Abstract
Dew water samples were collected during the winter season (December, 2014 to January, 2015) at an outflow location from the Indo-Gangetic Plain (IGP) to the Bay of Bengal (Coastal Bhola, Bangladesh). Physical properties of the dew water, including pH and electrical conductivity (EC), were measured. The concentrations of water soluble ions (Cl−, SO4 2−, NO3 −, HCO3 −, Na+, K+, Ca2+, and Mg2+) and trace metals (Zn, Fe, Mn, Cu, Cr, Pd, and Ni) were also measured. Source characterization of the chemical species was done by correlation analysis, enrichment factor analysis, percent source contribution calculation, and air mass trajectory analysis. The average pH and EC of the collected dew water were 6.81 and 154.80 μS cm−1, respectively. The average concentration of SO4 2− and NO3 − was 264.10 and 222.20 μeq L−1, respectively. The concentrations of water soluble ions followed the sequence: Ca2+ > Cl−> SO4 2 > Na+ > NO3 −> Mg2+ > K+ > HCO3 −. The concentrations of trace metals ranged in order with Zn > Fe > Mn > Cu, while the concentrations of Cr, Pb, and Ni were below detection limit in dew water. Regression analysis showed significant correlations among sea, soil, and anthropogenic species. High enrichment factors of SO4 2−, NO3 −, Zn, Mn, and Cu indicates anthropogenic sources. Percent source contribution of different species showed significant anthropogenic contribution for Cl−(1.80%), SO4 2−(83.5%), and NO3 −(99.3%). Air mass trajectory analysis supported that the regional urban pollutions have significant influence on the dew water chemistry at the coastal Bhola, Bangladesh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Indo-Gangetic Plain (IGP) encompasses 21% of the Indian subcontinent and hosts over 1/8th of the world’s population. The rapidly expanding economy in the IGP results in a wide range of emissions, including those from fossil fuel and biomass burning, industries, transport, mining, urbanization, and agricultural activities. Winter in this region is generally characterized by high aerosol optical depth (AOD), formation of persistent fogs, regional haze, and dew formation (Nair et al. 2007). Aerosol particles in the IGP region include those transporting in from overland and over ocean and are aged with continental as well as pristine ocean aerosol particles (Niranjan et al. 2006; Eck et al. 2001). The high aerosol loads make a significant contribution to the dew water chemical composition. Importance of the dew water chemistry has been reported in recent years (Lekouch et al. 2010; Yadav and Kumar 2014; Wentworth et al. 2016). Dew formation depends on the structure as well as chemistry of the substrate surface where condensation occurs (Beysens 1995). Contamination in condensed dew water is occurred by dry deposition, wet deposition (large fog droplet), and dissolution of gases from the atmosphere. Dew water collection is challenging, with many techniques for dew water collection having been reported, e.g., on wooden block (Duvdevani 1947), glass plate (Janssen and Römer 1991; Kidron 1998), nylon mesh (Zangvil 1996), aluminum sheet (Baier 1966), polyethylene sheet (Wagner et al. 1992), and Teflon sheet (Takeuchi et al. 2002).
Chemical composition of the dew water is strongly affected by the atmospheric condition of the sampling locations, e.g., temperature, humidity, dry deposition (Beysens et al. 2006; Takeuchi et al. 2003). Dew water is usually weakly acidic, having a pH close to 7, but can be more acidic (5.41–6.00) in urban locations. Concentrations of calcium and ammonium were found to be higher in previous studies (Table 1). Aerosol particles in the atmosphere act as condensation nuclei in the formation of dew and fog, making wet deposition a major pathway transporting pollutants from the atmosphere to the biosphere (Polkowska et al. 2008; Rubio et al. 2012; Lynam et al. 2015). Therefore, the chemical composition of dew water provides insight into local pollution and has been undertaken in most regions, including USA, Europe, Japan, China, etc. (Beysens et al. 2009; Polkowska et al. 2008; Xu et al. 2015). Unfortunately, there is very limited information on chemical composition of dew water in IGP region (Lakhani et al. 2012; Singh et al. 2006; Yadav and Kumar, 2014), even though winter time air pollution seriously impacts dew water chemical composition in IGP region. Severe air pollution situation in Bangladesh has already been reported (Salam et al. 2003a, 2008). For coastal Bhola, an IGP outflow location, chemical characterization of the aerosol particles was done, and relatively high concentrations of some chemical species were observed (Salam et al. 2003b). We decided to undertake a study of the dew water composition at this IGP outflow location in coastal Bhola, Bangladesh, and assess the impact of the aerosol particles on the dew water.
