach crawled over the skin of an allergic patient. They subsequently reported that 44 % of allergy patients in New York City had positive skin tests to cockroach [1]. Since that time, considerable evidence has confirmed that exposure to cockroach induced allergy and asthma [2, 3]. It has been shown that early life exposure to German cockroach (GC) allergens leads to sensitization and the incidence of asthma [4, 5]. Children who were both allergic to cockroach allergen and exposed to high levels of this allergen had greater hospitalization rates, unscheduled medical visits, and more days of wheezing than children allergic to and exposed to dust mites or cat dander [4], confirming cockroach as an important allergen. Cockroaches are found throughout the world and both the German cockroach (Blattella germanica) and the American cockroach (Periplaneta americana) have been associated with asthma. B. germanica is a widely distributed urban pest, which most commonly infest homes, apartments, restaurants and hotels in the United States. P. americana often reside outside in sewers, stream tunnels and drainage systems; they can also be found in commercial and large buildings such as grocery stores, restaurants and hospitals.
In this review, we describe the role of proteases associated with cockroach in the development of allergic disease. We will describe the current understanding of the role of cockroach-derived proteases in modulating allergic airway inflammation as well as present an overview of recent findings that cockroach proteases can regulate the innate immune response. This review will also highlight the role of protease-activated receptor (PAR)2 in modulating these responses.
Cockroach Proteases
Potential sources of cockroach allergens include saliva, feces, cast skins and dead bodies. We believe that the most likely source of cockroach allergens are feces (frass) because of the amount of excrement secreted from each cockroach, the potentially high number of cockroaches dwelling in homes and the fact that desiccated frass is likely to crumble and become airborne as dust. German cockroach (GC) frass and the commercially available whole body cockroach extract contain serine protease activity [6, 7]; however, it is important to note that none of the allergens from B. germanica have been showed to be proteolytically active. Bla g 2 was shown to have characteristics of an aspartic protease, but it was found to be inactive [8]. Three cockroach species from Korea (B. germanica, P. americana and T. fuliginosa) were confirmed to contain gelatinolytic activity [9]. Per a 10, an allergen from P. Americana, was shown to be proteolytically active [10]. Attempts at isolation and characterization of the serine protease are underway, and have led to the enrichment of the active protease, virtually devoid of endotoxin [11]. This preparation has been explored for the ability to induce an immune response. It is also important to note that it is currently unclear whether the active serine protease in GC frass is derived from the cockroach, or commensal bacteria in the gut of the cockroach.
Protease-Activated Receptor (PAR)2
A number of proteases have been shown to signal directly to cells by cleaving protease-activated receptors (PARs). PARs are 7-transmembrane G coupled protein receptors that are stimulated by a variety of extracellular proteases. Cleavage of an extracellular amino portion of the molecule by thrombin (for PAR-1, PAR-3 and PAR-4) or trypsin (PAR-2) results in a new amino terminus which acts as a tethered ligand that binds to the activation site of the receptor to activate the heterotrimeric G-proteins in the cell membrane [12]. PAR-2 has been implicated in allergic airway inflammation as mast cell tryptase (known to play a role in mediating allergic airway inflammation and airway reactivity) was also found to activate PAR-2 [13]. PAR-2 can also be activated, without proteolytic cleavage, using the peptides SLIGKV (human) or SLIGRL (murine) [14]. PAR-2 is expressed on airway epithelial cells [15], alveolar macrophages [16], mast cells [17], neutrophils [18] and dendritic cells [16]. GC frass was shown to activate PAR-2[19, 20] in human bronchial epithelial cells. The importance of PAR-2 expression in the activation of alveolar macrophages [21], mouse tracheal epithelial cells [11], and eosinophils [22] has recently been demonstrated. Knight et. al. demonstrated increased PAR-2 expression on the asthmatic bronchial epithelium compared with normal epithelium from biopsy sections [23]. In that study, they found no difference in the amount of staining of PAR-2 in samples obtained from steroid-dependent or steroid-free asthmatics. A recent study isolated American cockroach antigens Per a 1.0101 and Per a 1.0104 and found that these increased PAR-2 protein expression and release of IL-4 and IL-13 from P815 cells (a mouse lymphoblast-like mastocytoma cell line) [24]. However, there was no evidence presented that suggested these antigens acted directly on PAR-2. We recently showed that GC frass upregulated PAR-2 levels on pulmonary mDCs and on bone marrow-derived mDCs [25••], suggesting that allergen upregulation of PAR-2 could play a role in mediating the immune response.
