Abstract
The objective of this study was to identify hazards that occur due to surgical practices and assess exposure to surgical smoke. We investigated nine surgical specialties in their corresponding operating rooms (ORs) for on-line measurements of pollutants and off-line determination of PAHs. Surgery for the face and dentistry generated the smallest particle size with a GMD of 23.3 nm. Also, the highest levels of the lung deposition surface area (5.8 ± 6.8 μm2/cm3), particulate matter of < 10 μm (PM10; 6.46 ± 5.34 μg/m3), PM2.5 (1.82 ± 1.01 μg/m3), and black carbon (0.10 ± 0.05 μg/m3) were seen with surgery of the face and dentistry. For gaseous pollutants, we observed that gastroenterology had the highest levels of CO2 (869 ± 112 ppm) and total volatile organic compounds (3.70 ± 1.00 ppm) compared to the other operating rooms. Levels of CO (3.40 ± 1.20 ppm) and formaldehyde (0.90 ± 0.51 ppm) were highest in the urology OR. Average total PAHs were mainly present in the gaseous phase with the highest concentrations of 746.6~1045.8 ng/m3 for gynecology. Our results showed that most pollutant levels were relatively low. However, gaseous PAHs emitted from surgical practices can reach levels that may pose important cancer risks in terms of occupational health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrosurgery, laser ablation, and ultrasonic scalpel dissection are commonly used for surgical incision and dissection. However, significant amounts of pollutant byproducts are generated (Bigony 2007). Surgeons are transiently exposed to surgical smoke with high peak concentrations of pollutants. Other surgically related health care staff, such as nurses and anesthetists, also experience continuous and chronic exposure to surgical smoke throughout the course of a routine workday. The Occupational Safety and Health Administration (OSHA) in the USA estimates that 500,000 US workers are exposed to surgical smoke annually (OSHA 2003), and many health care professionals are chronically exposed to surgical smoke. A study reported that the surgical smoke-related cancer risk for surgeons and anesthetists exceeded the US Environmental Protection Agency (EPA) acceptable level of 10−6 for inhalation (Tseng et al. 2014; USEPA 1992). However, a large cohort study indicated that long-term exposure to surgical smoke did not increase the risk of lung cancer (Gates et al. 2007), but it may increase the risk of chronic pulmonary conditions such as asthma and pneumonia (Gates et al. 2007).
Previous reports indicated that surgical smoke has mutagenic potential and can cause inflammatory responses (Tomita et al. 1981; Wenig et al. 1993). Surgical smoke mainly contains fine (< 2.5 μm) and ultrafine particles (< 0.1 μm) and gases. A previous study showed that surgical smoke consists of 95% water in the liquid phase or steam, whereas the remaining 5% contained organic vapors and cellular debris in the form of particulate matter (PM) (Ulmer 2008). Electrosurgery generates numerous chemical compounds including benzene, formaldehyde (HCHO), toluene, acrolein, and polycyclic aromatic hydrocarbons (PAHs) (Mowbray et al. 2013; Tseng et al. 2014). Given the composition of surgical smoke and its mutagenic and inflammatory potential suggested previously, more research is required to identify the hazards of surgical practice and exposure assessment.
Chronic exposure to surgical smoke can cause pulmonary disorders in humans. The medical staff, such as operating nurses, spend all of their time in the same OR during the entire working day (more than 8 h), who is the population-at-risk for pulmonary exposure of surgical smoke. Previous reports showed that standard surgical masks do not adequately prevent/reduce exposure to particulate surgical smoke (Chen and Willeke 1992; Weber et al. 1993). The US National Institute for Occupational Safety and Health (NIOSH) recommends the use of smoke evacuation and filtering systems to reduce exposure to surgical smoke (NIOSH 1998). Even though there are systems to evacuate surgical pollutants, the pollutants still get produced and thus pose a potential health effect that needs to be controlled. Also, the level of exposure to surgical smoke is dependent on multiple factors, including the surgical procedure and the positioning of the smoke evacuator (Smith et al. 1989; Smith et al. 1990). The causal association of surgical smoke with human health remaining unknown may be due to a lack of comprehensive exposure assessment and accurate hazard identification. The objective of this study was to quantify hazardous pollutants generated by different surgical specialties. A comprehensive investigation of particulate and gaseous pollutants was performed by environmental monitoring for an exposure assessment in operating rooms (ORs). Also, the cancer risk due to inhalation of PAHs was estimated.