We collected dew water samples from December 2014 to January 2015 at this outflow location from the IGP into the Bay of Bengal. The objectives was to characterize the physicochemical properties of the dew water, to identify pollutant sources through apportionment studies, and to evaluate the influence of long range transported air pollutants on the dew water composition at this remote island location.
Experimental
Sampling location
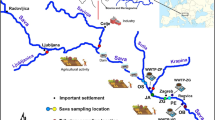
Dew samples were collected at Bhola (22°10′01″ N, 90°45′00″ E), Bangladesh, a rural delta island in the Bay of Bengal (Fig. 1). This location rests in the outflow path for pollutants from the IGP region to the Bay of Bengal (Fig. 1), making it an important monitoring location. Bhola has an area of 1441 km2 and a population primarily employed in agriculture and fishing. There are no major industries in Bhola Island, and no traffic was observed within 1 km of the sampling location. Biomass burning for cooking is the primary source of local pollution.
Meteorology of coastal Bhola, Bangladesh
The climate in Bangladesh is characterized by high temperatures, excessive humidity, and distinctly marked seasonal variations in precipitation. Bangladesh can be divided into four seasons: pre-monsoon (March–May), monsoon (June–September), post-monsoon (October–November), and winter (December–February). The average meteorological conditions in coastal Bhola during different seasons have been reported by Salam et al. (2003b). The average temperature varies between 4 and 25 °C in the winter and 24 to 36 °C in the summer, with an average of 25.9 °C, and daily high temperatures generally observed in the afternoon. The highest relative humidity occurs in July (99%) and the lowest in December (36%), with light precipitation occurring in March and April, and average annual precipitation of 230 mm. Our dew water samples were collected during winter season (December, 2014–January, 2015) in Bhola, Bangladesh. The temperature ranged from 9 to 17 °C, no rain events were recorded, and air mass back trajectories (NOAA/ARL 2005) indicated transport was mostly from north to southeast during sampling period.
Sampling procedure and analysis
Dew water samples were collected nightly in a stainless steel bowl (Fig. 2) from 20 December 2014 to 30 January 2015 at the IGP outflow location in Bhola Island, Bangladesh. Our first attempts used a collector made of a poly(ethylene) sheet, but the collection efficiency was not satisfactory. Therefore, we modified our system to collect dew water using a stainless steel container and during our sampling period were able to collect ten dew water samples having adequate volume (>30.0 mL) to analyze all for parameters. On the remaining days, weather conditions did not support enough dew formation in our collector. The stainless steel container was cleaned daily with de-ionized water prior to nightly deployment, and field blanks were collected to minimize errors in analysis. The collection system was installed at the top of a 9.0-m tower, built in 2013 in collaboration with NASA AERONET and ONR Global, USA, for aerosol and precipitation studies. As there is generally no dew in daytime, our sampling period was 9 to 10 h nightly. Dew condensed on the inclined surface of the bowl and collected at the bottom, from which the samples were removed and stored daily around 6:00 a.m. to preclude evaporation due to sunlight. The collected dew water samples were filtered using Whatman 41 filter paper. Then, the samples were split, physical properties and ions were measured from one part (e.g., pH, conductivity, anions, cations, etc.), while the other part was acidified with ultra-pure nitric acid (1% V/V) and stored separately for trace metal analysis. Samples were stored in the refrigerator with sealed container to avoid any chemical transformation. The field blanks were collected using a clean stainless steel collector having the same size as that used for the real sample. The collector was filled with 50 mL de-ionized water, covered with air tight lid, and then kept beside the real sampler during the whole sampling time.