Regulation of Allergic Airway Inflammation by Cockroach Proteases
Role of Serine Proteases and PAR-2 in the Regulation of Allergic Airway Inflammation.
The earliest report implicating allergen-derived proteases in modulating allergic airway inflammation showed that removal of protease from Aspergillus fumigatus resulted in decreased airway hyperresponsiveness (AHR) in a murine model [26]. Since that time, a few studies have shown the importance of active proteases in cockroach allergens in mediating allergic airway inflammation. Sensitization of mice to allergen can occur by intratracheal or intranasal instillation without the addition of adjuvant (type II allergens) or by binding allergen to aluminum hydroxide (alum) and administration by intraperitoneal injection (type I allergens). Comparison of naïve mice sensitized via intratracheal instillation with GC frass or GC frass devoid of serine protease activity (by preincubation with aprotinin), revealed that removal of the protease from GC frass led to decreased AHR and mucin production [27]. Interestingly, however, when mice were sensitized using GC frass or protease-devoid GC frass bound to alum and administered by intraperitoneal injection, there was no difference in AHR or mucin production between protease-containing or protease-devoid GC frass [28••]. These data implicate the importance of protease activity in the GC frass in the initiation of mucosal sensitization. The importance of PAR-2 in mediating allergic airway inflammation was recently demonstrated. Sensitization of wild type mice to GC frass resulted in increased AHR, serum IgE, Th2 cytokine production (IL-13, IL-4 and IL-5) and Th17 cytokine production (IL-17A), cellular infiltration (eosinophils, neutrophils and macrophages), and mucin production [28••]. Sensitization of PAR-2-deficient mice resulted in the significant decrease in all parameters of allergic airway inflammation. Comparing whole body cockroach extract (CE) to heat-inactivated CE, Arizmendi et. al. showed that sensitization to heat-inactivated CE induced significantly less eosinophilia when compared to untreated CE [29••]. They also confirmed a decrease in AHR, eosinophila and serum IgG1 in CE-exposed PAR-2-deficient mice, and confirmed the role of PAR-2 in allergic sensitization to CE using an anti-PAR-2 antibody. Sensitization with the proteolytically active Per a 10 from P. Americana was sufficient to induce AHR, cellular infiltration and both eosinophil peroxidase and myeloperoxidase activity levels [10]. In fact, the eosinophilia was higher in mice treated with the active Per a 10 compared to P. Americana extract. Together these data highlight an important regulatory aspect of cockroach-derived proteases and the activation of PAR-2 on regulating allergic airway inflammation. In the next sections, we will discuss the potential mechanisms by which proteases and PAR-2 activation stimulate the innate immune response leading to subsequent development of allergic asthma.
Regulation of the Early Innate Immune Response by Cockroach Proteases
Proteases Acting on the Airway Epithelium.
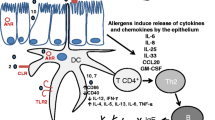
While it was once thought that the airway epithelium was a passive physical barrier, it is now thought that the epithelial barrier has a dynamic role in modulating airway inflammation. Early work confirmed the role of cockroach-derived proteases on expression of interleukin (IL)-8 and IL-6 from human airway epithelial cells [6, 19]. Activation of PAR-2 induced G-coupled protein led to increased signaling through mitogen-activated protein kinase (MEK), extracellular signal regulated kinase (ERK) [19] and nuclear factor for IL-6 (NF-IL-6) [30]. Recently, chemokine (C-C motif) ligand 20 (CCL20) and granulocyte macrophage-colony stimulating factor (GM-CSF) release were confirmed in primary mouse tracheal epithelial cells (MTEC) and this was dependent on active proteases and PAR-2 expression [11]. Since GM-CSF induced the development of pro-asthmatic myeloid DCs (mDC) [31] and CCL20 is a strong chemoattractant for lymphocytes and dendritic cells, it was hypothesized that GC frass proteases could directly affect the airway epithelium to initiate the development of allergic airway inflammation. The role of PAR-2 in regulating the innate immune response was further implicated by the finding that human β-defensin and CCL20 were significantly decreased in PAR-2-deficient gingival epithelial cells following treatment with Porphyromonas gingivalis [32]. Therefore, activation of PAR-2 by allergen-derived proteases could be one mechanism by which the airway epithelium responds to antigen exposure.
