Abstract
The Indus River Basin (IRB) with an area of 139,202 km2 is the lifeline river basin of Pakistan. An intensive study was conducted in six subcatchments of the IRB with five in the Upper Indus Basin (UIB) and one of the Lower Indus Basin (LIB; between Tarbela Dam and Panjand), i.e., the Gilgit River Basin (UIB-I), Hunza River Basin (UIB-II), UIB-III, UIB-IV and UIB-V, and LIB. A total of 84 surface water samples were collected from main stream and tributaries from June to August, 2016. The pH, electric conductivity (EC), and total dissolved solids (TDS) were measured in situ, whereas major ions (Ca2+, Mg2+, K+, Na+, Cl−, SO42−, and NO3−) and Si were analyzed in the laboratory. The results exhibited alkaline pH (8.55 ± 0.20) with diverse TDS (114.69 ± 77.65 mg L−1) and ion concentrations that were characterized primarily by the Ca-Mg-HCO3 type in the whole basin. The average TDS in the UIB and LIB were 93.99 ± 39.73 and 181.67 ± 167.82 mg L−1, respectively, under the influence of the arid to semi-arid climatic conditions and relatively higher anthropogenic interference in LIB compared to the UIB. The order of dominant major cations was different in the UIB and LIB, reflecting the diverse nature of geological formation. Gibbs plot, mixing diagrams, and ionic ratios were used to identify the controlling mechanism of river geochemistry in the IRB as carbonate weathering in general with different degrees of silicate weathering and minor contribution by evaporite dissolution. In addition, principal component/factor analysis also indicated that the major sources of dissolved loads in the basin are carbonates followed by silicates. Significant influences of silicate minerals were observed in the LIB, and there was a large contribution of evaporites in the UIB-II, UIB-III, and LIB. The suitability assessment showed that the river water fits the WHO permissible limits for drinking purposes from the perspective of major ions, whereas for irrigation purposes, most of the samples exhibited excellent and good levels except for a couple of permissible and doubtful levels from the Sawan and Deratang tributaries in the LIB, which may deteriorate the quality of soil and degrade the water quality downstream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The water quantity and quality of the Himalaya are among the most important and sensitive issues worldwide from the view of human well-being and ecological integrity (Barnett et al. 2005; Vörösmarty et al. 2010). The bedrock in the Himalaya is subjected to high weathering rates under the extreme climate conditions (Ali and De Boer 2007). Despite the fact that only 4% of the global land surface has been secured by the Himalaya, 25% of total dissolve loads entering into the oceans has been generated from the region (Raymo and Ruddiman 1992). Recent investigations of the Himalayan regions that focus on hydrochemical characteristics have revealed that surface water quality has been deteriorating in the past few decades due to rise in temperature, variable precipitation, poor land use, urbanization, deforestation, and mineral resources exploitation (Sun et al. 2012; Wu et al. 2012). Notably, the rivers and their tributaries that pass through the cities in the developing countries of South Asia such as Pakistan, Nepal, India, and Bangladesh receive large amount of contaminants released from industrial, domestic/sewage, and agricultural effluents (Karn and Harada 2001; Kambole 2003; Pekey et al. 2004; Richardson et al. 2015).
Investigations of river basins provide unique insights into the hydrologic functions of the individual parts of the catchment, because the geochemistry of rivers is regulated by complex interactions among various physical, chemical, and biological environments (Brezonik and Arnold 2011; Stumm and Morgan 2012). The notable studies on source identification and controlling factor of rivers worldwide are underscored as those of the Amazon (Stallard and Edmond 1987), the Upper Yangtze (Chen et al. 2002), the Mississippi (Battaglin et al. 2001), the Yenisei (Kimstach et al. 1998), the Yellow (Chen et al. 2005), and the Ganga and Yamuna Rivers (Dalai et al. 2002) and have stated that the major drivers of hydrochemical characteristics are climate, lithology, hydrology, and anthropogenic interferences (Gibbs 1970; Meybeck 1987; Qadir et al. 2008).
A basin-wise study was performed on weathering processes in the Indus River Basin (IRB) of Pakistan with a focus on carbon, sulfur, oxygen, and strontium isotopes (Karim and Veizer 2000). Some of the major tributaries of the IRB were studied by Subramanian (1979) and Pande et al. (1994) at a provincial level from India to Karachi in Pakistan. These studies used elemental and isotopic data and proposed that carbonate weathering and evaporite dissolution were controlling factors. Jacobson and Blum (2000) used major ions and strontium isotopes to determine disseminated calcite in Himalayan silicate rocks in order to evaluate the consumption of CO2 from the atmosphere. A subsequent geochemical study was reported by Qadir et al. (2008) in the Chenab River in which the spatiotemporal variations of water quality were explored. Apart from these studies, the water quality parameters in the Jamshoro area of Pakistan was evaluated by Baig et al. (2009), who found that Na+, K+, and SO42− concentrations were elevated because of the semi-arid environment and anthropogenic interferences.
The headwater areas in the Third Pole have been considered as one of the most pristine regions in the world (Paudyal et al. 2016). However, some recent studies indicated that South Asian regions with heavy pollution could be the source of contaminations, which could be long range transported to the remote Himalayan regions and deposited via wet and dry deposition through atmospheric circulations in the regions (Tripathee et al. 2014, 2016b).
It is noted from previous literatures that changing climatic conditions promote accelerated hydrochemical dynamics. In addition to the climate and lithology, as well as land use conditions, the IRB is also characterized with partial anthropogenic signatures, particularly in the downstream segment. Systematic studies on geochemical variabilities and implication of natural and anthropogenic factors are imperative to elucidate and protect the water quality in the basin. However, there are very limited published data available, which represent a significant research gap in understanding the geochemistry of the river. Therefore, the objective of this study is to evaluate the spatial variation of hydrochemical attributes, their sources, and controlling factors in the IRB. Since the basin is the lifeline of Pakistan and fundamentally supports economic activities, the suitability of the river water for human consumptions and irrigation purposes was also assessed.
Materials and methods
Study area
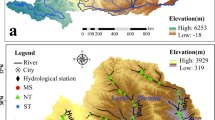
The Indus River originates from Lake Mansarovar in Tibetan Plateau, enters India, then flows through Pakistan, and finally empties into the Arabian Sea (Tahir et al. 2016). The IRB is located between latitudes 29° 30′ N and 37° 10′ N and between longitudes 70° 15′ E and 77° 0′ E, covering a total area of 139,202 km2 (with 8, 39, and 53% in China, India, and Pakistan, respectively). The area is confined between Kharmong and Panjnad, which is the confluence point of the Indus River with other rivers of Pakistan (Fig. 1). On the basis of climate, geology, land use, and topography, the IRB is classified into the Upper Indus Basin (UIB) (54%) and Lower Indus Basin (LIB) (46%), which are separated by the Tarbela Dam, situated at an elevation of 440 m (Ali and De Boer 2007) (Fig. 1). The LIB specified in this study is actually the mid-stream part of the whole IRB. The UIB lies between 440 and 8361 m asl, is mainly affected by westerlies, and is principally fed by glacier/snow melt (Liniger et al. 1998; Hewitt 2005). In contrast, the LIB lies between 70 and 440 m asl and is mostly characterized by an arid to semi-arid climate (Fig. A1), in which flow is primarily controlled by discharge from upstream, tributaries, and monsoon precipitation. The mean annual precipitation and air temperature range between 200 and 1400 mm and between 5 and 20 °C, respectively, in the UIB and between 200 and 1200 mm and between 10 and 32 °C, respectively, in the LIB, showing distinct differences between upper and lower segments (Baloch et al. 2006; Liu et al. 2012; Nadeem Faisal 2015; Lutz et al. 2016).