Field blanks were analyzed using the same procedure used for the dew, and results were excluded from the sample to avoid contamination error in the analysis. The pH and electrical conductivity were measured by using a pH meter (pH 211, Hanna Instruments) and conductivity meter (CM-5S, DKK-TOA Corporation). Concentration of water soluble cations (from filtered dew water) and trace metals (filtered dew water preserved with nitric acid) were determined by flame atomic absorption spectroscopy (model: AAnalyst 800, Perkin Elmer). Concentrations of water-soluble anions were determined by ion chromatography (model: 881 compact IC pro, Metrohm). Samples were filtered with a 0.45-μm Millipore membrane filter to exclude any remaining ultrafine particles before analysis of metals and ions. Concentration of HCO3 − was calculated from pH with Eq. (1) used by Kulshrestha et al. 2003:
Results and discussion
Physical properties
The dew water samples were analyzed for pH and electrical conductivity (EC). While slightly turbid before filtration, indicating presence of solid particulate material, the filtered dew water samples were transparent and odorless, indicating absence of volatile odorous species. The average pH of the dew water was 6.81 ± 0.3, nearly neutral. The acidity of dew water depends on dissolution of different gases (CO2, SOx, and NOx, etc.) and acidic aerosol (Beysens et al. 2006; Takeuchi et al. 2003), so the pH of the dew water is determined by the balance between acidic and neutralizing components, such as ammonium, bicarbonate, basic aerosol, etc. (Takeuchi et al. 2003). As a benchmark, rainwater pH is on the order of 5.6, owing to the formation of carbonic acid from CO2. The average EC of the collected dew water was 155 ± 62 μScm−1; corresponding to the total mineralization 119 ± 48 mg L−1 (≈0.77*EC). In Table 1, we compare our measured pH and EC to global dew water values. Our pH was comparable to that in Delhi and Agra (India) and rural Indiana (USA) but lower than in the other sites, except Bordeaux (France).
Chemical composition
The concentration of major ions in our dew water samples was measured and is presented in Table 1. The ionic concentrations of the measured cations and anions in our dew water samples reflect Ca2+ > Cl− > SO4 2− > Na+ > NO3 − > Mg2+ > K+ > HCO3 −. Concentration of measured total cations (1.3 × 103 μeq L−1) was 1.5 times higher than of total anions (0.84 × 103 μeq L−1). However, as we were unable to measure some ionic species, ion balance was not achieved in our data. From our measured ionic concentrations and the literature, in Table 1, we see that in Bhola, concentrations of NO3 − and K+ were higher than those in other locations, except for Negev, Israel. It is important to note here that most of the studies were conducted at urban sites. So, higher concentration of the anthropogenic species in dew water of Bhola is quite remarkable. In Bhola, K+ was 2–3 times higher than in Agra (India), Changchun (China), and Zadar (Croatia), while NO3 − was 1.5–2.5 times higher than in India (Delhi, Agra), Changchun (China), Yokohama (Japan), and Santiago (Chile) and much higher (5 to 27 times) than in all other locations. In Bhola, SO4 2− was 3–16 times higher than in Bordeaux (France), USA (Indiana, Arkansas), and Zadar (Croatia) but lower than in all other sites. Concentration of Na+ and Cl− were also higher in Bhola Island samples than in most other sites, except Zadar, Croatia (closer to the sea) and Negev, Israel (desert/semi desert region).