A number of studies have suggested that proteases in house dust mite (HDM) contributed to epithelial barrier dysfunction [33–35]. To date, not much is known regarding epithelial barrier dysfunction in regards to cockroach allergen. It is thought that proteases in HDM increased permeability via the detachment of Madin-Darby canine kidney (MDCK) cells [36]; however, this has not been explicitly investigated in the context of cockroach-derived proteases. It has been shown that cockroach extract antigen increased vascular endothelial growth factor (VEGF) in bronchial airway epithelial cells and increased electrical resistance in these cells [37], although in this study, a role for cockroach proteases was not addressed. Topical application of cockroach allergens to repeated tape-stripped hairless mice or humans resulted in a decreased barrier recovery rate in a PAR-2-specific manner [38]. In addition, topical application of a PAR-2 agonist peptide delayed recovery while accelerated barrier recovery was found in PAR-2-deficient mice compared to wild type controls [39]. Thus it is possible that cockroach-derived proteases could also alter lung epithelial barrier function, but further research is required in this area.
Early Innate Immune Response.
Within three hours following a single exposure of GC frass to naïve mice, an innate immune response occurs as evidenced by an increase in tumor necrosis factor (TNF)α, KC, CCL20 and GM-CSF levels in the BAL fluid [11, 40]. Chemokine and cytokine release in vivo was found to be protease- and PAR-2-dependent [11, 21]. We recently described a paradigm in which DCs play a crucial role in the initiation of the innate immune response via early release of TNFα, which modifies cytokine expression of airway epithelial cells and the ability of the cells to respond to allergen [41]. While the importance of proteases or PAR-2 was not investigated in that study, it is important to note that PAR-2-deficient bone marrow-derived DC (BMDC) produce less TNFα than wild type BMDC [25••]. Since the nature of DCs is to adapt to the highly specialized environments in which they are located [42•], the early release of TNFα from DCs could act directly on the TNFα receptor (TNFR1) on airway epithelial cells leading to increased expression of CCL20 and GM-CSF, an effect which would increase the recruitment and activation of subepithelial DCs (Fig. 1). This is supported by the finding that DCs cultured in the presence of lipopolysaccharides secreted exovesicles containing TNFα. which were then internalized by epithelial cells and resulted in chemokine release [43]. Thus, following exposure to allergen, both the DC and epithelium could play supporting roles for the initiation of allergic inflammation through the early release of TNFα from DCs which stimulates the airway epithelium to release mediators of DC maturation and recruitment. In addition, cockroach allergens have been shown to directly affect airway epithelial cytokine and chemokine production via PAR-2 [11, 19, 44]. These actions could be further complicated by the likely involvement of toll like receptors (TLR) and nucleotide oligomerization domain receptors (NOD).
Schematic of the mechanisms activated by cockroach-derived proteases and PAR-2. GC frass (blue circle) interacts with DC (in green) and airway epithelial cells (in yellow) via activation of PAR-2 (orange rectangle). In addition, proteases may increase epithelial permeability resulting in increased permeability and increased accessibility to allergen (shown on left). GC frass acts directly on the airway epithelium via PAR-2 to increase chemokine production leading to recruitment and maturation of DCs. Activated DCs increase production of TNFα, which also directly regulates chemokine production. GC frass-derived protease and activation of PAR-2 are important for upregulation of PAR-2 and CD80/CD86 expression on mDC and for the release of cytokines IL-23, IL-6 and TNFα
Recruitment, Development and Maturation of DCs in the Lung.