Materials and methods
Experimental design
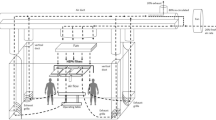
Characterization of surgical smoke was conducted from March to May 2016 at a hospital located in New Taipei City, Taiwan. Nine specialty ORs were investigated in the present study: outpatient surgery, otolaryngology, gynecology, orthopedics, face and dentistry, cardiac surgery, gastroenterology, neurosurgery, and urology. A nursing station served as a control. All of the environmental monitoring and exposure sampling were performed between 09:00 and 17:00 for 5 days (Monday to Friday). The ORs consisted of an anesthesia machine, electrocautery, and Mayo stand (Fig. 1). Environmental monitoring equipment was set up near the Mayo stand with an extended tube to the breathing area (approximately 1.4 m above ground level; Fig. 1). There were four HEPA-filtered ventilations (airflow outlet) in the OR. The HEPA-filtered clean air was positioned on the ceiling in the OR. The ventilation rate was controlled to 2100 m3/h in the ORs. All operating durations and procedures were recorded by OR nurses.
Environmental monitoring
To evaluate the environmental concentrations of particulate and gaseous pollutants emitted during surgical practices, continuous 8-h measurements were conducted per day in the ORs. All instruments were integrated in a single cart. Surgical pollutants were introduced through a unified stainless steel sampling tube, and the inlet was located at 1.4 m above the floor level. The particle size distribution (PSD), PM, and black carbon (BC) mass concentrations and lung-deposition surface area (LDSA) concentrations in the alveolar region were monitored for particulate pollutants (Chuang et al. 2018). Details of the particle monitors are provided in the Supplementary Information Table S1. A scanning mobility particle spectrometer (SMPS, TSI 3936, USA) was used to measure the particle number distribution in the size range of 13.6~736.5 nm. PM10 (PM of < 10 μm), and PM2.5 (< 2.5 μm) mass concentrations were determined by an optical dust monitor (Grimm 1.108, USA). BC mass concentrations were monitored with a portable Aethalometer (microAeth AE51, USA). An AeroTrak 9000 (TSI) was used to characterize the LDSA (in μm2/cm3). Particles introduced into the AeroTrak were charged, and the voltage of the ion trap was set to achieve the alveolar deposition fraction of total aerosols (Fissan et al. 2007; Leavey et al. 2013). For gaseous pollutants, total volatile organic compounds (TVOCs), HCHO, carbon dioxide (CO2), and carbon monoxide (CO) were monitored by an air quality monitor (SeeAiR, model M5–201, Chen-Wei VR International, Kaohsiung, Taiwan). The detection limit for TVOCs was 1 ppb, for HCHO was 10 ppb, for CO2 was 0.1 ppm, and for CO was 1 ppm. The sensitivity and accuracy for TVOCs were 1 ppb/6 s and ± 5%, for HCHO were 10 ppb/6 s and ± 10%, respectively. All particulate and gaseous monitors were calibrated before and after the measurements. The details for operating time in operating rooms of different specialties are provided in Supplementary Materials (Table S2).
Ventilation efficiency
To understand the ventilation efficiency for removing surgical smoke, levels of CO were used as a tracer gas to estimate the air change per hour (ACH) and the decay rate (h−1) as described in a previous report (Rydock 2004).
Exposure sampling
Two IOM samplers (SKC, USA) in parallel were used to collect 8-h particulate PAHs onto 25-mm Teflon substrates (Pall, USA) using an AirChek® XR5000 personal air sampling pump (SKC, Eighty-Four) at a flow rate of 2 L/min. Simultaneously, two XAD-2 sorbent tubes (SKC) in parallel were used to collect 8-h gaseous PAHs at a flow rate of 0.2 L/min.
Field emission-scanning electron microscopic and energy-dispersive X-ray microanalyses
The physicochemical features of PM collected during surgical practice were characterized by a portion of Teflon substrate. The preparation and analytical processes for field emission-scanning electron microscope (FE-SEM) (JEOL 2100, Japan) were previously reported (Chuang et al. 2011). FE-SEM was operated at an accelerating voltage of 15 kV. An elemental analysis was performed using an EDX Genesis Microanalysis System.
Particulate and gaseous PAHs
PAH extraction from Teflon substrates (n = 19) and XAD-2 sorbent tubes (n = 76) was conducted with methylene chloride followed by concentration, cleaning up, and re-concentration to 1 mL. Chromatography/mass spectrometry (GC/MS) (GCMS-QP2010; Shimadzu, Japan) was used to analyze PAHs according to a previous report (Yang et al. 2015). The 16 PAHs recommended by the USEPA were determined: naphthalene (Nap), acenaphthylene (AcPy), acenaphthene (Acp), fluorine (Flu), phenanthrene (Phen), anthracene (Ant), fluoranthene (FL), pyrene (Pyr), benzo(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3-cd)pyrene (INP), dibenzo(a,h)anthracene (DBA), and benzo(g,h,i)perylene (BghiP). Detection limits for particulate and gaseous PAHs were 0.028~0.115 and 5.54~22.6 ng/m3, respectively. Mean recovery yields for particulate and gaseous PAHs were 86.49 and 87.13%, respectively. Blank filters and XAD-2 were used to determine contaminants. The background level of blank filters and the blank front and back XAD-2 for all samples were subtracted from the PAH concentration.