Trace metals, including Zn, Fe, Mn, Cu, Cr, Pb, and Ni were measured in the Bhola dew water samples. The concentration order (in μgL−1) of trace metals followed the sequence Zn (345.5 ± 154.5) > Fe(267.5 ± 320.1) > Mn(186.2 ± 96.5) > Cu(75.0 ± 21.4), with concentrations of Cr, Pb, and Ni below minimum detection limit. The instrumental detection limits for Cr, Pb, and Ni were 100.0, 200.0, and 60.0 μg L−1, respectively. In the aerosol samples previously collected at the coastal Bhola site (Salam et al. 2003b), Fe and Zn concentrations were also very high. Correlation among different chemical species in dew water is given in Table 2. Significant correlation was observed between soil tracers Ca2+ and Mg2+ (0.62) indicating their common crustal origin. Correlation between K+ and soil tracer Ca2+ (0.65) suggests that a significant fraction of the K+ is of crustal origin. Strong correlation was also found between Na+ and K+ (0.87), Na+ and Ca2+ (0.70), and Na+ and Mg2+(0.86), suggesting some fraction had marine origin. Cl− also showed significant correlation with Na+ (0.73) indicating probable sea salt.
Significant correlation between anionic species SO4 2− and NO3 − and their individual correlation (at p = 0.03) with biomass marker K+ indicates their anthropogenic sources. Correlation is also obtained between different anionic (SO4 2−, Cl−, NO3 −) and cationic species (K+, Ca2+, Mg2+) indicating their common sources or association of acidic and basic species after neutralization. Among trace metals, only Fe and Zn show strong correlation with each other. Mn shows strong correlation with SO4 2− and NO3 −. Among other trace metals, only Cu shows significant correlation with anionic species (Cl−). No significant correlation was obtained between trace metals and other metals (Na, K, Ca, Mg, etc.). Correlation analysis of trace metals suggests anthropogenic sources as they showed little correlation with soil and/or sea-derived species.
Fractional acidity and neutralization factor
Fractional acidity of the dew water was calculated using the following formula (Yadav and Kumar 2014):
FA is 0 for maximum and 1 for no neutralization situation.
The average calculated value of FA in this study was 0.003 (highest 0.01, lowest 0.002), which indicates effective neutralization of acidic components (NO3 −, SO4 2−, etc.) in the dew water by basic components (NH4 +, Ca2+, Mg2+, and K+). The contribution of basic components to the neutralization process can be determined by calculating the neutralization factor (NF), via the equation (Yadav and Kumar 2014):
where X is the species whose neutralization factor is to be calculated.
The order by scale (larger to smaller) of neutralization factors found in dew water of Bhola Island is NFCa(1.42) > NFMg(0.42) > NF K (0.28). Dew forms near the ground, and thus collects more neutralizing particles (Ca2+, Mg2+) from soil than does water that forms at higher elevations (rain, cloud, etc.) (Yadav and Kumar 2014). Unfortunately, while NH4 + is another important neutralization species in dew water, its concentration was not measured in the current study.
Enrichment factor of major ions and trace metals
Enrichment factors were calculated to assist in identifying the origin of elements/ions in dew water (Kulshrestha et al. 1996). The calculation compares elemental ratios between ions/elements collected in the sample to reference material ratios. In coastal areas like Bhola, Na is commonly taken as a reference element for seawater since it is assumed to be primarily marine contribution in coastal regions (Kulshrestha et al. 2003). Al and Ca are typical lithophilic elements normally used as reference elements for continental crust (Cao et al. 2009). EFsea water and EFcrust of major cations and anions were calculated by the following equations, using Na and Ca as reference materials:
where X is the concentration of the species of interest. (X/Na)sea is the ratio from seawater composition (Kidron and Starinsky 2012), and (X/Ca)crust is the ratio from crustal composition (Taylor 1964).
An EF value much less than or much higher than 1 is considered to be diluted or enriched respectively relative to the reference source (Safai et al. 2004). EFsea water and EFcrust values are listed in Fig. 3.
Cl− has EFsea water value of 1.02 but has an EFcrust 135.5, which clearly indicates its marine origin. And, although the Cl−/Na+ value of dew water (1.19) is slightly higher than of sea water (1.16), this merely suggests very small contributions from other sources of Cl−, such as K+. The average EFcrust and EFseawater value of K+ and Mg2+ suggested primarily crustal sources, with partial contribution from marine sources. The high EFcrust and EFseawater values of SO4 2− and NO3 − indicated that both were enriched in dew water compared to soil and seawater, suggesting that their primary sources was anthropogenic.
