Abstract
Exposure to potentially toxic elements (PTEs) bound to PM2.5 can cause various health effects, including cardiovascular disease, allergies, and other related diseases. There have been several studies on the concentration of PTEs, including zinc (Zn), iron (Fe), and manganese (Mn) bound PM2.5 in the indoor air of urban schools. In this study, the concentration of Zn, Fe, and Mn in the indoor air of schools bound PM2.5 were meta-analyzed. PubMed and Scopus were used to retrieve papers related to the concentration of PTEs bound PM2.5 in the indoor air of urban schools from January 1, 2000 to March 10, 2020. The concentration of PTEs in PM2.5 was meta-analyzed based on the country subgroup in the random-effects model (REM). Thirty papers with 25 data reports were included in the study. The rank order of PTEs bound PM2.5 was Zn (17.32 ng/m3) > Fe (14.49 ng/m3) > Mn (7.40 ng/m3). The rank order of countries based on the concentration of Fe-bound PM2.5 in the indoor air of urban schools was China > Poland > Italy > Spain > Taiwan > Turkey > Iran) > Chile; Zn, Poland > Iran > Taiwan > Turkey > Spain > Italy > Chile; and for Mn, Poland > China > Iran > Taiwan > Spain > Italy > Chile. The pooled concentration of PTEs (Fe, Mn, and Zn) bound PM2.5 in the indoor air of urban schools in Poland and China was higher than in other countries, hence, therefore, it is recommended to carry out a PM2.5 concentration reduction program in the indoor air of schools in these countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical and microbial contamination of the environment, including water(Wang et al. 2022; Yang et al. 2021; Yu et al. 2022), food(Liu et al. 2022a; Sun et al. 2022; Wang et al. 2022), soil (Liu et al. 2022b), and air (Quan et al. 2022) can endanger human health. Air pollution in urban environments is one of the leading causes of health concerns, especially for sensitive people, especially children (Raysoni et al. 2017a). Ambient airborne particles are one of the main components of air pollutants that have significant adverse effects on human health (Chen et al. 2022b; Fang et al. 2018; Ghozikali et al. 2018; Liu et al. 2022c; Shang et al. 2021; Tian et al. 2022; Wang et al. 2022; Zhang et al. 2021). The presence of airborne particles in school classrooms is one of the primary pollutants affecting students’ health and indoor air quality (Di Gilio et al. 2017b). Various studies have shown that particulate matter of different sizes, such as PM10 and PM2.5, are associated with decreased lung function indices so that PM2.5 particles can penetrate deep into the lungs and lead to inflammation of the alveoli (Ghozikali et al. 2018).
Also, PTEs s are among the most harmful toxins in the environment, particularly airborne dust (Chen et al. 2022a; Gao et al. 2022; Huang et al. 2018; Wu et al. 2021; Yin et al. 2021; Zhang et al. 2021; Zoghi et al. 2022). The presence of trace metal elements in and on fine particles (PM2.5) is one of the main components that determine the toxicity of PM2.5 (Bi et al. 2018).
PM2.5 particles are produced by physical and mechanical processes and mainly originate from combustion (Ghozikali et al. 2018).In addition, these particles are produced from other sources such as traffic and various industries and natural resources such as dust storms (Hassanvand et al. 2015a). In general, indoor sources of PM2.5 emissions include cooking, combustion heat, and smoking, which are not present in school buildings. However, classroom indoor air pollution depends on various factors such as ventilation system, activities, number of occupants, concentration, and composition of outdoor PM2.5 (Di Gilio et al. 2017b). However, the sources of PM2.5 in the classroom may differ from elsewhere, and the metal compositions of PM2.5 may also be different (Bi et al. 2018). These trace elements may originate from various urban and industrial sources (Di Gilio et al. 2017b).
In general, particle exposure is widely associated with various cardiovascular, respiratory, and immunological health problems. These effects would be exacerbated by potentially toxic elements (PTEs) adsorbed on PM2.5 particles (Mesías Monsalve et al. 2018a). The trace metals zinc and manganese are carcinogenic and mutagenic (Raysoni et al. 2017a). These consequences are even more harmful to children’s health because their respiratory system is not yet fully developed (Di Gilio et al. 2017b).