DCs are the most potent antigen presenting cells (APC) and are thought to bridge the innate and adaptive immune response. Two major subsets of DCs are critical not only for the initiation of allergic airway responses, but also to drive immunity (myeloid, mDC) or tolerance (plasmacytoid, pDC). In the lung, immature DCs are located within and directly beneath the respiratory epithelium and are thus in a unique position to sample antigen [45]. The turnover of the DC population is high and immature DCs are continuously recruited from the circulation along chemokine gradients. DC recruitment into the lungs has been shown to be increased following allergen exposure in humans [46] and mice [47]. We found that the percentage of mDC in the lung was significantly increased following a single exposure to GC frass, and these levels were mildly, but significantly decreased in PAR-2-deficient mice [11]. Since the whole lung was studied in this context, it is possible that investigation of mDC levels in the microenvironment may highlight more substantial differences in mDC recruitment.
Fields et. al. reported that PAR-2 played a role in DC development and maturation. They showed that PAR-2-deficient mice failed to develop DCs; however, the addition of TNFα to the culture or crosslinking CD40 triggered DC development. In addition, the culture of bone marrow progenitor cells in the presence of soybean trypsin inhibitor (SBTI), a serine protease inhibitor, failed to result in mature DCs. Addition of TNFα to the SBTI-treated cells resulted in maturation of DCs [48]. These data suggested that serine protease activation of PAR-2 stimulated the development of DCs from bone marrow progenitor cells and may be important for DC maturation. Interestingly, we have never encountered an inability to culture mDCs from bones of PAR-2-deficient mice compared to wild type mice. Our culture method is similar to that of Fields et. al. [48]; however, we do not add IL-4 to our cultures. More research is needed in this area to determine the role of protease activation of DC development.
We recently showed that GC frass increased expression of CD80 and CD86 on pulmonary mDCs, but that PAR-2-deficient mDCs had a small, but statistically significant decrease in the level of these co-stimulatory markers compared to wild type mDCs [25••]. This complements the previous finding that a lack of protease/PAR-2 signaling leads to a lack of mature DCs [48]. In our study though, we used a complex antigen to stimulate pulmonary DCs, which likely stimulates the cells by a number of mediators and not just protease-PAR-2 activation. In unpublished work, we found decreased expression of CD80 and CD86 following stimulation with protease-free GC frass compared to untreated GC frass, but the frequency of mDCs expressing these markers was unaltered (K. Page and I. Lewkowich; unpublished observations). This would suggest that it is the activation of PAR-2 by protease and not necessarily just the presence of PAR-2 that is important for DC maturation. More research into this area will further identify the role for PAR-2 in DC maturation in the lung following allergen exposure.
Cytokine Production and Antigen Uptake by DCs.
APC stimulate naïve CD4+ T cells to direct their differentiation into T helper 1 (Th1), Th2 or Th17 cells. Th1 cells produce IFNγ and provide protective immunity against viruses and microbes, while Th2 cells produce IL-4, IL-5 and IL-13 primarily in response to helminths, allergens and extracellular microbes. Th17 cells produce IL-17 and are associated with an excessive inflammatory response leading to arthritis and other inflammatory diseases. GC frass [25••] and whole body cockroach extract (K. Page, unpublished observation) are sufficient to induce a Th2/Th17 response in murine lungs. PAR-2-deficient mice display reduced production of both Th2 and Th17-associated cytokines, suggesting a role for PAR-2 in influencing T cell responses [25••, 28••]. In addition, the production of IL-6, IL-23 and TNFα by mDCs was significantly decreased in PAR-2-deficient mice compared to wild type mice [25••], suggesting the potential importance of PAR-2 expression in regulating T cell differentiation. Gao et. al. [49•] recently investigated Th1/Th2 cytokine profile of co-cultured pDC and CD4+ T cells following treatment with cockroach antigen and found significantly elevated levels of IL-13, IL-10 and TNFα, but no increase in IL-12p70 or interferon (IFN)α. While the involvement of protease/PAR-2 was not addressed, the biological relevance of cockroach activation of pDCs may be important for future understanding of the role of pDC in mediating allergic airway inflammation.