The EF value of trace metals with respect to the crustal material (e.g., Al, Fe) is often used to evaluate the degree of anthropogenic influence (Huang et al. 2013). Enrichment factors (EF) of trace metals were calculated in this study using Fe as reference material by the following equation (Tripathee et al. 2014):
where X is the concentration of the species of interest. The ratios of (X/Fe)crust are taken from Salam et al. (2003a).
Elements with an EF >10 are considered to be of mainly anthropogenic origin (Huang et al. 2013). Based on EF, the trace elements can be classified as non-enriched (EF = 1 to 10), moderately enriched (EF = 10 to 100), and highly enriched (EF > 100) (Al-Momani 2003). Enrichment factors of trace metals in dew water collected from Bhola (see Fig. 3) suggest that their source is mainly anthropogenic (EF > 10). Moreover, EF of Cu and Zn were greater than 100, indicating highly enriched conditions, suggesting severe contamination due to human activities (Duce et al. 1975).
Source contributions
Marine contribution
In order to determine marine contribution to the chemical species present in dew water, the sea salt fraction (SSF) and non-sea salt fraction (NSSF) have been calculated. Bhola is an island surrounded by the Bay of Bengal, so significant marine contribution is expected in dew water. The equations used for calculating SSF and NSSF are taken from (Kulshrestha et al. 1996)
where X is the concentration of the species of interest, and (X/Na)sea is the ratio from seawater composition (Kidron and Starinsky 2012).
Na is used as reference, as this is a coastal site, and nearly all the sodium is of marine origin. From the value of %SSF, significant marine contributions are found for Cl− (97.47%), SO4 2− (11.57%), K+ (40.53%), and Mg2+ (5.23%) (see Fig. 4). The higher %NSSF value of SO4 2− (88.43%), K+(59.47%), Mg2+(94.77%), Ca2+(99.23%), and NO3 − (99.99%) suggest that these mostly came from non-marine sources.
Crustal and anthropogenic contribution
In order to estimate the crustal contribution and anthropogenic contribution of different species present in dew water, crustal fraction (%CF) and anthropogenic fraction (%AF) were calculated using the following equations (Lu et al. 2011):
where X is the concentration of the species of interest. Here, Ca is used as reference element for continental crust, since it is a natural source for lithophilic elements and its composition in soil is stable (Cao et al. 2009).
Potassium occurs in the coarse particles of soil, but fine particles of potassium could result from biomass burning, industrial sources, or sea spray. Here, the primary source of NSSF K+ was considered crustal, as we were unable to distinguish between crustal sources and biomass/wood combustion in the present study (Cao et al. 2009; Yadav and Kumar 2014). The source contribution for different species is shown graphically in Fig. 4. The calculated value of %CF suggested small contribution of soil originated SO4 2−, Cl−, and NO3 − in dew water. The %NSSF of K+ and Mg2+ is considered as having pure crustal origin (Lu et al. 2011; Safai et al. 2004). Natural dust makes an important contribution to the ions present in dew water as the crustal sources of Ca2+, Mg2+, and K+ are dust aerosols, originating from soil, which are suspended in the lower layer of the atmosphere, and transported on our collection surface by wind/deposition (Xu et al. 2015). Ca2+ and Mg2+ could be derived from the dissolution of both primary and secondary minerals (CaCO3, CaCO3·MgCO3, and CaSO4·2H2O) commonly present as aerosol particles (Yadav and Rajamani 2006). From the values of (%AF) in Fig. 4, we see that while a small fraction of Cl− (1.8%) potentially comes from anthropogenic sources, SO4 2− and NO3 − have the highest anthropogenic contribution with %AF values of 83.5 and 99.3%, respectively.