Incremental lifetime cancer risk
The incremental lifetime cancer risk (ILCR) was performed in accordance of the report of risk assessment guideline of the US EPA (USEPA 2005), which has been commonly used for the risk assessment of PAH exposure (without unit) (Hu et al. 2007; Pooltawee et al. 2017). The ILCR is a probability that a person will develop cancer per 1000,000 people from inhalation exposure to a carcinogen over a lifetime. Toxicity equivalency quantity (TEQ), equivalent to benzo[a]pyrene toxicity, was calculated according to a previous report (Nisbet and LaGoy 1992). TEQ and ILCR were calculated based on the PAHs determined in the particulate and gaseous phases. The ILCR of PAHs (ILCRPAHs) to health care workers was calculated as follows:
where ILCRPAHs is the total TEQ (ng/m3), equivalent to benzo[a]pyrene toxicity, which was the sum of the individual PAH concentrations multiplied by their own toxic equivalency factors (TEFs) as in a previous report (Nisbet and LaGoy 1992). The TEFs for the 16 PAHs were Nap (0.001), AcPy (0.001), Acp (0.001), Flu (0.001), Phen (0.001), Ant (0.01), FL (0.001), Pyr (0.001), BaA (0.1), Chr (0.01), BbF (0.1), BkF (0.1), BaP (1), INP (0.1), DBA (1), and BghiP (0.01). The inhalation rate (IR) was 1.25 m3/h, the exposure time (ET) was 8 h/day, exposure frequency (EF) (days/year) was 260 days/year (5 days/week), the exposure duration (ED) was 30 years, body weight (BW) was 70 kg, the average exposure time (AT) was 25,567 days (70 years), and the value of the slope factor of BaP (SFBap) was 3.1 (mg/kg/day)−1 (Castro et al. 2011; Wei See et al. 2006; Yu et al. 2015).
Results
Characterization of surgical smoke
Levels of the 8-h particulate and gaseous pollutants in the nursing station (background) and the nine ORs are shown in Table 1. We observed that all particulate pollutants determined at the nursing station were the highest compared to the ORs; however, gaseous pollutants were lower than for most of the ORs. The averaging particle number size distributions for nursing station, outpatient surgery, orthopedics, and cardiac surgery are provided in Fig. S1. We observed that the OR for orthopedics had the highest particle number concentration of 2065 cm−3, whereas the OR for gastroenterology had the lowest particle number concentrations of 180 cm−3. All the geometric mean diameter (GMD) measured in the ORs as well as the nursing station were below 70 nm, particularly surgery for the face and dentistry generated the smallest particle size with a GMD of 23.3 nm. Notably, we observed that the LDSA, PM10, PM2.5, and BC were at the highest levels at the OR for face and dentistry, and were 5.8 μm2/cm3 and 6.46, 1.82, and 0.10 μg/m3, respectively. ORs for outpatient surgery had the highest BC/PM2.5 ratio of 0.22 compared to the other ORs. For gaseous pollutants, we observed that the OR for gastroenterology had the highest levels of CO2 (869 ppm) and TVOCs (3.7 ppm) compared to the other ORs. Levels of CO and HCHO were the highest in the OR for urology, and were 3.40 and 0.90 ppm, respectively.
Ventilation efficiency
Ventilation efficiencies of the ORs are shown in Table 2. Volumes of the nine ORs were between 74.25 and 117.30 m3 with the ventilation rate of 2100 m3/h. To understand the ventilation efficiency of the ORs, we firstly calculated the ACH values of the ORs, which were between 17.09 (orthopedics) and 28.28 (outpatient surgery). However, experimental CO decay rates in the ORs were inconsistent with the order of ACHs. CO decay rates observed in the ORs were 0.90 ± 0.26 h−1 for otolaryngology, 1.01 ± 0.39 h−1 for gynecology, 1.20 ± 0.43 h−1 for orthopedics, 0.54 ± 0.16 h−1 for face and dentistry, and 1.30 ± 0.34 h−1 for cardiac surgery.