The UIB extends over parts of the Hindukush, Karakoram, and Western Himalayan ranges and is subjected to different microclimate regimes (Young and Hewitt 1990; Dimri and Chevuturi 2014). Within the UIB, the Gilgit (UIB-I) and Hunza (UIB-II) River basins are considered separately for detailed study because they are the largest subcatchments of the IRB. UIB-I originates in the Hindukush region, where the flow mostly depends upon rainfall (westerlies) and melt water (Hewitt 2005; Tahir et al. 2011). UIB-II originates near Khunjrab and then passes and flows through the center of the Karakoram ranges, where a substantial proportion of the flow is derived from glacial melt. The remainder of the UIB is further divided into three subcatchments in this study on the basis of their sources (UIB-III from glacial melt, UIB-IV from snowmelt, and UIB-V mostly from monsoonal precipitation). Maximum annual precipitation was observed at UIB-V and the northern part of LIB, whereas other parts of the investigation territory have low annual precipitation (Fig. A1). The land use of the IRB is classified into eight major classes, including water bodies, forests, shrublands, grasslands, agricultural areas, built-up area, snow/glaciers, and barren areas (Loveland et al. 2000). From north to south, the IRB is geologically split into five lithotectonic divisions (Fig. A2): (1) the Hindu Kush–Karakoram–Western Tibet Block (HKT), (2) the Kohistan–Ladakh Arcs (KLA), (3) the Higher Himalayas (HH), (4) the Lesser Himalayas and the Foothills (LHF), and (5) the Southern Sedimentary Basin (SSB) (Karim and Veizer 2000). Each division accommodates different rock formations, which contributes to the variety of dissolved loads in surface and groundwater. The details of the land use and geological formations of the IRB are explained in the supplementary file (Figs. A1 and A2).
Sampling and laboratory analysis
Water samples were collected from main stream and tributaries at 84 different sites (Fig. 1) throughout the IRB from June to August, 2016. Considering the diversity in climate, geology, land use, and anthropogenic activities, 17, 14, 15, 17, 10, and 11 water samples were collected from UIB-I, UIB-II, UIB-III, UIB-IV, UIB-V, and the LIB, respectively (Table A1), following the standard guideline values (APHA 2005; Duncan et al. 2007). For both the main stream and tributaries, three water samples were collected from each sampling site at depth of ≈ 30 cm beneath the surface (two from each bank and one from the center) and were thoroughly mixed. pH, electric conductivity (EC), total dissolved solids (TDS), and water temperature (Tem.) were measured on-site by using an Oakton PCSTestr 35. Samples were instantly filtered through a 0.45-μm polytetrafluoroethylene (PTFE) disk syringe filter and filled in high-density polyethylene (HDPE) bottles of 30 mL.
The concentrations of the major cations (Ca2+, Mg2+, Na+, K+) and Si were determined by inductively coupled plasma optical emission spectrometry (ICP-OES, Optima 5300 DV, PerkinElmer, Waltham, MA, USA) with a limit of detection of 0.01 mg L−1, and major anions (Cl−, NO3−, and SO4−) were determined using ion chromatography (ICS-2500, Thermo Fisher Scientific, Waltham, MA, USA) with LOD of 0.01 mg L−1 (Li et al. 2015; Tian et al. 2016) at the Institute of Tibetan Plateau Research, Chinese Academy of Sciences. Special care was taken during the sampling and laboratory analysis. All of the sample bottles were soaked overnight in 15% HCl and washed with deionized water before using in order to remove potential contaminants introduced during packing and shipping (APHA 2005). The sample bottles were sealed by using Parafilm and stored below 4 °C until laboratory analysis. Distilled deionized water was used to clean the equipment after every sample measurement on site. External standards for major cations and anions were prepared from the national standard solutions obtained from the Certified Reference Material Center, China (Tian et al. 2016).
For quality control and quality assurance, the standard operating procedures were strictly followed during sampling and laboratory analysis as directed by APHA (2005). In order to avoid contamination, powder-free nitrile exam gloves and mask was used during the sample collection and testing. At each sampling site, three water samples, i.e., at left bank, middle, and right bank of the river, were taken and mixed before a composite sample was prepared. The sample bottles were pre-rinsed three times with the same water before the final sample was acquired. Before the in situ measurements, the instruments were properly calibrated. Triplicate samples were run, and the average recovery of quality control analysis was 99 ± 4%, indicating the good quality of the data. In addition, four blank samples of deionized water filtered through 0.45-μm polytetrafluoroethylene (PTFE) disk syringe filter, filled in high-density polyethylene (HDPE) bottles, sealed with Parafilm were collected in the field and kept in the same environment with other water samples. The results of these field blank samples showed negligible contamination during the sampling, filtering, and storage processes, as the values of most hydrochemical variables were below the detection limit (Paudyal et al. 2016). The average analytical precision for both cations and anions was better than 2%. The alkalinity as HCO3− was estimated by charge balance (Li et al. 2016). To verify the accuracy, sums of major ions were calculated and compared to the TDS values (Σions = 1.06 TDS, r = 0.97), which illustrated highly acceptable data quality (Tripathee et al. 2014; Paudyal et al. 2016; Pant et al. 2018).
To interpret the data and develop a conclusive understanding of the geochemistry of the IRB, a series of statistical tests were performed using an IBM SPSS 22.0 (Xiao et al. 2012; Pant et al. 2018). These tests include a normality test, descriptive statistics (mean, max, min, SD etc.), Spearman correlation, and principal component analysis and factor analysis (PCA/FA) (Varol et al. 2013a). The normality test and correlation analysis were performed by considering all of the parameters to predict the degree of dependent of one variable on others with a correlation significance level of 0.01. PCA/FA was applied to group the changing patterns of major ions in order to explain the fluctuation in dataset with minimum loss of original information. PCA/FA is attained by analyzing the correlation matrix and transforming the original variables to uncorrelated ones, commonly called varifactors (VFs) (Helena et al. 2000). Additionally, the eigenvalues in PCA/FA define how much variance is present in associated VFs. The VF that holds the maximum eigenvalue is found to have the most covariability (Tripathee et al. 2016a). Suitability of the dataset for PCA/FA was tested by using the Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity methods which is run prior to PCA/FA. Furthermore, Gibbs, Piper, mixing diagrams, and ionic ratios were used for source identification and controlling factor analysis. The sodium ion percentage and sodium absorption ratio (SAR) were used to evaluate the irrigation water quality (Wilcox 1948; Richards 1954; Bauder et al. 2011; Thomas et al. 2014).