Various toxicological studies have shown that metal components in or on PM2.5 particles produce reactive oxygen species through the Fenton reaction, which in turn causes damage to cell DNA, lipids, and proteins (Gali et al. 2015). Furthermore, numerous studies have shown that particle-bound metals are involved in causing oxidative stress and mitochondrial damage, leading to increased mortality and cardiovascular disease (Di Gilio et al. 2017b).
It is estimated that adults spend approximately 60 to 80% of their time indoors and children at least 50% of their time in school. Thus, a significant portion of exposure to air pollutants occurs in school environments (Viana et al. 2014b), and exposure to fine particles and trace elements in the classroom can lead to health threats for children (Bi et al. 2018). Therefore, understanding the composition and concentration of suspended particles and trace elements and their relationships is important for evaluating personal exposure (Hassanvand et al. 2015a).
Although there are several investigations on the concentration of PM2.5 and PTEs bound in PM2.5 in the indoor air of urban schools (Abdel-Salam 2019, Bi et al. 2018, Chithra and Nagendra 2014, Ekmekcioglu and Keskin 2007, Ghozikali et al. 2018, Halek et al. 2009, Mohammadyan et al. 2017, Mohammadyan et al. 2013), a meta-analysis has not been conducted. The main aim of the current study was to meta-analysis the concentration of Zn, Fe, and Mn bound PM2.5 in the indoor air of urban schools.
Material and method
Searching strategy
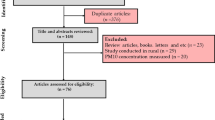
PRISMA guidelines were used to retrieve papers (Higgins and Green 2011) (Fig. 1). PubMed and Scopus were used to retrieve papers related to the concentration of PTEs bound PM2.5 in the indoor air of urban schools from January 1, 2000 to March 10, 2020. Keywords were “air pollution” OR “Particulate Matter” OR “PM2.5” OR “PM10” AND “toxic element” OR “trace element” OR “heavy metal” OR AND “residential” OR “school” OR “university.” Disagreement between two authors regarding excluding and select of papers was resolved by the final decision of the corresponding author(Aranega and Oliveira 2022, De Souza et al. 2021).
Inclusion and extraction of data
Our criteria measured Zn, Fe, Mn bound PM2.5 in indoor air; available full text; descriptive study; and presented mean and standard deviation PTEs in PM2.5 and investigation performed in school and urban areas. Extracted data from each paper included country, sample duration, mean and standard deviation concentration of heavy metal bound PM2.5, and measurement method.
Meta-analysis of data
The concentration of Zn, Fe, and Mn bound PM2.5 was meta-analyzed based on mean and standard deviation. Cochran’s Q test calculated the I2 index (heterogeneity statistic). As the I2 index was higher than 50%, heterogeneity was considered high (Higgins et al. 2008). Therefore, the random-effects model (REM) was used to estimate pooled effect size in the country subgroup. REM is a statistical model where the parameters of the model are random variables(Higgins et al. 2008). The concentration of PTEs bound to PM2.5 converted to ng/m3. Meta-analysis was conducted using Stata software (version 14; STATA Corp., College Station, TX).
Results
Five hundred and seventy articles were found in the initial search, and 505 were excluded due to duplicates in the databases. Then, in the screening step, 52 articles were excluded for reasons such as review articles, measurement of PM10, and measurement in rural areas. Finally, 13 articles with 25 data reports were included in the meta-analysis (Fig. 1 and Appendix 1). The “Results” revealed that the rank order of PTEs in bound PM2.5 based on overall concentration in the indoor air of urban school Zn (17.32 ng/m3) > Fe (14.49 ng/m3) > Mn (7.40 ng/m3). High concentrations of metals such as Zn and Fe in PM2.5 are mostly related to busy streets and high-traffic areas (areas that experience more than 20 vehicles from 2 or more axle vehicles). On high-traffic streets, vehicles are frequently stopped. Intermittent movement and stopping cause these metals to be released into the air from the exhaust and brake linings, the tires wear, and brake wear (Pastuszka et al. 2010). Also, many studies have stated that the reason for the high concentration of Zn compared to other metals in indoor air is the high use of,this metal in the following cases: as a coating to protect metals and wood from corrosion and UV rays; as a protector in paints to prevent mildew, the UV-protection of coatings, and zinc-rich coatings (Canha et al. 2014; Slezakova et al. 2011).
