Abstract
Size-segregated aerosol samples were collected at Raipur, India, during the period of October 2008 to September 2009, using eight-stage cascade impactor-type aerosol sampler at a flow rate of 28.3 l/min. The results give information about the mass concentrations, seasonal variations, and sources of water-soluble inorganic species viz. Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 in both PM2.5–10 (coarse) and PM2.5 (fine) fractions. Annual mean of mass concentrations of PM2.5–10 and PM2.5 aerosols were monitored to be 200.7 ± 55.1 and 185.9 ± 66.9 μg/m3, respectively. The annual mean concentrations of the inorganic components were monitored to be 5.4 ± 2.4, 6.2 ± 4.7, 5.3 ± 1.8, 3.2 ± 0.46, 40.8 ± 7.9, 9.2 ± 3.8, 21.6 ± 5.5, and 17.9 ± 4.0 μg/m3 in PM2.5–10 aerosols and 4.1 ± 2.1, 13.4 ± 4.2, 7.7 ± 2.0, 1.7 ± 0.4, 18.7 ± 5.4, 9.7 ± 3.1, 13.4 ± 5.7, and 36.8 ± 10.0 μg/m3, respectively, in PM2.5 aerosols, for the above ions. The concentration of Na+, Mg2+, and Ca2+ were higher in spring and summer. The seasonal variation of secondary components NH +4 , NO -3 , and SO 2-4 were similar; that means high concentration in winter and low concentration in fall. The results obtained from factor analysis suggested the presence of three main components/sources in the coarse and fine modes namely regional pollution, airborne road dust, and traffic emissions, which contributed 94.0% and 83.2% of the total variance of the system respectively for the PM2.5–10 and PM2.5 aerosols data set.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tropospheric concentrations of aerosol particles have increased significantly over the last 150 years and continue to increase further due to urbanization and industrialization, particularly in developing countries (Tiwari et al. 2009). Particulate matter (PM) is currently eliciting strong research interest due to their ability to alter the climate, the biochemical cycles, the chemistry of atmosphere, as well as cause for adverse health effects in humans (Shi et al. 2001; Kocak et al. 2007; Pope et al. 2002). Atmospheric aerosol particles exhibit wide range of sizes, from nanometer to micrometer range, while chemical composition differs greatly among the size ranges, and even among individual particles within a given size interval (Kim et al. 2002; Rinaldi et al. 2007). The sources, characteristics, and potential health effects of large coarse particles (>2.5 μm in diameter; PM2.5–10) are different from that of fine particles (<2.5 μm in diameter; PM2.5), as fine particles can penetrate deep into the lungs easily and therefore are likely to increase respiratory and mutagenic diseases (Wang et al. 2002; Akyuz and Cabuk 2009; Harrison and Yin 2000). However, the scientific understanding of how different PM2.5 components influence human health is still an area of active research (Franklin et al. 2008; Bell et al. 2009).
PM less than 10 μm, but greater than 2.5 μm in diameter (PM2.5–10; coarse PM) have also been shown to have health effects in the short-term while long-term effects are yet to be confirmed (Lin et al. 2005; Bai et al. 2007). Nevertheless, PM2.5 particle is one of the criteria pollutants in India with limit value of 65 μg/m3 as 24-h average and 40 μg/m3 as annual average (National Ambient Air Quality Standards; NAAQS, India; http://www.scorecard.org/env-release/def/cap_naaqs.html). Findings from various studies, though, showed that the concentrations of PM2.5 exceeded the NAAQS of India (Tiwari et al. 2009; Kulshrestha et al. 2009). Therefore, knowledge of the chemical composition of airborne particulate matters and their sources is necessary for scheduling policies to reduce pollution and its negative effect on human health and monuments. In this context, the study of air quality and the identification of pollution emission sources are also equally important (Bem et al. 2003; Kulmatov and Hojamberdiev 2010).
Water-soluble ions are chemical species which are easily soluble in water in the lower troposphere under certain conditions (Kulshrestha et al. 2009). It is known that aerosol and their associated ions play an important role in the content of chemical species in atmospheric ecosystem interfaces (Ruhling and Tyler 2004; Salam et al. 2008). In many urban and rural areas, water-soluble inorganic ions (Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 ) account for large fractions of atmospheric particle mass and associated with adverse effect on human health and acidification (Yao et al. 2002). Sulfate (SO 2-4 ), nitrate (NO -3 ) and ammonium (NH +4 ) are the most common components of secondary particles in the atmosphere which are usually formed from the direct emissions of sulfur dioxide (SO2), nitrogen dioxide (NO2), and ammonia (NH3) gases in the atmosphere (Wang et al. 2006).