Results and discussion
General geochemical characterizations
The descriptive statistics of major geochemical variables of the IRB and comparison with other river basins in the surrounding region are summarized in Table 1. Arithmetic mean values, geometric mean values, and median values were used for normally distributed datasets, log-transformed normally distributed datasets, and non-normally distributed datasets, respectively (Wang et al. 2017).
The pH of the IRB was found to be alkaline in nature (8.57 ± 0.20) and higher than the global mean (i.e., 8), which revealed the potential impact of carbonate-dominated lithologies in the basin (Li and Zhang 2008; Zhang et al. 2009). The degree of mineralization process in the basin is indicated by EC and TDS, which exhibited substantial variations with high standard deviations (158.5 ± 105.24 μS cm−1 and 114.69 ± 77.64 mg L−1, respectively). The higher TDS values in the IRB than that of the global mean values for the large rivers (i.e., 120 mg L−1) could be attributed to lithoclimatic settings and anthropogenic interferences in the basin (Jonnalagadda and Mhere 2001). The grand mean concentrations of TDS in the IRB were comparable to that of the Yarlung Tsangpo River in Tibet and global mean value, but were markedly lower than those of the other rivers in Pakistan and Tibet (Table 1).
Calcium (20.68 ± 8.86 mg L−1) was the most abundant major cation in the basin followed by sodium (5.21 ± 16.36 mg L−1), magnesium (3.77 ± 3.79 mg L−1), and potassium (3.56 ± 2.64 mg L−1), and Na+ had the most significant variation. Bicarbonate (60.41 ± 38.41 mg L−1) was the most dominant major anion in the basin followed by sulfate (18.53 ± 15.50 mg L−1), chloride (6.06 ± 15.60 mg L−1), and nitrate (1.46 ± 1.56 mg L−1), and Cl− had the most significant variation. The large variation in Na+ and Cl− concentrations could be attributed to multiple geogenic and anthropogenic sources (Tian et al. 2016). The concentrations of major ions in the IRB were more comparable than those of the Yarlung Tsangpo River and global mean values than to those of other rivers in surrounding regions (Table 1). However, the mean concentration of silica was found to be 1.79 ± 1.13 mg L−1, which was less than the global mean (7.63 mg L−1), indicating less intense silicate weathering in the IRB. The mean concentration of NO3− approximately two times that of the global mean and other rivers in the surrounding region, indicating possible enrichment from anthropogenic activities (Meybeck 2003; Wang et al. 2006; Liu et al. 2015). In summary, the major ionic concentrations of the IRB are within the range of WHO limits, and mostly comparable to the global mean values of the world’s major rivers (Table 1).
Spatial patterns of major ions
The descriptive statistics of major geochemical attributes at the subcatchment level in the IRB are shown in Table 2 and Fig. A3. The mean pH values of all subcatchments were alkaline and did not differ much. However, EC and TDS displayed a considerable variability. Elevated mean concentrations of TDS in the LIB (181.67 ± 167.82 mg L˗1) compared to the UIB (93.99 ± 39.73 mg L˗1) were chiefly ascribed to the arid to semi-arid climatic conditions and higher anthropogenic interferences supported by land use variations (Fig.2) (Gburek and Folmar 1999; Dalai et al. 2002; Chen et al. 2005). Remarkable differences exist in the relative abundance of cations in the UIB and LIB samples, in which cations in the UIB samples follow the order of Ca2+ > Mg2+ > K+ > Na+ and cations in the LIB water samples follow the order of Ca2+ > Na+ > Mg2+ > K+. The concentrations of all of the ions were remarkably higher in the LIB. Specifically, the Na+ and Cl− concentrations were ~ 10-fold higher than those in the UIB, which can be explained by the semi-arid environment, local sources of halite, and intensive anthropogenic interferences, particularly agricultural practices (Xiao et al. 2012). Remarkably higher concentrations of NO3− were observed in the LIB, which is characterized by intensive agriculture activities. Additionally, there is a strong correlation in the UIB between Ca2+-Mg2+ (r = 0.83), Ca2+-HCO3− (r = 0.87), and Mg2+-HCO3− (r = 0.91), whereas there is not a strong correlation between the aforementioned pairs in the LIB (Table A2), indicating the dominance carbonate weathering in the UIB (Varol et al. 2013b; Shammi et al. 2017).
Within UIB, the two glaciated subcatchment, i.e., UIB-II and UIB-III, illustrated higher TDS, indicating the high weathering rate in glaciated areas (Brown et al. 1996) and dilution by snowmelt and precipitation in UIB-I, UIB-IV, and UIB-V. Most of the major ions showed similar spatial patterns within UIB corresponding with the TDS values. For example, UIB-II and UIB-III represented higher SO42− contents than UIB-I, UIB-IV, and UIB-V indicating greater rock–water interaction time with pyrite dissolution. In contrast, LIB, UIB-IV, and UIB-V have higher Si concentrations compared with UIB-I, UIB-II, and UIB-III, indicating potential dissolution of silicate minerals from pedogenic processes (Reynolds and Johnson 1972; Xiao et al. 2015).
A strong positive correlation was observed between Ca2+-SO42− (r = 0.83, 0.74, 0.73, 0.91, and 0.80 for UIB-I, UIB-II, UIB-III, UIB-IV, and LIB, respectively) and Mg2+-SO42− pairs (r = 0.88, 0.66, 0.61, and 0.65 for UIB-I, UIB-II, UIB-IV, and LIB, respectively) (Table A2) indicating sulfate mineral dissolution and oxidation of pyrites as the common source of SO42− in the subcatchments (Xiao et al. 2015). In UIB-I, there was strong positive correlation between NO3−-Ca2+, NO3−-Mg2+, and NO3−-Na+ pairs (r = 0.66, 0.70, and 0.72, respectively), which could be resulted from the use of chemical fertilizers and leaching of soil salts due to intensive agricultural practices (Butz 1989; Sharma et al. 2012; Chen et al. 2015). In addition, the higher NO3− values in UIB-V also indicate more intensive anthropogenic activities supported by land use characteristics as compared with other subcatchments of the UIB (Fig. A1).