The rank order of countries based on concentration of Mn bound PM2.5 in indoor air of urban school was Poland (46.00 ng/m3) > China (26.56 ng/m3) > Iran (15.25 ng/m3) > Taiwan (6.49 ng/m3) > Spain (5.90 ng/m3) > Italy (4.93 ng/m3) > Chile (1.49 ng/m3) (Table 1).
Studies have shown that the release of Mn into the atmosphere depends more on natural resources (such as Earth’s crust erosion, excavation, volcanic activity, and mining). However, Ahmadi cited brake pads as the primary source of manganese emissions (Pastuszka et al. 2010, Rogula-Kozłowska et al. 2008). Similar to Pb emission, an abundance of Mn bound to PM2.5 was observed in March and April. Analysis of data in Chinese studies showed that two factors cause the presence of Mn on particulate matters in the atmosphere; one is the burning of coal as fuel in the cold seasons and the second is the re-suspension of dust on the dirt road and unpaved areas (Wang et al. 2020). Fang et al. (Fang et al. 2018) reported that the difference in the PM2.5 concentration and metals adsorbed on the PM2.5 surface in Taiwan is related to seasonal changes and weather conditions (temperature, humidity, wind speed). According to the findings, the PM2.5 concentration was highest in August and October, which coincided with school reopening. The concentration of Mn adsorbed on the PM2.5 was highest in August and September, attributed to the higher temperature in these months. The presence of low amounts of manganese-associated particles in the indoor air of Italian schools was attributed to its crustal origin (Cesari et al. 2012; Contini et al. 2014).
Soleimani et al. (Soleimani et al. 2018) reviewed the PTEs in the indoor air of Iran. They predicted that the Mn concentration associated with the particulate matter was higher in summer, fall, and winter. According to this research, high levels of manganese in the air were related to seasonal dust storms and strong monsoon winds. In contrast, Naderizadeh et al. (Naderizadeh et al. 2016) and Hassanvand et al. (Hassanvand et al. 2015b) reported sources of Mn emission in the air anthropogenic activities such as metal mining, industries that are metal-based, and vehicles. Numerous researchers in Chile have ascribed Mn emission in the atmosphere to erosion of the Earth’s surface, mining activities, and the distribution of their waste by wind (Castilla and Nealler 1978, Medina et al. 2005, Mesías Monsalve et al. 2018b, Neary and Garcia-Chevesich 2008, Ramirez et al. 2005). Steel, ferroalloy, and manganese manufacturing plants are the major industrial processes leading to high Mn loadings on particulate matter in the Spanish atmosphere (Arruti et al. 2010).
The rank order of countries based on concentration of Fe-bound PM2.5 in indoor air of urban school was China (1334.81 ng/m3) > Poland (567.80 ng/m3) > Italy (212.00 ng/m3) > Spain (200.00 ng/m3) > Taiwan (192.26 ng/m3) > Turkey (156.85 ng/m3) > Iran (102.20 ng/m3) > Chile (1.62 ng/m3) (Table 2).