Physicochemical characterization of PM
FE-SEM images for the PM collected from the ORs of the nine specialties are shown in Fig. 2. We observed that most of the filtered substrates were present but with small amounts of PM loading. Irregular PM was observed in otolaryngology and face and dentistry ORs, whereas fiber-like PM was observed in gynecology, orthopedics, and face and dentistry ORs. Different elements were detected in the PM collected from the nine operating rooms: Cr, Cd, and Pb for outpatient surgery; Mg, Ca, V, Ni, Cu, and Pb for otolaryngology; Cr, Mn, and Pb for gynecology; Cu, Cd, and Pb for orthopedics; Pb for face and dentistry; P, V, and Pb for cardiac surgery; Cr and Pb for gastroenterology; P, Cr, Co, Cu, Cd, and Pb for neurosurgery; and As and Pb for urology.
Gaseous and particulate PAHs
Figure 3 shows gaseous and particulate PAHs determined in the ORs of the nine specialties and the nursing station. The total of 16 gaseous PAHs at the nursing station was 659.7 ng/m3, but particulate PAHs were below the detection limit. The highest level of total gaseous PAH concentrations determined near the Mayo stand in the ORs was 1045.8 ng/m3 for gynecology, whereas the lowest PAH concentration was 368.2 ng/m3 for cardiac surgery. The highest concentration of total gaseous PAH concentrations determined near the anesthesia machine in the ORs was 746.6 ng/m3 for gynecology, whereas the lowest gaseous PAH concentration was 296.7 ng/m3 for cardiac surgery. For the particulate PAHs, we observe that the OR for gastroenterology had the highest level of 0.13 ng/m3 determined near the Mayo stand, whereas the OR for face and dentistry had the lowest concentration of 0.02 ng/m3. The OR for gynecology had the highest particulate PAHs (0.07 ng/m3) determined near the anesthesia machine, whereas the OR for otolaryngology had the lowest level of 0.02 ng/m3.
TEQ for PAHs
Figure 3 shows the TEQ for gaseous and particulate PAHs estimated in ORs of the nine specialties and the nursing station. The TEQ of the 16 gaseous PAHs for the nursing station was 0.660 ng/m3. The TEQ for the 16 gaseous PAHs determined near the Mayo stand ranged between 0.368 (cardiac surgery) and 1.046 ng/m3 (gynecology), whereas the TEQ for the 16 gaseous PAHs near the anesthesia machine ranged from 0.297 (cardiac surgery) to 0.747 ng/m3 (gynecology). The TEQ for the 16 particulate PAHs determined near the Mayo stand ranged from 0.00002 (face and dentistry) to 0.0001 ng/m3 (gastroenterology), whereas the TEQ for the 16 particulate PAHs near the anesthesia machine ranged from 0.00002 (otolaryngology) to 0.00007 ng/m3 (gynecology).
ILCR for PAHs
Figure 3 shows the ILCR for gaseous and particulate PAHs estimated in the ORs of the nine specialties and the nursing station. The ILCR of the 16 gaseous PAHs for the nursing station was 8.91 × 10−8. The ILCR for the 16 gaseous PAHs determined near the Mayo stand ranged between 4.97 × 10−8 (cardiac surgery) and 1.41 × 10−7 (gynecology), whereas the ILCR for the 16 gaseous PAHs near the anesthesia machine ranged from 4.01 × 10−8 (cardiac surgery) to 1.01 × 10−7 (gynecology). The ILCRs for the 16 particulate PAHs determined near the Mayo stand were 3.34 × 10−12 (face and dentistry) to 1.78 × 10−11 (gastroenterology), whereas the ILCRs for the 16 particulate PAHs near the anesthesia machine ranged from 3.34 × 10−8 (otolaryngology) to 9.59 × 10−8 (gynecology).
Discussion
ORs are micro-environments for indoor air quality in health care premises. Exposure levels to surgical smoke mainly depend upon the surgical process, ventilation efficiency, personal protective equipment (PPE), and working routines. In this study, particulate and gaseous pollutants of surgical smoke generated from nine specialties in ORs were characterized. The TEQ and ILCR were estimated based on the particulate and gaseous phases of PAHs. Three major findings are reported in the present study: (1) pollutant by-products emitted from surgical practice depend on the surgical specialty, (2) gaseous pollutants pose a more-significant risk than particulate pollutants, and (3) pulmonary exposure to gaseous PAHs has a higher cancer risk than the particulate phase.