Characterization of geochemical facies
Major cations and anions (milli-equivalent %) are plotted in a Piper diagram (Fig. 2), in which the cations and anions are illustrated in the bottom left and right triangles, respectively, and are further projected into the central diamond field to evaluate geochemical facies (Piper 1944). In the cation triangle, most of the samples lie in the left corner where Ca2+ values are higher (> 50%), revealing the dominance of calcium. Two samples from the LIB lie towards the right corner of the cation plot, displaying an increased concentration of sodium and potassium ions, which indicates the influence of local sources of Na+ and K+ (Karim and Veizer 2000; Selemani et al. 2017). In contrast, in the anion triangle, most of the samples lie on the left side of the triangle, showing a dominance of HCO3− over Cl− and SO42−. However, two samples from the LIB and one sample each from UIB-III, UIB IV, and the LIB showed higher concentrations of Cl− and SO42−, which could be due to the mixed sources of multiple geogenic and anthropogenic activities (Xiao et al. 2012). There are six subfields in the diamond-shaped Piper diagram (Fig. 2): (1) Ca˗Mg-HCO3, (2) Na˗Cl, (3) mixed Ca˗Mg˗Cl, (4) mixed Ca˗Na˗HCO3, (5) Ca˗SO4-Cl, and (6) Na˗HCO3 (Manoj et al. 2013). Mostly, the geochemical facies of the IRB are characterized by the Ca-Mg-HCO3− type (91.6%). Apart from this, two samples from UIB-I, one sample from UIB-III, and two samples from LIB are the Ca-Na-HCO3− type. All of these samples were taken from river reaches with relatively low discharge, or more anthropogenic activities. Only one sample lies in the Na-Cl type (LIB: Deratang tributary), and one lies in the Ca-SO4-Cl type (UIB-IV: Jaglot tributary). The Jaglot tributary was influenced by pyrite deposits and mining activities, whereas the Deratang tributary is recognized to have halite deposit within the sedimentary rocks as well as sewage waste from nearby urban land use. The hydrochemical facies clearly suggested spatial discrimination among different subcatchments with anthropogenic and geogenic signatures.
Mechanisms controlling the major ion chemistry of the IRB
Major ions of river water have multiple sources from physical, chemical, and biological processes in the basin, including the sea salts that are carried by atmosphere circulation and are deposited through precipitation, weathering of different types of rocks such as carbonates, silicates, and evaporites, and anthropogenic activities (Patz et al. 2005; Zhang et al. 2007).
Gibbs plot
Surface water chemistry and its controlling mechanisms were well defined by Gibbs (1970). The Gibbs plot (Fig. 3) explained three end-members: precipitation, rock dominance, and evaporation from bottom to top. In general, samples with low TDS (≈ 10.0 mg L−1) and high Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) (≈ 1.0) fall on the lower right corner, which reflects the influence of precipitation. Samples with medium amount of dissolved salts (70.0–300.0 mg L−1) with Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) ratios < 0.5 are located on the middle left side of the plot near the rock dominance end-member. When the TDS increases above 300.0 mg L−1 and the ratio of Na+/(Na+ + Ca2+) or Cl−/(Cl− + HCO3−) races approaches 1.0, evapocrystallization is dominant (Stallard and Edmond 1987). Figure 3 reveals that most of the water samples from the IRB were dominated by rock weathering. Two samples from the LIB lie towards the evaporation end-member, indicating the impact of arid to semi-arid environmental conditions.
Mixing diagram
Different combinations of major ions can result from weathering of different rocks. For instance, carbonate weathering produces Ca2+, Mg2+, and HCO3−, silicate weathering generates Na+, K+, Si, Ca2+, Mg2+, and HCO3−, and dissolution of evaporites or leaching of soil salts mainly produces SO42−, Ca2+, Mg2+, Na+, K+, Cl−, and NO3− (Karim and Veizer 2000; Dalai et al. 2002; Han and Liu 2004; Zhang et al. 2007). In addition, Na+, Cl−, and SO42− also occur from the dissolution of halite, pyrite, and sulfate minerals such as gypsum and anhydrite (Qadir et al. 2008; Paudyal et al. 2016). To show the origins of the riverine dissolved load produced by chemical weathering in a basin with complex lithologies, Na+-normalized molar ratios were plotted in the mixing diagram (Fig. 4) with respect to three representative lithologies (Gaillardet et al. 1999). The results clearly show that the majority of the IRB surface waters were dominated by carbonate weathering with influences of silicate weathering to different degrees and a minor contribution of evaporite weathering. Carbonate weathering is the controlling factor (> 90%) for the major dissolves load in UIB-I and UIB-II, as shown by the samples that were close to the end-member of carbonate. Mixing of carbonate (> 50%) and silicate weathering in UIB-II, UIB-IV, and UIB-V can be inferred from the parallel trend line between two end-members of these minerals, whereas the larger influence of silicate minerals was observed in the LIB based on samples near the silicate end-member. Slight shifting from the line between the carbonate and silicate end-members towards the evaporite end-member indicated a minor contribution by evaporite dissolution, especially in UIB-III, UIB-IV, UIB-V, and the LIB.
Ionic ratios
The sources of Ca2+, Mg2+, and HCO3− can be determined from the Ca2+ + Mg2+/HCO3− ratio (Fig. 5a). Almost all the samples lie close to the 1:1 line, indicating that the controlling mechanism of Ca2+, Mg2+, and HCO3− is carbonate weathering (Varol et al. 2013b). The high correlation between Ca2+ and Mg2+ (Fig. 5b) clearly indicated that both ions are mostly from same source region (Zhang et al. 2007).
A plot of Na+ + K+ vs. Si (Fig. 5c) revealed an increasing trend of Si with respect to Na+ + K+ in the LIB indicating silicate weathering from clastic sedimentary rocks. However, clustered data points of the UIB samples revealed relatively less favorable conditions for dissolving silica. The mean Si/(Na+ + K+) ratio in UIB-I, UIB-II, UIB-III, UIB-IV, UIB-V, and the LIB was 0.70, 0.27, 0.53, 0.62, 0.58, and 0.21, respectively, indicating less intensive silicate weathering and additional input of Na+ and K+ from multiple sources (Na-bearing salts and anthropogenic activities), particularly in the LIB.
The Ca2+ + Mg2+/Na+ + K+ ratio (Fig. 5d) is widely used to evaluate the relative contribution of different types of rock in a basin (Ahmad et al. 1998; Han and Liu 2004; Zhang et al. 2007). For rivers where carbonate is the dominant source, the ratio is high such as 6.7 in the Yarlung Tsangpo River (Qu et al. 2017), 6.0 in part of the Indus River in India (Ahmad et al. 1998), 14.6 in the Upper Han River (Li and Zhang 2008), and 9.72 in the Tigris River (Varol et al. 2013a). In the evaporite-dominated areas with arid environments, Ca2+ + Mg2+/Na+ + K+ is low, such as 0.6 in the Ejina Basin in northwest China (Si et al. 2009). In this study, UIB-I, UIB-II, UIB-III, UIB-IV, and UIB-V lie in carbonate weathering zones where Ca2+ + Mg2+/Na+ + K+ ratios were 12.24, 8.55, 8.96, 6.83, and 6.18, respectively. In the LIB, the ratio abruptly decreased to 3.74, affirming that the LIB is influenced more by evaporite dissolution in an arid to semi-arid environment (Habib and Kuper 1998; Habib 2006).