Air mass trajectories analysis
Backward air mass trajectories were calculated for selected sampling days (for samples having the highest and lowest concentrations of SO4 2−, NO3 −, and trace metals) with the NOAA HYSPLIT model (hybrid single-particle lagrangian integrated trajectory model) (http://www.arl.noaa.gov/). The 48-h air mass trajectories were calculated at 500, 1500, 2500 meters AGL (above ground level). Figure 5a represents trajectories for the sample having the lowest concentrations of SO4 2−, NO3 −, and trace metals. That sample had 6.2 times lower concentration of (SO4 2− + NO3 −) than average and 5.8 times lower concentration of trace metals (sum of concentration of all trace metals) than average. From Fig. 5a, the calculated air masses at all levels were coming from the Bay of Bengal. On this sampling day, dew water was significantly influenced by clean air from over ocean, hence the observed very low concentrations of measured chemical species. The second case, having a lower than average concentration of (SO4 2−+NO3 −), is shown in Fig. 5b. Here, the air mass was coming from the IGP region to the sampling location carrying polluted air masses. The third case, Fig. 5c, represents sampling days when the concentration of (SO4 2− + NO3 −) was 1.25 times higher than average. Back trajectories for this case show that air masses came from the north (500 MAGL) and west directions (1500 and 2500 MAGL) on that sampling day. Figure 5d represents the fourth case: sampling days when concentration of (SO4 2− + NO3 −) was 1.63 times higher than average. Figure 5e represents our fifth case: the sampling day when the concentration of trace metals was highest in the dew water. The concentration of total trace metals in Fig. 5e was 2.02 times higher than the average total trace metal concentration. The air masses on that day came directly from north.
a Air mass trajectories when SO4 2−, NO3 −, and trace metals were lowest. b Air mass trajectories when SO4 2−+NO3 − was lower than the average value. c Air mass trajectories when SO4 2−+ NO3 − was higher than the average value. d Air mass trajectories when SO4 2− + NO3 − was highest. e Air mass trajectories when trace metals were highest
Bhola is a remote rural Island of the Bay of Bengal and does not host much industrial activity.
The most probable major sources of the anthropogenic species in dew water in Bhola are air masses coming from different parts of IGP, as seen through the trajectory analysis. Although, biomass burning for cooking and agriculture activities could be responsible for some anthropogenic contributions; if it is the main source, then dew water samples should have very low concentrations of SO4 2−, NO3 −,and trace metals. Along the back trajectory of the air masses (Fig. 5), there are some big cities in Bangladesh and India. Moreover, winter time post-harvest agriculture burning could also have significantly impacted aerosol as well as dew water composition over the IGP (Rajput et al. 2011). High anthropogenic contributions of SO4 2− and NO3 − in dew water (the “Crustal and anthropogenic contribution” section) is an indicator for emissions of SOx and NOx into the atmosphere from different sources upwind in IGP, e.g., coal combustion, biomass burning, industrial emission, and vehicular exhaust (Yadav and Kumar 2014; Rubio et al. 2002). Dissolution of precursor gases or their oxidized products is likely responsible for the very higher concentrations of NO3 − as well as SO4 2− in dew water in coastal Bhola. The sources of trace metals in dew water are likely similar contamination from aerosol particles from upwind sources. It appears that dew water in coastal Bhola during winter time is contaminated when exposed to long-range transported air masses from polluted IGP regions.
Conclusion
Dew water collected in coastal Bhola, Bangladesh, during winter time was almost neutral with an average pH of 6.81. Concentrations of NO3 −, K+, Na+, and Cl− in dew water were relatively higher in Bhola. Further analyses for enrichment factor, percent source contribution, and correlation analyses revealed that the elemental composition of dew water at coastal Bhola had substantial contributions from anthropogenic sources. Air mass trajectories analyses showed that regional urban pollution has a significant influence on the dew water chemical composition in this remote, rural, IGP outflow location.