The continued use of wood and coal for cooking are unavoidable issues in developing countries (Kumar et al. 2007), and India is no exception to it. Several investigators have studied the chemical composition, mass, and size distribution of atmospheric aerosols both in India (Rastogi and Sarin 2005; Parmar et al. 2001) and abroad (Yao et al. 2002; Sun et al. 2004; Wang et al. 2005). Most of the earlier studies reported in India are only focused on the physical characteristics of aerosols (Moorthy et al. 1991; Parmeshwaran et al. 1998; Pilli et al. 2002) except few studies which have been conducted at urban sites and industrial location for chemical characterization of aerosols (Parmar et al. 2001; Sharma and Maloo 2005; Kumar et al. 2007; Tiwari et al. 2009).
However, the information on air quality in terms of water-soluble ionic composition in PM2.5–10 and PM2.5 aerosols are inadequate for a rapidly growing industrial city Raipur, Chhattisgarh, situated at the eastern central region of India. In order to address the needs indicated above, size-segregated aerosol samples were collected between the period of October 2008 and September 2009 at sampling site Raipur (Chhattisgarh), India in four different seasons, winter (October to January), spring (February to March), summer (April to June), and fall (July to September) to study the mass concentrations, seasonal variation, and sources of PM2.5–10 and PM2.5 aerosols and its important water-soluble ionic constituents.
Experimental
Sampling location
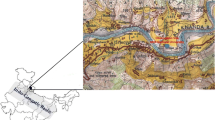
Figure 1 shows the location of the sampling site selected at Raipur (21o14’N, 81o38’E, 297 m above sea level), India, for continuous sampling of aerosols to study the ambient air quality. Raipur, the study area, has population of 1.2 million with a huge population growth rate especially after it became the capital of a new state Chhattisgarh, in India. With the rapid urbanization and motorization of Raipur, this is manifested by increasing energy consumption and sharp increase of motor vehicles, the PM pollution in Raipur remains at a much higher level than the National Ambient Air Quality Standards of India (Deshmukh et al. 2010). The collection of atmospheric aerosols was performed at the terrace of the building School of Studies in Chemistry, Pandit Ravishankar Shukla University, at an altitude of approximately 15 m from the ground level. There is the National Highway, namely, Great Eastern Road, which adjoins Kolkata and Mumbai, approximately 400 m away from the sampling site with frequent heavy vehicular traffic volume of nearly 25,000 vehicles per day. The study area is literally sandwiched between two industrial complexes on its eastern and western outskirts extended at distances of about 20–30 km, with some big industries such as Bhilai Steel Plant, Jamul Cement Factory, Century Cement, Larson and Toubro Cement, Monnet Sponge Iron and Power Plant, Woolworth Textile Plant, etc. Apart from this, a large number of fertilizer plants, oil extraction plants, agro-forest plants, and electrical goods manufacturing plants are also set within the circumference of 30 km. A detailed description of the site is given elsewhere (Deshmukh et al. 2010).
Sample collection
The aerosol samples were collected from October 2008 to September 2009 using eight-stage cascade impactor-type aerosol sampler (Model TE 20–800 Tisch Air Pollution Monitoring Equipment, USA), which separated particles into eight size fractions between 0.4 and 10.0 μm, according to the following equivalent aerodynamic cut-off diameter of stages, 10.0–9.0 (stage 0), 9.0–5.8 (stage 1), 5.8–4.4 (stage 2), 4.4–2.5 (stage 3), 2.5–2.1 (stage 4), 2.1–1.0 (stage 5); 1.0–0.7 (stage 6), and 0.7–0.4 μm (stage 7). The flow rate was maintained at 28.3 L min-1 during the collection of the samples. The sampling frequency was two samplings per week at fixed-day intervals throughout the year covering all four prevailing seasons (winter, spring, summer, and fall) in this region. Therefore, in total, during the period of study, 96 samplings (96 × 8 size fractions = 768 samples of eight different size fractions) were carried out. The sum of particles of the upper four stages of the eight-stage cascade impactor (stage 0 to stage 3) are considered as coarse particles (2.5 < Da < 10.0 μm). Similarly, the sum of particles of the lower four stages (stage 4 to stage 7) are considered as fine particles (Da < 2.5 μm; see cut-off diameter of each stage given above). Thus, after summing up the above samples, a total of 96 samples each of coarse and fine particles were analyzed. The sampler was run for the period of 24 h (started in the morning 6 am) so that analytical amount of samples could be obtained. A set of field blank filter was also collected. The samples were collected on dried and pre-weighed 80 mm Whatman 41 glass filters. The filters were kept in vacuum desiccators for 24 h to remove any moisture content before mounting them on the air sampler. After sampling, the filters were immediately transferred to vacuum desiccators to again de-moisture in the same manner. Before and after sampling, the filters were weighed using an analytical balance (Sartorius CP225D) with a reading precision of 10 μg after stabilizing under a constant temperature (20 ± 5°C) and humidity (40 ± 2%). The exposed filters and field blanks were placed into polyethylene bottles and kept frozen at −20°C during storage and transported to the Public Health and Research Institute of Kobe City, Japan, for analysis.