Figure 5e shows that Na+ + K+ was positively correlated with Cl− + SO42−, suggesting a common source of evaporites for Na+, K+, Cl−, and SO42−. If halite dissolution is responsible for the Na+ concentration in any natural water, then the molar ratio of Na+/Cl− is approximately 1.0, and if it is > 1.0, then the source of Na+ is other Na-bearing salts (Xiao et al. 2012). In this study, all of the subcatchments have Na+/Cl− ratios that are much greater than 1.0 (UIB-I = 1.89, UIB-II = 3.821, UIB-III = 1.82, UIB-IV = 1.91, UIB-V = 2.04, and LIB = 3.55), indicating that Na+ was released from the weathering of Na-bearing salts, especially in UIB-II and the LIB (Meybeck 1987). In addition, the decreasing order of Ca2+/Na+ ratio in different subcatchment is UIB-I > UIB-III > UIB-II > UIB-IV > UIB-V > LIB (i.e., 14.69, 13.23, 12.94, 9.1, 8.47, and 2.62, respectively), revealing the substantial enrichment of Na-bearing salts in the LIB in spite of the dominance of carbonate weathering in the IRB (Table A3).
Principal component/factor analysis
The mechanism governing the chemical compositions of surface water can be assessed by identifying interrelationships and co-variations of parameters (Table A4) using different VFs from PCA/FA (Li and Zhang 2008; Sharma et al. 2016). Factor loading is classified as strong and moderate corresponding to absolute loading values > 0.75 and 0.75–0.5, respectively (Liu et al. 2003).
PCA/FA was firstly applied to the entire normalized data set (n = 84) of 11 parameters. The KMO value (0.825) validated the applicability of PCA/FA for the whole data. The analysis evolved two VFs with eigenvalues > 1, explaining about 81.97% of the total variance in the dataset (Fig. 6). The VF1 accounting for 42.97% of the total variance was strongly correlated (> 0.75) with EC, TDS, Ca2+, Mg2+, SO42−, and HCO3−. The colocation of VF1 components indicate their major contribution to the TDS of the basin and common origin of carbonate rock weathering and sulfate dissolution. Since limestone and gypsum are widely distributed in the soils of the basin, pedogenic activities could also be the potential sources of these components (Singh et al. 2004; Huang et al. 2009; Tripathee et al. 2016a, 2017). The VF2 accounting for 39% of the total variance was strongly correlated with Na+, Cl−, NO3−, and Si. The colocation of VF2 components indicated silicate weathering and breakdown of silicate dominated clay minerals in the basin as their common sources. K+ is moderately correlated with both VF1 and VF2, with slightly higher loading with VF2, indicating its affinity towards carbonate rocks, since the volcanics and plutonic rocks rick with ultrapotassic garnet and gneisses are widely found in the region (Andrews-Speed and Brookfield 1982; Ahmad et al. 2001).
The results also showed a good agreement with the PCA/FA findings of the subcatchment dataset. For instance, each of the UIB-I, UIB-II, UIB-III, UIB-IV, UIB-V, and LIB have two varifactors explaining the different level of the total variance. In all of the subbasins in the UIB, VF1 had strong loading on Ca2+, Mg2+, Na+, SO42−, and HCO3−, representing the carbonate weathering as a dominant source of dissolved loads in the basin. The VF2 of most of the subbasins in the UIB had strong loading on Cl−, NO3−, K+, Na+, and Si, indicating the contribution of silicate weathering. The subbasin-wise analysis of PCA/FA, corresponding VFs, variable loadings, and explained variance, are presented in Table A5 and Fig. A4.
Drinking and irrigation water quality
The river waters were suitable for drinking purposes based on TDS and ionic contents, as a majority of the parameters were comparable to the global mean and all the parameters fall within the threshold limit of the World Health Organization (Table 1) (Han and Liu 2004; WHO 2011; Varol et al. 2013b; Paudyal et al. 2016).
The high saturation of Na+ in soil is due to adsorption from water by clay particles, displacing Ca2+ and Mg2+, tends to reduce soil permeability, and eventually results in poor internal drainage (Collins and Jenkins 1996; Saleh et al. 1999). The combination of Na+ with CO32− or with Cl− tends to form more saline soil of both the carbonate and halite types which are not suitable for cropland (Wilcox 1948; Oster 1994). The sodium percentage (Na %) is therefore calculated to assess suitability for irrigation (Eq. 1).
The classification of irrigation waters with respect to sodium percentage by Richards (1954) and Wilcox (1948) is listed in Table 3, where all the samples from UIB-I and a majority of the samples from other subcatchments lie in the “excellent” category, whereas nine samples lie in the “good” category (one each from UIB-III and UIB-V, two each from UIB-II and UIB-IV, and three from the LIB). In the LIB, two samples from major tributaries (Sawan and Deratang) lie in the “permissible” and “doubtful” categories, indicating the low quality of water for irrigation. These tributaries could deteriorate the quality of soil and its agrarian path in the future and could also degrade the agriculture water quality in the downstream direction.
If the waters with high Na+ and low Ca2+ or Mg2+ concentration were used for agriculture, the ion exchange in soil may cause saturation of Na+ and increase the salinity which eventually reduces the osmotic activity of plants and interferes with water and nutrient uptake (Saleh et al. 1999). The Na+/alkali hazard, expressed in terms of sodium adsorption ratio (SAR), has been widely used to assess water quality for irrigation purposes (Xiao et al. 2015). Hem (1985) defines SAR as a measure of cation exchange of irrigation water (Eq. 2):
In the IRB, the mean SAR values in UIB-I, UIB-II, UIB-III, UIB-IV, UIB-V, and the LIB were 0.078 ± 0.019, 0.12 ± 0.04, 0.14 ± 0.08, 0.13 ± 0.05, 0.18 ± 0.16, and 0.994 ± 1.58, respectively. Based on the Bouwer (1978) classification, all of the water samples belong to the “no problem category” of irrigation water quality, i.e., SAR < 6.0. Water samples from the Deratang tributary in the LIB exhibited higher SAR values of 5.46, which are close to threshold limit, indicating that the water quality of the tributary could be a concern in the future for farmland productivity (Nazeer et al. 2014).
Conclusions
River water in the IRB exhibited an alkaline pH with Ca2+ and HCO3− as the dominant cation and anion, respectively. The concentrations of major ions in the IRB were more comparable to those of the Yarlung Tsangpo River and the global mean values than those of the other rivers in surrounding regions. The chemical compositions of the river water exhibited distinct spatial patterns from upstream to downstream. Elevated mean concentrations of TDS in the LIB were chiefly ascribed to the arid to semi-arid climatic conditions and relatively higher anthropogenic interference compared to the UIB. The average concentrations of major ions follow the same order of HCO3− > SO42− > Cl− > NO3− for anions. However, different orders of cation contents of Ca2+ > Mg2+ > K+ > Na+ and Ca2+ > Na+ > Mg2+ > K+ occurred in the UIB and LIB, respectively, reflecting the diverse nature of geological formation.