References
Al-Momani IF (2003) Trace elements in atmospheric precipitation at northern Jordan measured by ICP-MS: acidity and possible sources. Atmos Environ 37:4507–4515
Baier W (1966) Studies on dew formation under semi-arid conditions. Agric Meteorol 3:103–112
Beysens D, Ohayon C, Muselli M, Clus O (2006) Chemical and biological characteristics of dew and rain water in an urban coastal area (Bordeaux, France). Atmos Environ 40:3710–3723
Beysens D, Lekouch I, Mileta M, Milimouk I, Muselli M (2009) Dew and rain water collection in South Croatia. Int J Chem Mol Nucl Mater Metall Eng 3:141–147
Beysens D (1995) The formation of dew. Atmos Res 39:215–237
Cao YZ, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94:462–469
Duce RA, Hoffman GL, Zoller WH (1975) Atmospheric trace metals at remote northern and southern hemisphere sites: pollution or natural? Science 187:339–342
Duvdevani S (1947) An optical method of dew estimation. Q J R Meteor Soc 73:282–296
Eck TF, Holben BN, Dubovik O, Smirnov A, Slutsker I, Lobert JM, Ramanathan V (2001) Column-integrated aerosol optical properties over the Maldives during the northeast monsoon for 1998–2000. J Geophys Res 106:28555–28566
Huang J, Kang S, Zhang Q, Guo J, Chen P, Zhang G, Tripathee L (2013) Atmospheric deposition of trace elements recorded in snow from the Mt. Nyainqentanglha region, southern Tibetan plateau. Chemosphere 92:871–881
Janssen LHJM, Römer FG (1991) The frequency and duration of dew occurrence over a year: model results compared with measurements. Tellus Ser B 43:408–419
Jiries A (2001) Chemical composition of dew in Amman, Jordan. Atmos Res 57:261–268
Kidron GJ (1998) A simple weighing method for dew and fog measurements. Weather 53:428–433
Kidron GJ, Starinsky A (2012) Chemical composition of dew and rain in an extreme desert (Negev): cobbles serve as sink for nutrients. J Hydrol 420–421:284–291
Kulshrestha UC, Sarkar AK, Srivastava SS, Parashar DC (1996) Investigation into atmospheric deposition through precipitation studies at New Delhi (India). Atmos Environ 30:4149–4154
Kulshrestha UC, Kulshrestha MJ, Sekar R, Sastry GSR, Vairamani M (2003) Chemical characteristics of rainwater at an urban site of south-central India. Atmos Environ 37:3019–3026
Lakhani A, Parmar RS, Prakash S (2012) Chemical composition of dew resulting from radiative cooling at a semi-arid site in Agra, India. Pure Appl Geophys 169:859–871
Lekouch I, Mileta M, Muselli M, Milimouk-Melnytchouk I, Šojat V, Kabbachi B, Beysens D (2010) Comparative chemical analysis of dew and rain water. Atmos Res 95:224–234
Lu X, Li LY, Li N, Yang G, Luo D, Chen J (2011) Chemical characteristics of spring rainwater of Xi’an city, NW China. Atmos Environ 45:5058–5063
Lynam MM, Dvonch JT, Hall NL, Morishita M, Barres JA (2015) Trace elements and major ions in atmospheric wet and dry deposition across central Illinois, USA. Air Qual Atmos Health 8:135–147
Nair VS, Moorthy KK, Alappattu DP, Kunhikrishnan PK, George S, Nair PR, Babu SS, Abish B, Satheesh SK, Tripathi SN, Niranjan K, Madhavan BL, Srikant V, Dutt CBS, Badarinath KVS, Reddy RR (2007) Wintertime aerosol characteristics over the Indo-Gangetic Plain (IGP): impacts of local boundary layer processes and long-range transport. J Geophys Res 112:205
Niranjan K, Sreekanth V, Madhavan BL, Krishna MK (2006) Wintertime aerosol characteristics at a north Indian site Kharagpur in the Indo-Gangetic plains located at the outflow region into Bay of Bengal. J Geophys Res 111:209
Okochi H, Kajimoto T, Arai Y, Igawa M (1996) Effect of acid deposition on urban dew chemistry in Yokohama, Japan. Bull Chem Soc Jpn 69:3355–3365