Another important aspect of dendritic cell activation is the uptake of allergen by the DC. One report eloquently showed that DC uptake of AlexaFluor 488-labeled OVA was enhanced when a selective PAR-2 agonist was added [50]. They stained for CD11c + cells and measured uptake of AF488 OVA in DCs in the draining lymph node and spleen. Using AlexaFluor 405-labeled GC frass, it was reported that there was no difference between wild type and PAR-2-deficient pulmonary mDC in their uptake of allergen [25••]. While these two findings appear to contrast, it is likely that pinpointing a target in the absence of other signals (i.e. the use of a PAR-2 agonist) would give a different result that when a real world allergen (which contains endotoxin, proteases, a TLR2 agonist and a variety of unknown components) is used. This finding does suggest, however, that involvement in antigen uptake may not be a primary role for PAR-2 when a naïve DC encounters a complex allergen.
Role of DCs in Sensitization.
Animal models have shown the importance of DC in modulating allergic airway inflammation [51]. Transfer of antigen-pulsed mDC into the airways of naïve mice is sufficient to sensitize mice [52, 53], while transfer of antigen-pulsed pDC induced tolerance [54]. GC frass-pulsed mDC are sufficient to sensitize mice for subsequent development of allergic airway inflammation [25••].
PAR-2 expression was upregulated on mDC, but not pDC following a single exposure to GC frass, suggesting the importance of protease/PAR-2 activation on sensitization to allergen [25••] Adoptive transfer of GC frass-stimulated wild-type and PAR-2-deficient mDCs showed that the lack of PAR-2 on sensitizing DCs markedly impacted Th2 cytokine production thus providing evidence that PAR-2 on mDCs is involved in promoting Th2 immune responses. In addition, we recently were successful in partially isolating the serine protease from GC frass and importantly this preparation was almost completely devoid of endotoxin [11]. GC frass-derived protease enhanced Th2 sensitization to a normally tolerogenic antigen ovalbumin (OVA), suggesting its role as an adjuvant for specific Th2 sensitization [25••]. This was confirmed by increased AHR, serum IgE levels, and Th2 cytokine (IL-13, IL-4 and IL-5) production. Interestingly, IL-17A levels were not increased by the GC frass-derived protease, suggesting a complicated dynamic between Th2 and Th17 cytokine activation. Together these data highlight the crucial role for DC activation by cockroach-dependent proteases acting via PAR-2 in the initiation of allergic airway inflammation.
Natural Inhibitors of Allergen-Derived Protease
A1AT. Alpha1 antitrypsin (A1AT) is a naturally occurring protease inhibitor in serum and the main function of A1AT is thought to maintain the physiological balance of proteinase-antiproteinase. A1AT inhibits neutrophil elastase, mast cell tryptase and cathepsin G and other serine proteases. Interestingly, many of the proteases that A1AT inactivates are proteases that have the ability to activate PAR-2. No research to date has been conducted to determine if there is a correlation between A1AT levels and activation of PAR-2 by endogenous proteases. A1AT was shown to inhibit antigen-dependent AHR in sheep [55], and therapeutic A1AT augmentation therapy has proven successful for at least one bronchial asthma patient [56]. We previously showed that A1AT inhibited GC frass activity by 30–50 %; however, GC frass was also capable of cleaving A1AT in a matter of 5 minutes [7]. This data would indicate that while A1AT levels may be increased to inhibit the altered protease/antiprotease balance, GC frass proteases may be a powerful inhibitor of the body’s natural response to quench protease activity and inhibit the activation of PAR-2.
SLPI.