Spatial variation patterns and correlation analysis suggested that major ionic concentrations are primarily controlled by the natural processes along with some anthropogenic modifications to NO3−. Piper plot showed that chemical facies of river water in the IRB are primarily characterized by the Ca-Mg-HCO3− type. The Gibb’s plot revealed that most of the water samples in the IRB were dominated by rock weathering, whereas a few samples from the LIB indicated slight evaporation and crystallization processes under the arid to semi-arid climate conditions. The mixing plots illustrated that geochemical compositions of river water in the IRB were dominated by carbonate weathering with the influence of silicate weathering to different degrees and a minor contribution of evaporite dissolution. PCA/FA also indicated that the major sources of dissolved loads in the basin are carbonates followed by silicates in general. In particular, a significant influence of silicate minerals was observed in the LIB, and a higher contribution of evaporites was observed in UIB-II, UIB-III, and the LIB. Ionic ratios illustrated that the major geochemical source is carbonate weathering for Ca2+, Mg2+, and HCO3−, silicate weathering in general with additional geogenic and anthropogenic sources in the UIB for Na+ and K+, and Na-bearing salts and evaporites (other than halite) for Cl−, and SO42−.
From the perspective of major ions, the surface water quality of the IRB was found to be within the WHO permissible limits for drinking purposes except for the high pH. For irrigation, the river water exhibited excellent to good levels in the UIB but excellent to doubtful levels in the LIB. As a result, periodic monitoring of the geochemistry of the IRB is important for the sustainability of riverine ecology and livelihoods of people in the region.
References
Ahmad T, Khanna PP, Chakrapani GJ, Balakrishnan S (1998) Geochemical characteristics of water and sediment of the Indus river, Trans-Himalaya, India: constraints on weathering and erosion. J Asian Earth Sci 16:333–346. https://doi.org/10.1016/S0743-9547(98)00016-6
Ahmad MN, Fujiwara Y, Paudel LP (2001) Remagnetization of igneous rocks in Gupis area of Kohistan arc, northern Pakistan. Earth, Planets and Space 53(5):373–384
Ali KF, De Boer DH (2007) Spatial patterns and variation of suspended sediment yield in the upper Indus River basin, northern Pakistan. J Hydrol 334:368–387
Andrews-Speed CP, Brookfield ME (1982) Middle Paleozoic to Cenozoic geology and tectonic evolution of the northwestern Himalaya. Tectonophysics 82:253–275
APHA (2005) Standard methods for the examination of water and wastewater. 21st ed. American Public Health Association, Washington DC. Stand Methods Exam Water Wastewater 21st ed Am Public Heal Assoc Washingt DC 1220
Ashouri H, Hsu K-L, Sorooshian S, Braithwaite DK, Knapp KR, Cecil LD, Nelson BR, Prat OP (2015) PERSIANN-CDR: daily precipitation climate data record from multisatellite observations for hydrological and climate studies. Bull Am Meteorol Soc 96:69–83. https://doi.org/10.1175/BAMS-D-13-00068.1
Baig JA, Kazi TG, Arain MB, Afridi HI, Kandhro GA, Sarfraz RA, Jamal MK, Shah AQ (2009) Evaluation of arsenic and other physico-chemical parameters of surface and ground water of Jamshoro, Pakistan. J Hazard Mater 166:662–669
Baloch MS, Awan IU, Hassan G (2006) Growth and yield of rice as affected by transplanting dates and seedlings per hill under high temperature of Dera Ismail Khan, Pakistan. J Zhejiang Univ B 7:572–579
Barnett TP, Adam JC, Lettenmaier DP (2005) Potential impacts of a warming climate on water availability in snow-dominated regions. Nature 438:303–309. https://doi.org/10.1038/nature04141
Battaglin BW, Kendall C, Chang CCY, et al (2001) Isotopic and chemical composition of inorganic and organic water-quality samples from the Mississippi River Basin, 1997–98. USGS Water Resour Invest Rep 57
Bauder TA, Waskom RM, Sutherland PL, et al (2011) Irrigation water quality criteria. Serv action; no 0506
Bouwer H (1978) Groundwater hydrology. McGraw-Hill, New York
Brezonik P, Arnold W (2011) Water chemistry: an introduction to the chemistry of natural and engineered aquatic systems. OUP, USA
Brown GH, Sharp M, Tranter M (1996) Subglacial chemical erosion: seasonal variations in solute provenance, Haut Glacier d’Arolla, Valais, Switzerland. Ann Glaciol 22:25–31
Butz D (1989) The agricultural use of melt water in Hopar settlement, Pakistan. Ann Glaciol 13:35–39
Chen H, Xia Q, Ingrin J (2015) Water content of the Xiaogulihe ultrapotassic volcanic rocks, NE China: implications for the source of the potassium-rich component. Sci Bull 60:1468–1470. https://doi.org/10.1007/s11434-015-0862-4
Chen J, Wang F, Meybeck M, et al (2005) Spatial and temporal analysis of water chemistry records (1958–2000) in the Huanghe (Yellow River) basin. Wiley Online Library
Chen J, Wang F, Xia X, Zhang L (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187:231–255
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the Middle Hills of the Himalayas, Nepal. J Hydrol 185:71–86
Dalai TK, Krishnaswami S, Sarin MM (2002) Major ion chemistry in the headwaters of the Yamuna river system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochim Cosmochim Acta 66:3397–3416. https://doi.org/10.1016/S0016-7037(02)00937-7
Dimri AP, Chevuturi A (2014) Model sensitivity analysis study for western disturbances over the Himalayas. Meteorog Atmos Phys 123:155–180
Duncan D, Harvey F, Walker M (2007) EPA guidelines: regulatory monitoring and testing. Water and wastewater sampling. Environ Prot Auth J 3:58
Gaillardet J, Dupré B, Louvat P, Allègre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159:3–30. https://doi.org/10.1016/S0009-2541(99)00031-5
Gburek WJ, Folmar GJ (1999) Flow and chemical contributions to streamflow in an upland watershed: a baseflow survey. J Hydrol 217:1–18
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(80):1088–1090. https://doi.org/10.1126/science.170.3962.1088
Habib Z (2006) Water management and reservoirs in Pakistan. South Asian J 11
Habib Z, Kuper M (1998) Performance assessment of the water regulation and distribution system in the Chishtian sub-division at the main and secondary canal levels. IWMI
Han G, Liu C-Q (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem Geol 204:1–21. https://doi.org/10.1016/j.chemgeo.2003.09.009
Helena B, Pardo R, Vega M et al (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34:807–816