Studies conducted in Poland have shown that the proximity of schools to crossroads and traffic sites can be the primary cause of high levels of Fe associated with fine particles (Pastuszka et al. 2010, Rogula-Kozłowska et al. 2008). Specific research in Italy has shown that iron-bound particles in indoor air are due to the activity of industries (especially steelmaking and metal smelting) near the city. These studies showed that when wind speeds are high in autumn and winter, wind blow lead to re-suspension and transfer of dust-containing metal to indoor air (Di Gilio et al. 2017a). It has been reported that the presence of Fe production industries and the re-suspension of dust and soil are responsible for the high level of this metal on PM2.5 in urban air. Hassan et al. (Hassanvand et al. 2015b) reported that since industries in Iran are located outside the city, the main reason for iron-on PM2.5 in indoor air is the re-suspension of dust from roads. Studies in Taiwan have reported that the average concentration of Fe-bound PM2.5 is highest in summer when temperatures are high (Fang et al. 2003, 2018, 2014). The proximity of schools to dirt roads, unpaved streets and playgrounds, and sweeping the street was reported by Amato et al. (Amato et al. 2014) as the leading cause of the release of Fe into the indoor air of Spanish schools. In research papers conducted in Spain, dust re-suspension through solid wind, moving vehicles, and playing with children were reported to cause iron release into the indoor air. Raysoni et al. (Raysoni et al. 2017b) attributed the Fe contents associated with PM2.5 emitted from non-anthropogenic sources such as erosion of the Earth’s crust, volcano eruption, and excavation to heavy traffic and metallurgical industries. In addition, Monsalve et al. (Mesías Monsalve et al. 2018b) and Amato et al. (Amato et al. 2014) revealed that Fe’s presence in PM2.5 in the air had been caused due to natural sources, urbanization activities, and natural crustal elements. They discussed that Fe emissions in the air were not associated with industrialization.
The rank order of countries based on concentration of Zn-bound PM2.5 in indoor air of urban school was Poland (267.00 ng/m3) > Iran (68.90 ng/m3) > Taiwan (57.29 ng/m3) > Turkey (48.54 ng/m3) > Spain (42.10 ng/m3) > Italy (15.50 ng/m3) > Chile (1.41 ng/m3) (Table 3).
Studies in Iran have shown that zinc emissions in the air are related to heavy traffic, brake wear, and vehicle tire wear. Also, because zinc is an additive in engine oil, this metal can be released into the environment (Hassanvand et al. 2015b; Norouzi et al. 2017; Soleimani et al. 2018). Studies in Taiwan have shown that the highest concentration of zinc-bound particles was associated with late summer, which was related to high temperature and the reopening of schools (Fang et al. 2003, 2018, 2012). Viana et al. (Viana et al. 2014a) and Querol et al. (Querol et al. 2004) attributed the release of Zn-bound particulate matter in indoor air in Spain to vehicle emissions wall painting, painting colors, and the presence of metal furniture in the classroom. Most studies in Poland have attributed identified heavy traffic that causes abrasion of car’s internal components (such as pad, brake, engine, and tires) as the leading cause of Zn-PM2.5 emission into the air (Pastuszka et al. 2010, Rogula-Kozłowska et al. 2008, Zwoździak et al. 2013). Studies reviewed in Chile reported that Zn could be released into the atmosphere due to smelting and mining activities, extraction of ores, natural oxidation of minerals, and Cu ore processes (Gidhagen et al. 2002, Jorquera and Barraza 2013, Mesías Monsalve et al. 2018b). Ekmekcioglu and Keskin (Ekmekcioglu and Keskin 2007) reported the Automotive Industry as a significant source of airborne Zn due to its use in many auto parts. Ergenekon and Ulutas (Ergenekon and Ulutaş 2014) have stated that emission of anthropogenic sources such as application in building materials, burning heavy oil, motor oil, generator activities, and power plants was associated with the Zn-PM2.5 emission in indoor dust. Contini’s study in Italy reported that Zn emissions were caused by sintering processes and heavy traffic (Contini et al. 2014).
Conclusion
In the current study, the concentration of Zn, Fe, and Mn in the indoor air of schools bound PM2.5 were meta-analyzed. The concentration of Zn bound to PM in schools’ indoor air was higher than in other PTEs. Therefore, it is recommended to carry out plans to identify zinc emission sources and reduce its emission. The pooled concentration of PTEs (Fe, Mn, and Zn) bound PM2.5 in the indoor air of urban schools in Poland and China was higher than in other countries. Hence, it is recommended to perform PM2.5 concentration control plans in schools’ indoor air, especially in these countries.