Surgical smoke is produced by all electrosurgical tools in the operating room, such as monopolar, bipolar, and argon diathermy and other devices (Gallagher et al. 2011). To identify hazards of air pollutants generated in different surgical specialties in ORs, nine specialties were investigated in the present study: outpatient surgery, otolaryngology, gynecology, orthopedics, face and dentistry, cardiac surgery, gastroenterology, neurosurgery, and urology. For comparison between the general indoor air quality in the hospital and in ORs, a nursing station for the OR was used as a control. On-line environmental monitoring for particulate and gaseous pollutants and off-line exposure sampling for particulate and gaseous PAHs were conducted for 5 consecutive days in the ORs.
First, we characterized particulate and gaseous pollutants at the nursing station. We observed that particulate pollutants present at the nursing station were higher than in all of the ORs, whereas most of the gaseous pollutants were lower at the nursing station than in the ORs. These results suggest that the OR is a relative clean environment in terms of PM levels, but with significant gaseous pollution during surgery. Importantly, we observed that the emitted surgical pollutant by-products depend upon the surgical specialty. We observed that orthopedics surgery generated the highest concentrations of particles. The GMD of all the particles size distributions measured from the nine ORs ranged 23.3~60.0 nm, suggesting that the surgical PM is dominated by nano-sized fractions. This observation is consistent with previous reports (Eshleman et al. 2017; Ragde et al. 2016). The nano-sized PM emitted from surgical practice has high potential to be inhaled into the deeper lungs and alveolar region (Brook et al. 2010), leading to interaction with alveolar epithelial cells. To confirm this, we measured alveolar deposition of inhaled surgical PM (LDSA results). We observed that PM with the smallest size of 23.3 nm emitted from face and dentistry surgery had the highest concentrations that were able to deposit in the alveolar region of humans. Previous reports indicated that surface area concentrations of alveolar deposition range 30~70 μm2/cm3 in urban areas (Ntziachristos et al. 2007; Sabbagh-Kupelwieser et al. 2010). The LDSA measured in the OR for face and dentistry was 5.8 μm2/cm3 in the present study, which is relatively lower than urban levels. Also, levels of PM10, PM2.5, and BC were the highest for face and dentistry among the nine surgical specialties in the present study. These particle concentrations were relatively lower than previously reported occupational (Chuang et al. 2018) and hospital levels (Wang et al. 2006). However, the highest BC/PM2.5 ratio was measured with outpatient surgery. This result suggests that monopolar and/or bipolar diathermy could be the main electrosurgical tools used in this specialty, and the heating process is the main mechanism for generating surgical particles.
Similar to particle numbers and mass concentrations, FE-SEM images presented sparse PM collected onto filter substrates. Nano-sized PM was mainly observed in samples collected from ORs. The emission sources of surgical PM depend on the surgical procedures. Notably, this PM contained distinct metal elements dependent upon the surgical specialty with Pb consistently presented in all samples. Pb was been previously observed in surgical gloves (Mehra et al. 2011); however, the sources of Pb emitted into the ambient air of ORs remain unclear. A report observed that surgical PM with a size range of 0.3~0.5 μm may potentially penetrate through medical masks into human respiration (Tseng et al. 2014). Therefore, engineering control for reducing surgical PM is important for protecting human health. Further investigation is required to identify the metal elements from different emission sources by surgical procedures.
Similar to particulate pollutants, levels of gaseous pollutant emissions were dependent on the surgical specialty. We observed that the highest levels of CO2 and TVOC were generated during gastroenterological procedures, whereas measured levels of CO and HCHO were the highest in the urology OR. The 8-h mean levels of CO2 and CO measured in all ORs were below the California Division of Occupational Safety and Health Administration (CA/OSHA) permissible exposure limit-time weighted average (PEL-TWA) (OSHA 2018), which are 5000 ppm for CO2 and 25 ppm for CO. CO2 and CO levels were similar to those of a previous investigation conducted in a respiratory care ward at a hospital of Taiwan (Shen et al. 2014). Currently, there is no TVOC regulation for occupational settings. The mean TVOC levels determined in the complaint area of an university hospital in Thailand was 9.5 ppm (Ekpanyaskul 2010), which was higher than our results determined in the ORs. A previous study found that CO was mainly produced from urological endoscopic diathermy with a maximum level of HCHO of 0.0056 ppm (Weston et al. 2009). Notably, we observed that the 8-h mean HCHO in ORs for gastroenterology (0.80 ppm) and urology (0.90 ppm) exceeded the CA/OSHA PEL-TWA of 0.75 ppm (OSHA 2018). Together, hazards from gaseous pollutants should be controlled/removed during surgical practice to protect human health.