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. Department of the Interior, US Geological Survey
Hewitt K (2005) The Karakoram anomaly? Glacier expansion and the elevation effect, Karakoram Himalaya. Mt Res Dev 25:332–340
Huang X, Sillanpää M, Gjessing ET, Vogt RD (2009) Water quality in the Tibetan Plateau: major ions and trace elements in the headwaters of four major Asian rivers. Sci Total Environ 407:6242–6254
Iqbal HH, Shahid N, Qadir A, Ahmad SR, Sarwar S, Ashraf MR, Arshad HM, Masood N (2017) Hydrological and ichthyological impact assessment of Rasul barrage, river Jhelum, Pakistan. Polish J Environ Stud 26:107–114
Jacobson AD, Blum JD (2000) Ca/Sr and 87Sr/86Sr geochemistry of disseminated calcite in Himalayan silicate rocks from Nanga Parbat: influence on river-water chemistry. Geology 28:463–466
Jonnalagadda SB, Mhere G (2001) Water quality of the Odzi River in the eastern highlands of Zimbabwe. Water Res 35:2371–2376
Kambole MS (2003) Managing the water quality of the Kafue River. Phys Chem Earth, Parts A/B/C 28:1105–1109
Karim A, Veizer J (2000) Weathering processes in the Indus River basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem Geol 170:153–177
Karn SK, Harada H (2001) Surface water pollution in three urban territories of Nepal, India, and Bangladesh. Environ Manag 28:483–496
Kiem A, Ishidaira H, Hapuarachchi HP et al (2008) Future hydroclimatology of the Mekong River basin simulated using the high-resolution Japan Meteorological Agency (JMA) AGCM. Hydrol Process 22:1382–1394
Kimstach VA, Meybeck M (Michel), Baroudy E (1998) A water quality assessment of the former Soviet Union. E & FN Spon
Li F, Xu Z, Yang H, Liu M (2011) Observed (1961–2009) and projected (2010–2100) climate change over the Yarlung Tsangpo River basin. In: Water Resource and Environmental Protection (ISWREP), 2011 International Symposium on IEEE, pp 2337–2340
Li S, Zhang Q (2008) Geochemistry of the upper Han River basin, China, 1: spatial distribution of major ion compositions and their controlling factors. Appl Geochem 23:3535–3544
Li X, Ding Y, Yu Z, Mika S, Liu S, Shangguan D, Lu C (2015) An 80-year summer temperature history from the Xiao Dongkemadi ice core in the central Tibetan Plateau and its association with atmospheric circulation. J Asian Earth Sci 98:285–295
Li X, He X, Kang S et al (2016) Diurnal dynamics of minor and trace elements in stream water draining Dongkemadi Glacier on the Tibetan Plateau and its environmental implications. J Hydrol 541(Part):1104–1118. https://doi.org/10.1016/j.jhydrol.2016.08.021
Liniger H, Weingartner R, Grosjean M, et al (1998) Mountains of the world: water towers for the 21st century:[a contribution to global freshwater management]. Mountain Agenda c/o Institute of geography University of Berne
Liu C-W, Lin K-H, Kuo Y-M (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313:77–89
Liu H, Li J, Li X, Zheng Y, Feng S, Jiang G (2015) Mitigating greenhouse gas emissions through replacement of chemical fertilizer with organic manure in a temperate farmland. Sci Bull 60:598–606. https://doi.org/10.1007/s11434-014-0679-6
Liu Z, Ostrenga D, Teng W, Kempler S (2012) Tropical rainfall measuring mission (TRMM) precipitation data and services for research and applications. Bull Am Meteorol Soc 93:1317–1325
Loveland TR, Reed BC, Brown JF, Ohlen DO, Zhu Z, Yang L, Merchant JW (2000) Development of a global land cover characteristics database and IGBP DISCover from 1 km AVHRR data. Int J Remote Sens 21:1303–1330
Lutz AF, Immerzeel WW, Kraaijenbrink PDA, Shrestha AB, Bierkens MFP (2016) Climate change impacts on the upper Indus hydrology: sources, shifts and extremes. PLoS One 11:e0165630. https://doi.org/10.1371/journal.pone.0165630
Manoj K, Ghosh S, Padhy PK (2013) Characterization and classification of hydrochemistry using multivariate graphical and hydrostatistical techniques. Res J Chem Sci ISSN 2231:606X
Meybeck M (2003) Global occurrence of major elements in rivers. Treatise Geochem 5:207–223
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428. https://doi.org/10.2475/ajs.287.5.401
Moon S, Huh Y, Zaitsev A (2009) Hydrochemistry of the Amur River: weathering in a northern temperate basin. Aquat Geochem 15:497–527. https://doi.org/10.1007/s10498-009-9063-6
Nadeem Faisal (2015) Annual report on 2015 Pakistan’s Monsoon Rainfall
Nazeer S, Hashmi MZ, Malik RN (2014) Heavy metals distribution, risk assessment and water quality characterization by water quality index of the river Soan, Pakistan. Ecol Indic 43:262–270
Oster JD (1994) Irrigation with poor quality water. Agric Water Manag 25:271–297
Pande K, Sarin MM, Trivedi JR, Krishnaswami S, Sharma KK (1994) The Indus river system (India-Pakistan): major-ion chemistry, uranium and strontium isotopes. Chem Geol 116:245–259
Pant RR, Zhang F, Rehman FU et al (2018) Spatiotemporal variations of hydrogeochemistry and its controlling factors in the Gandaki River Basin, Central Himalaya Nepal. Sci Total Environ 622:770–782
Patz JA, Campbell-Lendrum D, Holloway T, Foley JA (2005) Impact of regional climate change on human health. Nature 438:310–317. https://doi.org/10.1038/nature04188
Paudyal R, Kang S, Sharma CM, Tripathee L, Huang J, Rupakheti D, Sillanpää M (2016) Major ions and trace elements of two selected rivers near Everest region, southern Himalayas, Nepal. Environ Earth Sci 75:1–11. https://doi.org/10.1007/s12665-015-4811-y
Pekey H, Karakaş D, Bakoglu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar Pollut Bull 49:809–818
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. EOS Trans Am Geophys Union 25:914–928
Qadir A, Malik RN, Husain SZ (2008) Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab, Pakistan. Environ Monit Assess 140:43–59. https://doi.org/10.1007/s10661-007-9846-4
Qu B, Zhang Y, Kang S, Sillanpää M (2017) Water chemistry of the southern Tibetan Plateau: an assessment of the Yarlung Tsangpo river basin. Environ Earth Sci 76:74. https://doi.org/10.1007/s12665-017-6393-3
Raymo ME, Ruddiman WF (1992) Tectonic forcing of late Cenozoic climate. Nature 359:117–122
Reynolds RC, Johnson NM (1972) Chemical weathering in the temperate glacial environment of the northern Cascade Mountains. Geochim Cosmochim Acta 36:537–554
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. LWW
Richardson M, Hausfather Z, Nuccitelli DA, Rice K, Abraham JP (2015) Misdiagnosis of earth climate sensitivity based on energy balance model results. Sci Bull 60:1370–1377. https://doi.org/10.1007/s11434-015-0806-z