Data availability
Data openly available in a public repository.
References
Abdel-Salam MM (2019) Investigation of indoor air quality at urban schools in Qatar. Indoor Built Environ 28:278–288
Amato F, Rivas I, Viana M, Moreno T, Bouso L, Reche C, Àlvarez-Pedrerol M, Alastuey A, Sunyer J, Querol X (2014) Sources of indoor and outdoor PM2.5 concentrations in primary schools. Sci Total Environ 490:757–765
Aranega JP, Oliveira CA (2022) Occurrence of mycotoxins in pastures: a systematic review. Qual Assurance Safety Crops Foods 14:135–144
Arruti A, Fernández-Olmo I, Irabien A (2010) Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain). J Environ Monitor 12:1451–1458
Bi D, Qiu Y, Cheng H, Zhou Q, Liu X, Chen J, Cui X, Liu M, Zhu Z (2018) Seasonal characteristics of indoor and outdoor fine particles and their metallic compositions in Nanjing, China. Build Environ 137:118–126
Canha N, Almeida SM, Freitas MdC, Trancoso M, Sousa A, Mouro F, Wolterbeek HT (2014) Particulate matter analysis in indoor environments of urban and rural primary schools using passive sampling methodology. Atmos Environ 83:21–34
Castilla JC, Nealler E (1978) Marine environmental impact due to mining activities of El Salvador copper mine, Chile. Mar Pollut Bull 9:67–70
Cesari D, Contini D, Genga A, Siciliano M, Elefante C, Baglivi F, Daniele L (2012) Analysis of raw soils and their re-suspended PM10 fractions: characterisation of source profiles and enrichment factors. Appl Geochem 27:1238–1246
Chen F, Aqeel M, Maqsood MF, Khalid N, Irshad MK, Ibrahim M, Akhter N, Afzaal M, Ma J, Hashem M (2022a) Mitigation of lead toxicity in Vigna radiata genotypes by silver nanoparticles. Environ Pollut 308:119606
Chen Z, He X, Ge J, Fan G, Zhang L, Parvez AM, Wang G (2022b) Controllable fabrication of nanofibrillated cellulose supported HKUST-1 hierarchically porous membranes for highly efficient removal of formaldehyde in air. Ind Crops Prod 186:115269
Chithra V, Nagendra SS (2014) Seasonal trends of indoor particulate matter concentrations in a naturally ventilated school building. WIT Trans Ecol Environ 183:341–351
Contini D, Cesari D, Donateo A, Chirizzi D, Belosi F (2014): Characterization of PM10 and PM2.5 and their metals content in different typologies of sites in south-eastern Italy. Atmosphere 5
De Souza C, Khaneghah AM, Oliveira CAF (2021) The occurrence of aflatoxin M1 in industrial and traditional fermented milk: a systematic review study. Ital J Food Sci 33:12–23
Di Gilio A, Farella G, Marzocca A, Giua R, Assennato G, Tutino M, de Gennaro G (2017a) Indoor/outdoor air quality assessment at school near the steel plant in Taranto (Italy). Adv Meteorol 2017:1526209
Di Gilio A, Farella G, Marzocca A, Giua R, Assennato G, Tutino M, De Gennaro G (2017b): Indoor/outdoor air quality assessment at school near the steel plant in Taranto (Italy). Adv Meteorol 2017b
Ekmekcioglu D, Keskin SS (2007) Characterization of indoor air particulate matter in selected elementary schools in Istanbul, Turkey. Indoor Built Environ 16:169–176
Ergenekon P, Ulutaş K (2014) Heavy metal content of total suspended air particles in the heavily industrialized town of Gebze, Turkey. Bull Environ Contam Toxicol 92:90–95
Fang G-C, Chang C-N, Chu C-C, Wu Y-S, Fu PP-C, Yang IL, Chen M-H (2003) Characterization of particulate, metallic elements of TSP, PM2.5 and PM2.5-10 aerosols at a farm sampling site in Taiwan Taichung. Sci Total Environ 308:157–166