Efficient ventilation is important to remove pollutants released into occupational environments. We observed that the ACH in the nine ORs ranged 17.90~28.28 times, all of which are above the 15 times of ACH requirement for ORs reported by the US Centers for Disease Control and Prevention (CDC 2018). Notably, we used CO emitted from surgical practice as a tracer gas to estimate the decay rate in ORs. The reasons for employing CO to estimate ACH are twofold. One is that CO is a concern in laparoscopic procedures in which high concentrated CO could be trapped and concentrated in the peritoneal cavity. It is a recognized chemical of electrosurgical smoke, and generally co-emitted with soot/black carbon. The trend of CO concentration could reveal local ventilation process, while particulate pollutants cannot, majorly due to their inertial property. Therefore, the ACH estimated by CO could reflect the “local” flow field/ventilation condition. The inequity between the nominal ACH and estimated ACH suggests that localized high concentration of CO is the real exposure scenario for workers in OR. Second, CO is the only monitoring gas species which is suitable for estimating ACH. Results showed that the OR for face and dentistry had the lowest CO decay rate. These values are generally about one order of magnitude smaller than the calculated ACH, which also suggests that air in ORs was poorly mixed, and emitted air pollutions could accumulate longer than expected. The low decay rate may have been due to continuous CO emissions from surgical processes and the localized high concentration of CO measured in this study. In other words, the emitted CO was not instantaneously removed by ventilation. Also, the ventilation air flow field might not be as efficient as expected. Such phenomena could lead to the accumulation of surgical pollutants, as the highest levels of particulate pollutants (i.e., LDSA, PM10, PM2.5, and BC) were present in the OR for face and dentistry. Persistent pollutants in ORs could increase pulmonary hazards for health care professionals. Notably, the method of estimating the CO decay rate in the present study slightly differed from the standard method (Rydock 2004) of filling the experimental room with a tracer gas followed by turning the ventilation on. However, our observation represents real conditions in ORs. Our findings suggest that even when the ACH is higher than the CDC recommendation, emitted pollutants can still accumulate locally in ORs. Therefore, local exhaust ventilation may be an essential method to remove the surgical smoke as soon as it is generated.
Next, levels of 16 PAHs in gaseous and particulate phases detected in the operating rooms for the nine specialties were determined. In the present study, gaseous PAH concentrations were 368.2~1045.8 ng/m3 for measurements near the Mayo stand and 296.7~746.6 ng/m3 measurements near the anesthesia machine. A previous report showed that a diesel engine emitted 16.66 ng/m3 of nano-sized particles of 16 bulk PAHs (Chuang et al. 2012), which was significantly higher than the PAHs determined in ORs. A previous study indicated that surgeons are exposed to 487~2257 ng/m3 of total gaseous PAHs during breast surgery (Tseng et al. 2014). Additionally, an increased amount of bleeding was associated with higher PAH emissions during surgical practice (Naslund Andreasson et al. 2012). Our results further indicated that PAH emissions may depend on the surgical specialty. Therefore, the resultant cancer risk after chronic pulmonary exposure to surgically emitted PAHs may differ with surgical specialty. We estimated ILCRs for gaseous and particulate PAHs in the nine ORs based on the TEQ. None of the ILCRs for the nine specialties exceeded the USEPA level of 10−6 for inhalation (USEPA 1992). However, Tseng and colleagues indicated that ILCRs of gaseous and particulate PAHs ranged 131 × 10−6~992 × 10−6 during breast surgery (Tseng et al. 2014). The difference with our results may have been due to the sampling stage, surgical types, and environmental conditions. Although the cancer risk was relatively lower than the USEPA level, chronic exposure to PAHs by the lungs, eyes, and skin should be a concern in ORs, particularly to gaseous PAHs.
There are a few limitations of this study, which are listed here. We estimated the CO decay rates based on CO emissions from surgical processes, which is not the recommend standard method for investigating ventilation efficiency. But we can better understand the pollutant removal efficiency in the actual micro-environment during different surgical specialties in ORs. The settings/locations of different surgical equipment could affect pollutant removal/monitoring. To avoid the influence of surgical procedures during an entire week of monitoring, PAH sampling was set up near the anesthesia machine, which was used to estimate personal exposure levels. The exposure assessment might not truly represent exposure levels of health care staff. We investigated all surgical processes in the same surgery, but details of the surgical procedures were not discussed in the present study.