Saleh A, Al-Ruwaih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Selemani JR, Zhang J, Muzuka ANN, Njau KN, Zhang G, Maggid A, Mzuza MK, Jin J, Pradhan S (2017) Seasonal water chemistry variability in the Pangani River basin, Tanzania. Environ Sci Pollut Res 24:26092–26110. https://doi.org/10.1007/s11356-017-0221-x
Shammi M, Rahman MM, Islam MA, Bodrud-Doza M, Zahid A, Akter Y, Quaiyum S, Kurasaki M (2017) Spatio-temporal assessment and trend analysis of surface water salinity in the coastal region of Bangladesh. Environ Sci Pollut Res 24:14273–14290. https://doi.org/10.1007/s11356-017-8976-7
Sharma A, Singh AK, Kumar K (2012) Environmental geochemistry and quality assessment of surface and subsurface water of Mahi River basin, western India. Environ Earth Sci 65:1231–1250
Sharma S, Roy A, Agrawal M (2016) Spatial variations in water quality of river Ganga with respect to land uses in Varanasi. Environ Sci Pollut Res 23:21872–21882. https://doi.org/10.1007/s11356-016-7411-9
Si J, Qi F, Xiaohu W et al (2009) Major ion chemistry of groundwater in the extreme arid region northwest China. Environ Geol 57:1079–1087
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38:3980–3992
Stallard RF, Edmond JM (1987) Geochemistry of the Amazon: 3. Weathering chemistry and limits to dissolved inputs. J Geophys Res 92:8293. https://doi.org/10.1029/JC092iC08p08293
Stumm W, Morgan JJ (2012) Aquatic chemistry: chemical equilibria and rates in natural waters. John Wiley & Sons, Hoboken
Subramanian V (1979) Chemical and suspended-sediment characteristics of rivers of India. J Hydrol 44:37–55
Sun B, Zhang L, Yang L, Zhang F, Norse D, Zhu Z (2012) Agricultural non-point source pollution in China: causes and mitigation measures. Ambio 41:370–379
Tahir AA, Adamowski JF, Chevallier P, Haq AU, Terzago S (2016) Comparative assessment of spatiotemporal snow cover changes and hydrological behavior of the Gilgit, Astore and Hunza River basins (Hindukush–Karakoram–Himalaya region, Pakistan). Meteorog Atmos Phys 128:793–811
Tahir AA, Chevallier P, Arnaud Y, Neppel L, Ahmad B (2011) Modeling snowmelt-runoff under climate scenarios in the Hunza River basin, Karakoram Range, Northern Pakistan. J Hydrol 409:104–117
Thomas J, Joseph S, Thrivikramji KP, Manjusree TM, Arunkumar KS (2014) Seasonal variation in major ion chemistry of a tropical mountain river, the southern Western Ghats, Kerala, India. Environ Earth Sci 71:2333–2351
Tian Y, Yu C, Zha X, Wu J, Gao X, Feng C, Luo K (2016) Distribution and potential health risks of arsenic, selenium, and fluorine in natural waters in Tibet, China. Water 8:568
Tripathee L, Kang S, Huang J, Sillanpää M, Sharma CM, Lüthi ZL, Guo J, Paudyal R (2014) Ionic composition of wet precipitation over the southern slope of central Himalayas, Nepal. Environ Sci Pollut Res 21:2677–2687
Tripathee L, Kang S, Rupakheti D, Cong Z, Zhang Q, Huang J (2017) Chemical characteristics of soluble aerosols over the central Himalayas: insights into spatiotemporal variations and sources. Environ Sci Pollut Res 24:24454–24472
Tripathee L, Kang S, Rupakheti D, Zhang Q, Bajracharya RM, Sharma CM, Huang J, Gyawali A, Paudyal R, Sillanpää M (2016a) Spatial distribution, sources and risk assessment of potentially toxic trace elements and rare earth elements in soils of the Langtang Himalaya, Nepal. Environ Earth Sci 75:1332
Tripathee L, Kang S, Sharma CM, Rupakheti D, Paudyal R, Huang J, Sillanpää M (2016b) Preliminary health risk assessment of potentially toxic metals in surface water of the Himalayan rivers, Nepal. Bull Environ Contam Toxicol 97:855–862. https://doi.org/10.1007/s00128-016-1945-x
Varol M, Gökot B, Bekleyen A (2013a) Dissolved heavy metals in the Tigris River (Turkey): spatial and temporal variations. Environ Sci Pollut Res 20:6096–6108
Varol M, Gökot B, Bekleyen A, Şen B (2013b) Geochemistry of the Tigris River basin, Turkey: spatial and seasonal variations of major ion compositions and their controlling factors. Quat Int 304:22–32. https://doi.org/10.1016/j.quaint.2012.12.043
Vörösmarty CJ, McIntyre PB, Gessner MO et al (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Wang G, Zeng C, Zhang F, Zhang Y, Scott CA, Yan X (2017) Traffic-related trace elements in soils along six highway segments on the Tibetan plateau: influence factors and spatial variation. Sci Total Environ 581–582:811–821. https://doi.org/10.1016/j.scitotenv.2017.01.018
Wang J, Yan W, Jia X (2006) Modeling the export of point sources of nutrients from the Yangtze River basin and discussing countermeasures. Acta Sci Circumst 26:658–666
WHO (2011) Guidelines for drinking-water quality. Geneva: world health organization
Wilcox LV (1948) The quality of water for irrigation use. United States Department of Agriculture, Economic Research Service
Wu L, Long T, Liu X, Guo J (2012) Impacts of climate and land-use changes on the migration of non-point source nitrogen and phosphorus during rainfall-runoff in the Jialing River watershed, China. J Hydrol 475:26–41
Xiao J, Jin Z-D, Ding H et al (2012) Geochemistry and solute sources of surface waters of the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. J Asian Earth Sci 54:162–173
Xiao J, Jin ZD, Wang J, Zhang F (2015) Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quat Int 380:237–246
Young GJ, Hewitt K (1990) Hydrology research in the upper Indus basin, Karakoram Himalaya, Pakistan. Hydrol Mt Areas. IAHS Publ 190:139–152
Zhang Q, Xu C-Y, Yang T (2009) Variability of water resource in the Yellow River basin of past 50 years, China. Water Resour Manag 23:1157–1170
Zhang S-R, Lu XX, Higgitt DL, Chen CTA, Sun HG, Han JT (2007) Water chemistry of the Zhujiang (Pearl River): natural processes and anthropogenic influences. J Geophys Res 112:F01011. https://doi.org/10.1029/2006JF000493
Funding
This research was financially supported by the International Partnership Program of Chinese Academy of Sciences (Grant No. 131C11KYSB20160061), the National Natural Science Foundation of China (Grant No. 41422101), and the Chinese Academy of Sciences-The World Academy of Scicences (CAS-TWAS) President’s Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 1977 kb)
Rights and permissions
About this article
Cite this article
Rehman Qaisar, F.U., Zhang, F., Pant, R.R. et al. Spatial variation, source identification, and quality assessment of surface water geochemical composition in the Indus River Basin, Pakistan. Environ Sci Pollut Res 25, 12749–12763 (2018). https://doi.org/10.1007/s11356-018-1519-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1519-z