Methods
One fourth of the filter samples were extracted into 10 ml ultrapure water (specific resistance, 18 MΩ cm) by using an ultrasonic bath (UT-105 S, Sharp, Japan) and a shaker (EYELA Multi Shaker, Rikakikoi Co., Tokyo, Japan) to further measure various water-soluble inorganic ions. The ultrasonic and mechanical agitations were operated for 45 min in each case. The extracted solutions were filtered through microporous membranes (pore size, 0.45 μm; diameter, 25 mm). The pH of the filtrate solution was measured employing a pH meter (Systronic India, model 324). After the pH measurement, the filtered samples were preserved at 4°C in a refrigerator.
The major anions (Cl-, NO -3 , and SO 2-4 ) and cations (Na+, NH +4 , K+, Mg2+ and Ca2+) were quantitatively determined by Ion Chromatograph (Dionex, DX-120, USA). The determination of anions and cations was performed using separating columns, Ionpac AS 12A and CS 16A, respectively. Eluents of 2.7 Na2CO3 and 0.3 mM NaHCO3 (Wako, Japan) were prepared and used for the detection of anions with a pump flow rate of 1.5 ml min-1. A 2.6-mM methane sulphonic acid (CH3SO3H) solution was used as an eluent for cation analysis with a pump flow rate of 1 ml min-1. Ions were identified based on their retention time. The method detection limit (DL) was calculated for the Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 ions. A 1 ppm of the laboratory prepared calibration standard was used to calculate DL. The obtained DL were 0.01, 0.03, 0.02, 0.01, 0.01, 0.01, 0.01, and 0.02 μg/m3 for Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 , respectively. Blank concentration was measured using field blank samples. Blank filters were extracted and passed through similar procedures as followed for the extraction and preparation of the regular particulate sample to analyze with the ion chromatography. The overall mean concentrations for field blank samples collected over a year were observed to be 0.05, 0.10, 0.01, 0.00, 0.01, 0.23, 0.00 and 0.21 μg/m3 for Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 , respectively. All the reported PM mass and water-soluble ion concentrations have been corrected using field blanks.
Meteorology of the sampling location
The meteorological data, including temperature (T, °C), vapor pressure (VP, kPa), rainfall (RF, mm), relative humidity (RH, %), wind speed (WS, m/s), and wind direction (WD, degree) were obtained from the Department of Agrometeorology, Indira Gandhi Agricultural University, Raipur, India, which is located at an aerial distance of approximately 4 km in north–east direction from the sampling site. Figure 2 shows monthly variations in T, RH, RF, VP, WS, and WD during the study period, where vertical bars indicate standard deviations from their respective mean. December is a cold winter month with an average temperature of 20.7°C that rises in summer and attains a maximum 35.3°C in May, shown in the upper panel of Fig. 2. The annual mean temperature was found to be about 27.7°C during the study period. The observed highest vapor pressure was 3.1 kPa in August and September and the lowest were 1.2 and 1.3 kPa in April and February, respectively. The relative humidity, shown in the middle panel of Fig. 2, varied from 27% (April) to 85% (August), with an annual mean of 57% during the study period. In Raipur, fall was relatively wet with monthly average of 127.3, 66.6, and 24.3 mm in July, August, and September, respectively, while winter was very dry with precipitation of 2.5, 0.0, 0.0, and 0.0 mm in October, November, December, and January, respectively. Wind speed and direction were plotted in the lower panel of Fig. 2. The prevailing wind were south–west (SW) during the study period with wind speed varying from 1.9 (December) to 10.8 m/s (July). The annual mean wind speed was found to be 5.1 m/s during the study period. Wind rose plots (Fig. 3) were generated by using a WRPLOT View (Lakes Environmental, Canada). Figure 3 also indicated that the winter and spring are relatively calm (58.70% and 48.89%) than summer and fall seasons.