Conclusions
In conclusion, we provide results of a comprehensive investigation of gaseous and particulate pollutants in ORs generated from nine common surgical specialties. The OR usually supplies a relative clean HEPA-filtered air with a good ventilation purification system, which is able to reduce the effects of outdoor air pollution. However, we observed that gaseous pollutants including PAHs may pose a more significant hazard to OR health care staff compared to particulate pollutants. Also, hazardous by-products depend on the surgical specialty. Removal of gaseous pollutants should be considered a first priority for ORs. For example, a better ventilation design and local ventilation system are essential to improve the air quality of ORs. Higher levels of personal protective equipment are a second line of respiratory protection against particulate pollutants. Notably, accumulation of surgical pollutants emitted outside the ORs should be recognized due to the positive pressure design of ORs. Our findings suggest that surgical smoke, especially gaseous pollutants, generated from surgical practices should be further controlled in order to reduce risks of human health impacts on surgical health care staff.
References
Bigony L (2007) Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J 86:1013–1020
Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD, on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism (2010) Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121:2331–2378
Castro D, Slezakova K, Delerue-Matos C, Alvim-Ferraz MC, Morais S, Pereira MC (2011) Polycyclic aromatic hydrocarbons in gas and particulate phases of indoor environments influenced by tobacco smoke: Levels, phase distributions, and health risks. Atmos Environ 45:1799–1808
CDC (2018) Guidelines for Environmental Infection Control in Health-Care Facilities. https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html#tableb2
Chen CC, Willeke K (1992) Aerosol penetration through surgical masks. Am J Infect Control 20:177–184
Chuang HC, Jones TP, Lung SC, BéruBé KA (2011) Soot-driven reactive oxygen species formation from incense burning. Sci Total Environ 409:4781–4787
Chuang HC, Fan CW, Chen KY, Chang-Chien GP, Chan CC (2012) Vasoactive alteration and inflammation induced by polycyclic aromatic hydrocarbons and trace metals of vehicle exhaust particles. Toxicol Lett 214:131–136
Chuang HC, Su TY, Chuang KJ, Hsiao TC, Lin HL, Hsu YT, Pan CH, Lee KY, Ho SC, Lai CH (2018) Pulmonary exposure to metal fume particulate matter cause sleep disturbances in shipyard welders. Environ Pollut 232:523–532
Ekpanyaskul C (2010) Etiological investigation of unintentional solvent exposure among university hospital staffs. Indian J Occup Environ Med 14:100–103
Eshleman EJ, LeBlanc M, Rokoff LB, Xu Y, Hu R, Lee K, Chuang GS, Adamkiewicz G, Hart JE (2017) Occupational exposures and determinants of ultrafine particle concentrations during laser hair removal procedures. Environ Health 16:30
Fissan H, Neumann S, Trampe A, Pui DYH, Shin WG (2007) Rationale and principle of an instrument measuring lung deposited nanoparticle surface area. In: Maynard AD, Pui DYH (eds) Nanotechnology and Occupational Health. Springer Netherlands, Dordrecht, pp 53–59. https://doi.org/10.1007/978-1-4020-5859-2_6
Gallagher K, Dhinsa B, Miles J (2011) Electrosurgery. Surgery 29:70–72
Gates MA, Feskanich D, Speizer FE, Hankinson SE (2007) Operating room nursing and lung cancer risk in a cohort of female registered nurses. Scand J Work Environ Health 33:140–147
Hu Y, Bai Z, Zhang L, Wang X, Zhang L, Yu Q, Zhu T (2007) Health risk assessment for traffic policemen exposed to polycyclic aromatic hydrocarbons (PAHs) in Tianjin, China. Sci Total Environ 382:240–250
Leavey A, Fang J, Sahu M, Biswas P (2013) Comparison of Measured Particle Lung-Deposited Surface Area Concentrations by an Aerotrak 9000 Using Size Distribution Measurements for a Range of Combustion Aerosols. Aerosol Sci Technol 47:966–978
Mehra A, Deakin DE, Khan A, Sheehan TM, Nightingale P, Deshmukh SC (2011) Lead contamination of surgical gloves by contact with a lead hand. ISRN Orthop 2011:946370
Mowbray N, Ansell J, Warren N, Wall P, Torkington J (2013) Is surgical smoke harmful to theater staff? a systematic review. Surg Endosc 27:3100–3107
Naslund Andreasson S, Mahteme H, Sahlberg B, Anundi H (2012) Polycyclic aromatic hydrocarbons in electrocautery smoke during peritonectomy procedures. J Environ Public Health 2012:929053
NIOSH (1998) Control of smoke from laser/electric surgical procedures. http://www.cdc.gov/niosh/hc11.html