Fang G-C, Chiang H-C, Chen Y-C, Xiao Y-F, Zhuang Y-J (2014) Particulates and metallic elements monitoring at two sampling sites (Harbor, Airport) in Taiwan. Environ Forensics 15:296–305
Fang G-C, Chen Y-C, Lo C-T, Cho M-H, Zhuang Y-J, Tsai K-H, Huang C-Y, Xiao Y-F (2018) Concentrations and analysis of health risks of ambient air metallic elements at Longjing site in central Taiwan. Environ Geochem Health 40:461–472
Fang GC, Huang YL, Huang JH, Liu CK (2012) Dry deposition of Mn, Zn, Cr, Cu and Pb in particles of sizes of 3 μm, 5.6 μm and 10 μm in central Taiwan. J Hazard Mater 203–204:158–168
Gali NK, Yang F, Jiang SY, Chan KL, Sun L, Ho K-f, Ning Z (2015) Spatial and seasonal heterogeneity of atmospheric particles induced reactive oxygen species in urban areas and the role of water-soluble metals. Environ Pollut 198:86–96
Gao L, Huang X, Wang P, Chen Z, Hao Q, Bai S, Tang S, Li C, Qin D (2022) Concentrations and health risk assessment of 24 residual heavy metals in Chinese mitten crab (Eriocheir sinensis). Qual Assurance Safety Crops Foods 14:82–91
Ghozikali MG, Ansarin K, Naddafi K, Nodehi RN, Yaghmaeian K, Hassanvand MS, Kashani H, Jaafari J, Atafar Z, Faraji M (2018) Short-term effects of particle size fractions on lung function of late adolescents. Environ Sci Pollut Res 25:21822–21832
Gidhagen L, Kahelin H, Schmidt-Thomé P, Johansson C (2002) Anthropogenic and natural levels of arsenic in PM10 in Central and Northern Chile. Atmos Environ 36:3803–3817
Halek F, Kavousi A, Hassani F (2009) Evaluation of indoor-outdoor particle size distribution in Tehran’s elementary schools. World Acad of Sci Eng and Tech 57:463–466
Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, Gholampour A, Arhami M, Kashani H, Zare A (2015a) Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci Total Environ 527:100–110
Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, Gholampour A, Arhami M, Kashani H, Zare A, Niazi S, Rastkari N, Nazmara S, Ghani M, Yunesian M (2015b) Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci Total Environ 527–528:100–110
Higgins J, White IR, Anzures-Cabrera J (2008) Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med 27:6072–6092
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions. Wiley
Huang B-F, Chang Y-C, Han A-L, Hsu H-T (2018) Metal composition of ambient PM2.5 influences the pulmonary function of schoolchildren: a case study of school located nearby of an electric arc furnace factory. Toxicol Ind Health 34:253–261
Jorquera H, Barraza F (2013) Source apportionment of PM10 and PM2.5 in a desert region in northern Chile. Sci Total Environ 444:327–335
Liu G, Nie R, Liu Y, Mehmood A (2022a) Combined antimicrobial effect of bacteriocins with other hurdles of physicochemic and microbiome to prolong shelf life of food: a review. Sci Total Environ 154058
Liu J, Chen Y, Wang X (2022b) Factors driving waste sorting in construction projects in China. J Clean Prod 336:130397
Liu Y, Tian J, Zheng W, Yin L (2022c) Spatial and temporal distribution characteristics of haze and pollution particles in China based on spatial statistics. Urban Climate 41:101031
Medina M, Andrade S, Faugeron S, Lagos N, Mella D, Correa JA (2005) Biodiversity of rocky intertidal benthic communities associated with copper mine tailing discharges in northern Chile. Mar Pollut Bull 50:396–409
Mesías Monsalve S, Martínez L, Yohannessen Vásquez K, Alvarado Orellana S, Klarián Vergara J, Martín Mateo M, Costilla Salazar R, Fuentes Alburquenque M, Cáceres Lillo DD (2018a) Trace element contents in fine particulate matter (PM2.5) in urban school microenvironments near a contaminated beach with mine tailings, Chañaral. Chile Environ Geochem Health 40:1077–1091