Results and discussion
Mass concentrations of size-segregated aerosols
Table 1 shows a statistical summary of mass concentrations in the different size ranges in Raipur. As Table 1 shows, the size distribution of the aerosol mass for atmospheric particles Da < 10 μm was bimodal (or monomodal) centered on stage 3. Overall the 51.9% and 48.1% of the aerosol mass was distributed in the coarse (stage 0–3) and fine (stage 4–7) size range, respectively, to the total aerosol mass. However, stage 3 showed the maximum value and the minimum value was observed on stage 7. This difference in mass distribution is apparently dependent on the aerosol air concentrations and resuspended soil dusts could be the possible source for the particles Da < 10 μm near the sampling site.
Overall mass concentration and distribution of PM2.5–10 and PM2.5 atmospheric aerosol particulate matters
Concentrations of PM2.5–10, PM2.5 and ratios of PM2.5/PM10 in Raipur from October 2008 to September 2009 are shown in Table 2. Mass concentrations of PM2.5–10 and PM2.5 in Raipur were in the range of 101.2–298.4 and 104.5–298.6 μg/m3, with a mean and standard deviation of 200.7 ± 55.1 and 185.9 ± 66.9 μg/m3, respectively. Frequencies of 24-h PM2.5 mass concentrations exceeding 65 μg/m3 (National Ambient Air Quality Standards of India, NAAQS; http://www.scorecard.org/env-releases/def/cap_naaqs.html) at Raipur were 100% during the study period with a further distribution of 50%, 19%, 25%, and 6% for samples more than two, three, four, and five times, respectively, that of the above prescribed limit. As shown in Table 2, the average loading of PM2.5–10 and PM2.5 showed higher deposition during winter than spring and summer seasons. However, the lowest concentrations of PM2.5–10 and PM2.5 were found in monsoon season. The data shown in Table 2 indicates clearly that the airborne particulate pollution is very serious at Raipur, and it is much worse in winter than in summer. The high concentrations of PM2.5–10 and PM2.5 during winter season is expected to be caused by the combined effects of the elevated emissions from fossil fuel and coal burning and also due to the prevailing meteorological conditions such as lower temperature, wind speed, and mixing height. The lower inversion layer caused by low mixing height limits the dilution and dispersion of PM2.5 pollutants during winter season. The high concentrations of PM2.5 in the present study may be due to the contribution from heavy traffic and other human particle-generating activities in nearby areas. The low concentration in fall was likely related to more precipitation in this season.
The ratio of PM2.5 to PM10 is used to determine the contribution of fine particulate to the respirable particulate matter with aerodynamic particle size less than 10 μm. Table 2 shows the ratio of PM2.5/PM10 for different seasons in Raipur, India. It can be seen that PM2.5 accounted for 51%, 48%, 44%, and 46% of PM10 in winter, spring, summer, and fall, respectively. The mean PM2.5/PM10 ratio was found to be 0.48. Evidently, fine particles accounted for about half of the respirable particles in spring and in winter. Such high value is expected to be due to the high concentration of PM2.5 in the atmosphere at Raipur region that may be attributed mainly to the combustion of fossil fuel during winter. As mentioned above, the low level inversion is one of the important causes responsible for higher concentration of PM2.5 in winter. Such high proportions of fine particles implied that the particulate pollution at Raipur would have a great effect on the health of human being and possibly on the global environmental change.
Ionic load distribution in PM2.5–10 and PM2.5 aerosols
The observed primary data on water-soluble inorganic ions in the coarse (10 > D a > 2.5 μm) and in fine (D a < 2.5 μm) fractions of particulate matter are presented in Table 3. The percentage contribution of fine and coarse fractions to the concentration of water-soluble ions is shown in Fig. 4. Overall, the half portion of atmospheric mass is associated with coarse particles. Crust originated water-soluble ions (Na+, Mg2+, Ca2+, and NO -3 ) are extensively found in coarse particles (50%), as expected on the basis of their source and formation mechanism. On an average, about 62% of particulate nitrate (NO -3 ) is associated with coarse particles, strongly indicating that it is chemically combined mostly with alkaline ion species (Pakkanen et al. 1999). The most likely formation pathway for particulate nitrate is the reaction of gaseous nitric acid or some other nitrogen compounds with sea salt particles and mineral dust particles. The mass concentration of sulfate is mainly found in the fine mode (67%). The fine mode also dominates the ammonium (NH +4 ), chloride (Cl-), and potassium (K+) mass concentrations (68%, 51%, and 59%, respectively). Regarding sodium (Na+), magnesium (Mg2+), and calcium (Ca2+), 57%, 65%, and 69%, respectively, of the mass concentrations are found in the coarse fractions.