Polkowska Ż, Błaś M, Klimaszewska K, Sobik M, Małek S, Namieśnik J (2008) Chemical characterization of dew water collected in different geographic regions of Poland. Sensors 8:4006–4032
Rajput P, Sarin MM, Rengarajan R (2011) High precision GC-MS analysis of atmospheric polycyclic aromatic hydrocarbons (PAHs) and isomer ratios from biomass burning emissions. J Environ Prot 2:445–453
Rubio MA, Lissi E, Villena G (2002) Nitrite in rain and dew in Santiago city, Chile. Its possible impact on the early morning startof the photochemical smog. Atmos Environ 36:293–297
Rubio MA, Lissi E, Herrera N, Pérez V, Fuentes N (2012) Phenol and nitrophenols in the air and dew waters of Santiago de Chile. Chemosphere 86:1035–1039
Safai PD, Rao PSP, Momin GA, Ali K, Chate DM, Praveen PS (2004) Chemical composition of precipitation during 1984–2002 at Pune, India. Atmos Environ 38:1705–1714
Salam A, Bauer H, Kassin K, Ullah SM, Puxbaum H (2003a) Aerosol chemical characteristics of a mega-city in Southeast Asia (Dhaka–Bangladesh). Atmos Environ 37:2517–2528
Salam A, Bauer H, Kassin K, Ullah SM, Puxbaum H (2003b) Aerosol chemical characteristics of an island site in the Bay of Bengal (Bhola-Bangladesh). J Environ Monit 5:483–490
Salam A, Hossain T, Siddique MNA, Alam AMS (2008) Characteristics of atmospheric trace gases, particulate matter, and heavy metal pollution in Dhaka, Bangladesh. Air Qual Atmos Health 1:101–109
Singh SP, Khare P, Kumari KM, Srivastava SS (2006) Chemical characterization of dew at a regional representative site of north-central India. Atmos Res 80:239–249
Takeuchi M, Okochi H, Igawa M (2002) Controlling factors of weak acid base concentrations in urban dew water: comparison of dew chemistry with rain and fog chemistry. Bull Chem Soc Jpn 75:757–764
Takeuchi M, Okochi H, Igawa M (2003) Deposition of coarse soil particles and ambient gaseous components dominating dew water chemistry. J Geophys Res 108:4319
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochimica Cosmochimica Acta 28:1273–1285
Tripathee L, Kang S, Huang J, Sillanpää M, Sharma CM, Lüthi ZL, Guo J, Paudyal R (2014) Ionic composition of wet precipitation over the southern slope of central Himalayas, Nepal. Environ Sci Pollut Res 12:2677–2687
Wagner GH, Steele KF, Peden ME (1992) Dew and frost chemistry at a midcontinent site, United States. J Geophys Res 97:20591–20597
Wentworth GR, Murphy JG, Benedict K, Bangs EJ, Collett JL Jr (2016) The role of dew as a nighttime reservoir and morning source for atmospheric ammonia. Atmos Chem Phys Discuss 16:7435–7449
Xu Y, Zhu H, Tang J, Lin Y (2015) Chemical compositions of dew and scavenging of particles in Changchun, China. Adv Meteorol 2015 Article ID 104048
Yadav S, Rajamani V (2006) Air quality and trace metal chemistry of different size fractions of aerosols in N–NWIndia-implications for source diversity. Atmos Environ 40:698–712
Yadav S, Kumar P (2014) Pollutant scavenging in dew water collected from an urban environment and related implications. Air Qual Atmos Health 7:559–566
Zangvil A (1996) Six years of dew observations in the Negev Desert, Israel. J Arid Environ 32:361–371
Acknowledgements
The authors acknowledge the financial support from the Office of the Naval Research Global (ONRG), USA, for the construction of the Bhola Observatory. Thanks to the sampling crews. Thanks also to the Centre for Advanced Research in Sciences (CARS), Dhaka University, for helping with the chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shohel, M., Simol, H.A., Reid, E. et al. Dew water chemical composition and source characterization in the IGP outflow location (coastal Bhola, Bangladesh). Air Qual Atmos Health 10, 981–990 (2017). https://doi.org/10.1007/s11869-017-0487-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-017-0487-7