Mesías Monsalve S, Martínez L, Yohannessen Vásquez K, Alvarado Orellana S, Klarián Vergara J, Martín Mateo M, Costilla Salazar R, Fuentes Alburquenque M, Cáceres Lillo DD (2018b) Trace element contents in fine particulate matter (PM(2.5)) in urban school microenvironments near a contaminated beach with mine tailings, Chañaral. Chile Environ Geochem Health 40:1077–1091
Mohammadyan M, Alizadeh Larimi A, Etemadinejad S, Yosefinejad R (2013) Respirable particle concentrations in primary schools’ classrooms in Sari. J Mazandaran Univ Med Sci 23:67–75
Mohammadyan M, Alizadeh-Larimi A, Etemadinejad S, Latif MT, Heibati B, Yetilmezsoy K, Abdul-Wahab SA, Dadvand P (2017) Particulate air pollution at schools: indoor-outdoor relationship and determinants of indoor concentrations. Aerosol Air Qual Res 17:857–864
Naderizadeh Z, Khademi H, Ayoubi S (2016) Biomonitoring of atmospheric heavy metals pollution using dust deposited on date palm leaves in southwestern Iran. Atmósfera 29:141–155
Neary DG, Garcia-Chevesich P (2008) Hydrology and erosion impacts of mining derived coastal sand dunes, Chañaral Bay. Arizona-Nevada Academy of Science, Chile
Norouzi S, Khademi H, Ayoubi S, Cano AF, Acosta JA (2017) Seasonal and spatial variations in dust deposition rate and concentrations of dust-borne heavy metals, a case study from Isfahan, central Iran. Atmos Pollut Res 8:686–699
Pastuszka JS, Rogula-Kozłowska W, Zajusz-Zubek E (2010) Characterization of PM10 and PM2.5 and associated heavy metals at the crossroads and urban background site in Zabrze, Upper Silesia, Poland, during the smog episodes. Environ Monit Assess 168:613–627
Quan Q, Liang W, Yan D, Lei J (2022) Influences of joint action of natural and social factors on atmospheric process of hydrological cycle in Inner Mongolia. China Urban Climate 41:101043
Querol X, Alastuey A, Rodríguez S, Viana MM, Artíñano B, Salvador P, Mantilla E, García do Santos S, Fernandez Patier R, de Rosa J, Sanchez de la Campa A, Menéndez M, Gil JJ (2004) Levels of particulate matter in rural, urban and industrial sites in Spain. Sci Total Environ 334–335:359–76
Ramirez M, Massolo S, Frache R, Correa JA (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine. Chile Mar Pollut Bull 50:62–72
Raysoni AU, Armijos RX, Weigel MM, Echanique P, Racines M, Pingitore NE, Li W-W (2017) Evaluation of sources and patterns of elemental composition of PM2.5 at three low-income neighborhood schools and residences in Quito, Ecuador. Int J Environ Res Public Health 14:674
Raysoni AU, Armijos RX, Weigel MM, Echanique P, Racines M, Pingitore NE, Li WW (2017b) Evaluation of sources and patterns of elemental composition of PM(2.5) at Three low-income neighborhood schools and residences in Quito, Ecuador. Int J Environ Res Public Health 14
Rogula-Kozłowska W, Pastuszka JS, Talik E (2008) Influence of Vehicular traffic on concentration and particle surface composition of PM10 and PM2.5 in Zabrze. Poland Pol J Environ Stud 17:539–548
Shang K, Chen Z, Liu Z, Song L, Zheng W, Yang B, Liu S, Yin L (2021) Haze prediction model using deep recurrent neural network. Atmosphere 12:1625
Slezakova K, Pires JCM, Martins FG, Pereira MC, Alvim-Ferraz MC (2011) Identification of tobacco smoke components in indoor breathable particles by SEM–EDS. Atmos Environ 45:863–872
Soleimani M, Amini N, Sadeghian B, Wang D, Fang L (2018) Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City Iran. J Environ Sci 72:166–175