Secretory leukocyte protease inhibitor (SLPI) is found in large quantities in bronchial and mucosal fluids and is thought to play a number of important roles in the lung. SLPI is a natural inhibitor of trypsin, mast cell tryptase, and neutrophil elastase that may play a role in allergic asthma. SLPI is constitutively expressed in mucosal tissues and immune cells. In a recent study, it was shown that the cleaved portion of SLPI (cSLPI) was increased in subjects with allergic rhinitis and asthma compared to healthy controls [57]. Overexpression of SLPI was shown to prevent the development of AHR and prevented OVA-mediated IgE, while ablation of the SLPI gene led to more severe responses to OVA [58]. In our murine model, we find that a single intratracheal instillation of GC frass leads to increased SLPI mRNA levels in the lung within 3 hr (3.6 ± 0.3 fold increase compared to PBS-treated mice) and we find that increased SLPI mRNA is independent of GC frass proteases or PAR-2 expression (K. Page, unpublished observation). The importance of this finding is that GC frass-derived proteases may serve to cleave and inactivate the natural inhibitors in the lung thus allowing for increased damage to the airway epithelium or increased interaction with the mucosal DCs. Interestingly a group showed administration of SLPI prior to antigen prevented antigen-induced AHR, cellular infiltration of eosinophils and neutrophils, and decreased mucus production in sheep [59]. In addition, they found that administration of aerosolized SLPI one hour after allergen challenge also decreased bronchoconstriction and mucus velocity. SLPI is a broad spectrum serine protease inhibitor, and it is unclear whether therapeutic benefits would be derived from inhibiting the protease activity in an allergen. It is perhaps more likely that the role of these endogenous protease inhibitors would be to inhibit the systemic increase of protease activity, likely derived from mast cell and leukocyte proteases. Additional studies would be required to determine a role for SLPI in GC frass activation of PAR-2 in vivo.
Conclusions
Currently there are no conditional knockouts of the PAR-2 mice that allow the gene to be inactivated in a specific tissue (i.e. the airway epithelium or the dendritic cell); however, these types of studies will lead to a more comprehensive study of the importance of PAR-2 on mediating this disease. Investigations of how to clinically target PAR-2 and the eventual importance of those inhibitors on novel therapeutic interventions need to be further evaluated. It is important to remember that not only can PAR-2 be activated at the time of inhalation of allergen, but PAR-2 is also expressed on other disease-mediating cells including eosinophils [60] and mast cells [61]. Thus, activation of PAR-2 by the allergen could lead to the initiation of disease and further activation of PAR-2 by neutrophil proteases, or proteases derived from a number of potential sources could lead to exacerbation or chronic disease. Thus, it will be important to clearly identify when to target PAR-2 activation therapeutically. The idea of therapeutically targeting allergen-derived protease activity to lessen the disease state has been recently reviewed [62]. In addition, a comprehensive study of PAR-2 with TLRs and NODs in the context of GC frass needs to be completed. It is known that GC contains both TLR4 and TLR2 [40], and it has recently been suggested that PAR-2 and TLR4 signal cooperatively [63•, 64]. This may open up novel areas of study and may lead to the development of new therapeutics for the treatment of allergic airway disease.
Abbreviations
- AHR:
-
airway hyperresponsiveness
- A1AT:
-
alpha 1 antitrypsin
- CE:
-
cockroach extract
- CR:
-
cockroach
- DC:
-
dendritic cell
- GC:
-
German cockroach
- mDC:
-
myeloid dendritic cell
- PAR:
-
protease activated receptor
- pDC:
-
plasmacytoid dendritic cell
- SLPI:
-
secretory leukocyte protease inhibitor
- TLR:
-
toll like receptor
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bernton H, Brown H. Insect allergy-Preliminary studies of the cockroach. J Allergy. 1964;35:506–13.
Arruda LK, Vailes LD, Ferriani VPL, et al. Cockroach allergens and asthma. Curr Rev Allergy Clin Immunol. 2001;107:419–28.
Crain EF, Walter M, O'Connor GT, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Env Health Persp. 2002;110:939–45.
Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen is causing morbidity among inner city children with asthma. N Eng J Med. 1997;336:1356–63.
Finn PW, Boudreau JO, He H, et al. Children at risk for asthma: home allergen levels, lymphocyte proliferation, and wheeze. J Allergy Clin Immunol. 2000;105:933–42.
Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33:35–42.
Hughes VS, Page K. German cockroach frass proteases cleave pro-matrix metalloproteinase-9. Exp Lung Res. 2007;33:135–50.
Pomes A, Chapman MD, Vailes LD, et al. Cockroach allergen Bla g 2; structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165:391–7.
Jeong KY, Kim C, Yong TS. Enzymatic activities of allergen extracts from three species of dust mites and cockroaches commonly found in Korean home. Korean J Parasitol. 2010;48:151–5.
Sudha VT, Arora N, Singh BP. Serine protease activity of Per a 10 augments allergen-induced airway inflammation in a mouse model. Eur J Clin Invest. 2009;39:507–16.