Nisbet IC, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
Ntziachristos L, Polidori A, Phuleria H, Geller MD, Sioutas C (2007) Application of a Diffusion Charger for the Measurement of Particle Surface Concentration in Different Environments. Aerosol Sci Technol 41:571–580
OSHA (2003) Safety and health topics: laser/electrosurgery plume. https://www.osha.gov/SLTC/laserelectrosurgeryplume/
OSHA (2018) Permissible Exposure Limits. https://www.osha.gov/dsg/annotated-pels/tablez-1.html
Pooltawee J, Pimpunchat B, Junyapoon S (2017) Size distribution, characterization and risk assessment of particle-bound polycyclic aromatic hydrocarbons during haze periods in Phayao Province, northern Thailand. Air Qual Atmos Health 10:1097–1112
Ragde SF, Jorgensen RB, Foreland S (2016) Characterisation of Exposure to Ultrafine Particles from Surgical Smoke by Use of a Fast Mobility Particle Sizer. Ann Occup Hyg 60:860–874
Rydock JP (2004) Tracer Study of Proximity and Recirculation Effects on Exposure Risk in an Airliner Cabin. Aviat Space Environ Med 75:168–171
Sabbagh-Kupelwieser N, Horvath H, Szymanski WW (2010) Urban Aerosol Studies of PMi Size Fraction with Reference to Ambient Conditions and Visibility. Aerosol Air Qual Res 10:425–432
Shen JH, Wang YS, Lin JP, Wu SH, Horng JJ (2014) Improving the indoor air quality of respiratory type of medical facility by zeolite filtering. J Air Waste Manag Assoc 64:13–18
Smith JP, Moss CE, Bryant CJ, Fleeger AK (1989) Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med 9:276–281
Smith JP, Topmiller JL, Shulman S (1990) Factors affecting emission collection by surgical smoke evacuators. Lasers Surg Med 10:224–233
Tomita Y, Mihashi S, Nagata K, Ueda S, Fujiki M, Hirano M, Hirohata T (1981) Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res 89:145–149
Tseng HS, Liu SP, Uang SN, Yang LR, Lee SC, Liu YJ, Chen DR (2014) Cancer risk of incremental exposure to polycyclic aromatic hydrocarbons in electrocautery smoke for mastectomy personnel. World J Surg Oncol 12:31
Ulmer BC (2008) The hazards of surgical smoke. AORN J 87:721–734 quiz 735-728
USEPA (1992) Guidelines for exposure assessment vol 57. Washington, DC URL: https://www.epa.gov/sites/production/files/2014-11/documents/guidelines_exp_assessment.pdf. Accessed July 2018
USEPA (2005) Guidelines for carcinogen risk assessment vol 70. Washington, DC URL: https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf. Accessed July 2018
Wang X, Bi X, Sheng G, Fu J (2006) Hospital indoor PM10/PM2.5 and associated trace elements in Guangzhou, China. Sci Total Environ 366:124–135
Weber A, Willeke K, Marchioni R, Myojo T, McKay R, Donnelly J, Liebhaber F (1993) Aerosol penetration and leakage characteristics of masks used in the health care industry. Am J Infect Control 21:167–173
Wei See S, Karthikeyan S, Balasubramanian R (2006) Health risk assessment of occupational exposure to particulate-phase polycyclic aromatic hydrocarbons associated with Chinese, Malay and Indian cooking. J Environ Monit 8:369–376
Wenig BL, Stenson KM, Wenig BM, Tracey D (1993) Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg Med 13:242–245
Weston R, Stephenson RN, Kutarski PW, Parr NJ (2009) Chemical composition of gases surgeons are exposed to during endoscopic urological resections. Urology 74:1152–1154
Yang TT, Lin ST, Lin TS, Chung HY (2015) Characterization of polycyclic aromatic hydrocarbon emissions in the particulate and gas phase from smoldering mosquito coils containing various atomic hydrogen/carbon ratios. Sci Total Environ 506-507:391–400
Yu K-P, Yang KR, Chen YC, Gong JY, Chen YP, Shih H-C, Candice Lung S-C (2015) Indoor air pollution from gas cooking in five Taiwanese families. Build Environ 93:258–266
Acknowledgements
The authors wish to thank Miss Yi-Syuan Lin and all the medical staffs in the operating room for technical assistance with this research.
Funding
This study was founded by the Institute of Labor, Occupational Safety and Health, Ministry of Labor, Taiwan (IOSH105-A304).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the concept and design of the study, drafting of the article, and critical revision of the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOC 198 kb)
Rights and permissions
About this article
Cite this article
Yang, TT., Chuang, KJ., Chang, NY. et al. Exposure assessment of particulate and gaseous pollutants emitted during surgery in operating rooms of different specialties. Air Qual Atmos Health 11, 937–947 (2018). https://doi.org/10.1007/s11869-018-0597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-018-0597-x