Sun Y, Li J, Zhu L, Jiang L (2022) Cooperation and competition between CRISPR-and omics-based technologies in foodborne pathogens detection: a state of the art review. Curr Opin Food Sci 44:100813
Tian J, Liu Y, Zheng W, Yin L (2022) Smog prediction based on the deep belief-BP neural network model (DBN-BP). Urban Climate 41:101078
Viana M, Rivas I, Querol X, Alastuey A, Sunyer J, Álvarez-Pedrerol M, Bouso L, Sioutas C (2014) Indoor/outdoor relationships and mass closure of quasi-ultrafine, accumulation and coarse particles in Barcelona schools. Atmos Chem Phys 14:4459–4472
Wang K, Wang W, Li L, Li J, Wei L, Chi W, Hong L, Zhao Q, Jiang J (2020) Seasonal concentration distribution of PM1.0 and PM2.5 and a risk assessment of bound trace metals in Harbin, China: effect of the species distribution of heavy metals and heat supply. Sci. Rep. 10:8160
Wang Y, Wu X, Liu J, Zhai Z, Yang Z, Xia J, Deng S, Qu X, Zhang H, Wu D (2022) Mo-modified band structure and enhanced photocatalytic properties of tin oxide quantum dots for visible-light driven degradation of antibiotic contaminants. J Environ Chem Eng 10:107091
Wu X, Liu Z, Yin L, Zheng W, Song L, Tian J, Yang B, Liu S (2021) A haze prediction model in chengdu based on LSTM. Atmosphere 12:1479
Yang Y, Zhu H, Xu X, Bao L, Wang Y, Lin H, Zheng C (2021) Construction of a novel lanthanum carbonate-grafted ZSM-5 zeolite for effective highly selective phosphate removal from wastewater. Microporous Mesoporous Mater 324:111289
Yin L, Wang L, Huang W, Liu S, Yang B, Zheng W (2021) Spatiotemporal analysis of haze in Beijing based on the multi-convolution model. Atmosphere 12:1408
Yu C, Chen X, Li N, Zhang Y, Li S, Chen J, Yao L, Lin K, Lai Y, Deng X (2022) Ag3PO4-based photocatalysts and their application in organic-polluted wastewater treatment. Environ Sci Pollut Res 1–17
Zhang Z, Tian J, Huang W, Yin L, Zheng W, Liu S (2021) A haze prediction method based on one-dimensional convolutional neural network. Atmosphere 12:1327
Zoghi A, Salimi M, Mirmahdi RS, Massoud R, Khosravi-Darani K, Mohammadi R, Rouhi M, Tripathy AD (2022) Effect of pretreatments on bioremoval of metals and subsequent exposure to simulated gastrointestinal conditions. Qual Assurance Safety Crops Foods 14:145–155
Zwoździak A, Sówka I, Krupińska B, Zwoździak J, Nych A (2013) Infiltration or indoor sources as determinants of the elemental composition of particulate matter inside a school in Wrocław, Poland? Build Environ 66:173–180
Funding
Tobacco and Health Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran, is the financer of this project (IR.HUMS.REC.1399.415).
Author information
Authors and Affiliations
Contributions
Searching in databases was performed by Amenh Bahreini; data extraction by Amenh Bahreini; meta-analysis of data by Yadolah Fakhri and prepared manuscript by Trias Mahmudiono; Yadolah Fakhri, Zoha Hedari Nejad, Hasti Daraei, Amin Mousavi Khaneghah.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was approved from Tobacco and Health Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran (IR.HUMS.REC.1399.415).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fakhri, Y., Akhlaghi, M., Daraei, H. et al. The concentration of potentially toxic elements (zinc, iron, manganese) bound PM2.5 in the indoor air of urban schools: a global systematic review and meta-analysis. Air Qual Atmos Health 16, 77–84 (2023). https://doi.org/10.1007/s11869-022-01257-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-022-01257-1