Day SB, Ledford JR, Zhou P, et al. German cockroach proteases and protease-activated receptor-2 regulated chemokine production and dendritic cell recruitment. J. Innate Immun. 2012;4:100–10.
Scarborough RM, Naughton MA, Teng W, et al. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem. 1992;267:13146–9.
Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–9.
Hollenberg MD. Protease-mediated signalling: new paradigms for cell regulation and drug development. Trends in Pharmacolog Sci. 1996;17:3–6.
Asokananthan N, Graham PT, Stewart DJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–8.
Colognato R, Slupsky JR, Jendrach M, et al. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–52.
D'Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranuation cascade. Biotech Histochem. 2000;75:85–90.
Howells GL, Macey MG, Chinni C, et al. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–7.
Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and ERK. J Allergy Clin Immunol. 2003;112:1112–8.
Hong JH, Lee SI, Kim KE, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004;113:315–9.
Day SB, Zhou P, Ledford JR, Page K. German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2. J Innate Immun. 2010;2:495–504.
Wada K, Matsuwaki Y, Yoon J, et al. Inflammatory responses of human eosinophils to cockroach are mediated through protease-dependent pathways. J Allergy Clin Immunol. 2010;126:169–72.
Knight DA, Lim S, Scaffidi AK, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803.
He S, Zhang Z, Zhang H, et al. Analysis of properties and proinflammatory functions of cockroach allergens Per a 1.01s. Scand J Immunol. 2011;74:288–95.
•• Lewkowich IP, Day SB, Ledford JR. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir Res. 2011;12:122. This report showed that the isolated protease from GC frass acted as an adjuvant to induce allergic airway inflammation in a murine model. It also confirmed upregulation of PAR-2 on mDC following allergen exposure and reported alterations in cytokine production and co-stimulatory molecule expression in PAR-2-deficient mDCs.
Kheradmand F, Kiss A, Xu J, et al. A protease-activated pathway underlying Th2 cell type activation and allergic lung disease. J Immunol. 2002;169:5904–11.
Page K, Lierl K, Herman N, Wills-Karp M. Differences in susceptibility to German cockroach frass and its associated proteases in induced allergic inflammation in mice. Respir Res. 2007;8:91.
•• Page K, Ledford JR, Zhou P, Wills-Karp M. Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respir Res. 2010;11:62. Two important findings were noted. First, this was the first report confirming that allergic airway inflammation was partially dependent on PAR-2 using a murine model. Second, this report showed that allergen-derived proteases were crucial for allergen sensitization only when delivered mucosally.
•• Arizmendi NG, Abel M, Mihara K, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186:3164–72. This manuscript shows the effective use of an anti-PAR-2 antibody in relieving allergic airway inflammation in mice.
Page K, Hughes VS, Odoms KK, et al. German cockroach proteases regulate IL-8 expression via NF-IL6 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2005;32:225–31.
Esashi E, Wang YH, Perng O, et al. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity. 2008;28:509–20.
Dommisch H, Chung WO, Rohani MG, et al. Protease-activated receptor 2 mediates human beta-defensin 2 and CC chemokine ligand 20 mRNA expression in response to proteases secreted by Porphyromonas gingivalis. Infect Immun. 2007;75:4326–33.
Wan H, Wilton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33.
Wan H, Winton HL, Soeller C, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–94.
Post S, Nawijn MC, Hackett TL, et al. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax. 2011; E pub ahead of print.
Herbert CA, King CM, Ring PC, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12:369–78.
Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol. 2002;110:589–95.
Jeong SK, Kim HJ, Youm JK, et. al. Mite and cockroach allergens activate protease activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Derm. 2008;128:1930–9.
Hachem JP, Houben E, Crumrine D, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–86.
Page K, Lierl KM, Hughes VS, et al. TLR2-mediated activation of neutrophils in response to German cockroach frass. J Immunol. 2008;180:6317–24.
Lutfi R, Ledford JR, Zhou P, et al. Dendritic cell-derived tumor necrosis factor alpha modifies airway epithelial cell responses. J Innate Immun. 2012; [EPub ahead of print].
• Soloff AC, Barratt-Boyes SM. Enemy at the gates: dendritic cells and immunity to mucosal pathogens. Cell Res. 2010;20:872–85. Excellent review of the role of DCs in their response to pathogens and the control at the mucosa.
Obregon C, Rothen-Rutishauser B, Gerber P, et al. Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Path. 2009;175:696–705.
Lee MF, Wang NM, Liu SW, et al. Induction of interleukin 8 by American cockroach allergens from human airway epithelial cells via extracellular signal regulatory kinase and jun N-terminal kinase but not p38 mitogen-activated protein kinase. Ann Allergy Asthma Immunol. 2010;105:234–40.
Jahnsen FL, Strickland DH, Thomas JA, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–7.
Jahnsen FL, Moloney ED, Hogan T, et al. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823–6.
Weckmann M, Collison A, Simpson JL, et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nature Med. 2007;13:1308–15.
Fields RC, Schoenecker JG, Hart JP, et al. Protease-activated receptor-2 signaling triggers dendritic cell development. Am J Path. 2003;162:1817–22.
• Gao P, Grigoryev DN, Rafaels NM, et al. CD14, a key candidate gene associated with a specific immune response to cockroach. Clin Exp Allergy. 2010;40:1353–64. This study reported cytokine expression levels from pDCs following allergen challenge and found a strong Th2 cytokine profile.
Ebeling C, Lam T, Gordon JR, et al. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007;179:2910–7.
van Rijt LS, Jung S, Kleinjan A, et al. In vivo depletion of lung CD11c + dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–91.
Lambrecht BN, Pauwels RA. Fazekas de St. Groth B: Induction of rapid T cell activation, division, and recirculation by intratracheal injection of dendritic cells in a TCR transgenic model. J Immunol. 2000;164:2937–46.
Lambrecht BN, Peleman RA, Bullock GR, Pauwels RA. Sensitization to inhaled antigen by intratracheal instillation of dendritic cells. Clin Exp Allergy. 2000;30:214–24.
de Heer HJ, Hammad H, Soullié T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98.
Forteza R, Botvinnikova Y, Ahmed A, et al. The interaction of alpha 1-proteinase inhibitor and tissue kallikrein in controlling allergic ovine airway hyperresponsiveness. Am J Respir Crit Care Med. 1996;154:36–42.
Blanco I, Lara B, de Serres F. Efficacy of alpha1-antitrypsin augmentation therapy in conditions other than pulmonary emphysema. Orphanet J Rare Dis. 2011;6:14.
Belkowski SM, Boot JD, Mascelli MA, et al. Cleaved secretory leucocyte protease inhibitor as a biomarker of chymase activity in allergic airway disease. Clin ExpAllergy. 2009;39:1179–86.
Marino R, Thuraisingam T, Camateros P, et al. Secretory leukocyte protease inhibitor plays an important role in the regulation of allergic asthma in mice. J Immunol. 2011;186:4433–42.
Wright CD, Havill AM, Middleton SC, et al. Secretory leukocyte protease inhibitor prevents allergen-induced pulmonary responses in animal models of asthma. J Pharmacol Exp Ther. 1999;289:1007–14.
Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–22.
Carvalho RF, Nilsson G, Harvima IT. Increased mast cell expression of PAR-2 in skin inflammatory diseases and release of IL-8 upon PAR-2 activation. Exp Derm. 2010;19:117–22.
Vliagoftis H, Forsythe P. Should we target allergen protease activity to decrease the burden of allergic airway inflammation? Inflamm Allergy Drug Targets. 2008;7:288–95.
• Nhu QM, Shirey K, Teijaro JR, et al. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. Very interesting report showing potential cooperation between PAR2 and TLR2, TLR3 or TLR4.
Rallabhandi P, Nhu QM, Toshchakov VY, et al. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction; a novel paradigm for receptor coopertivity. J Biol Chem. 2008;283:24314–25.
Acknowledgment
Dr. Page has received grant support from the National Institutes of Health (grant no. HL75568).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Page, K. Role of Cockroach Proteases in Allergic Disease. Curr Allergy Asthma Rep 12, 448–455 (2012). https://doi.org/10.1007/s11882-012-0276-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11882-012-0276-1