Ionic composition of PM2.5–10 and PM2.5 atmospheric aerosol particulate matters
Water-soluble ions comprise a large part of aerosol particles and play an important role in the atmospheric chemistry. The concentration of water-soluble inorganic ions in PM2.5–10 and PM2.5 aerosols are presented in Table 3. As shown in Table 3, the total water-soluble inorganic ions accounted for 46.4% and 52.2% of the total mass of PM2.5–10 and PM2.5, respectively. Of all anions and cations, in the terms of concentration, SO 2-4 is the dominant component followed by Ca2+ and NO -3 in the PM2.5 particles, while concentration of SO 2-4 is lower than Ca2+ and NO -3 in PM2.5–10 particles. For PM2.5–10, the concentration of the most abundant ionic species followed the order \( {\text{C}}{{\text{a}}^{{{2} + }}} > {\text{NO}}_3^{ - } > {\text{SO}}_4^{{2 - }} > {\text{C}}{{\text{l}}^{ - }} > {\text{NH}}_4^{ + } > {\text{N}}{{\text{a}}^{ + }} > {{\text{K}}^{ + }} > {\text{M}}{{\text{g}}^{{{2} + }}} \) while in the PM2.5 these were \( {\text{SO}}_4^{{2 - }} > {\text{C}}{{\text{a}}^{{{2} + }}} > {\text{NO}}_3^{ - } > {\text{NH}}_4^{ + } > {\text{C}}{{\text{l}}^{ - }} > {{\text{K}}^{ + }} > {\text{N}}{{\text{a}}^{ + }} > {\text{M}}{{\text{g}}^{{{2} + }}} \).
The annual mean concentrations, in terms of percentage of the total mass of the analyzed ionic species, of SO 2-4 , Ca2+, and NO -3 were 34.8%, 17.7%, and 12.8%, respectively in PM2.5 and 16.3%, 37.2%, and 19.7%, respectively, in PM2.5–10. NO -3 was dominant in coarse mode (10.0% of total PM2.5–10 mass) resulting from gaseous HNO3 reaction involving large particle such as sea salt, as shown in reaction 1 (Kouyoumdjian and Saliba 2006). Though, the present study area is closer to anthropogenic emissions, but still NO -3 was predominantly found in coarse mode. Hence, in addition to reaction 1, it is also possible that gaseous HNO3 might have reacted with carbonates, such as CaCO3, on mineral particles to form coarse mode nitrate through reaction 2 (Kouyoumdjian and Saliba 2006).
With respect to cations, Ca2+ is the most prominent component. The Ca2+ ratio of PM2.5/PM2.5–10 is 0.46. This value indicated that calcium was higher in PM2.5–10 aerosols than that in PM2.5 aerosols at Raipur. The possible source of calcium in this region is the soil rich in lime stone and dust emitting from cement plants that are established in the surroundings of Raipur. The available lime stone belt in the earth crust is main reason behind the establishment of many cement plants in this region.
NH +4 was the second most prominent cations in both PM2.5–10 and PM2.5. Ammonia is an important alkaline gas in the atmosphere. Ammonia in fine particles could have been produced from reactions between gaseous ammonia and acidic species like H2SO4 and HNO3 (Zhuang et al. 1999).
Comparison of values reported for other high elevation sites
Table 4 shows a comparison between the average concentrations of the chemical species observed in this study with those at other high elevation sites located in the world to extract some characteristic features of the aerosol at eastern central state in India.
It could be seen clearly that the aerosol pollution is more serious in Raipur. In PM2.5–10 aerosol, the species (Ca2+, NO -3 , and SO 2-4 ) exhibited the highest concentration in Raipur compared with other sites, which was likely due to heavy traffic and other local anthropogenic activities (crustal resuspension from construction works, etc.) in Raipur. The concentration of SO 2-4 was also higher in Raipur than the other sites. This suggested that SO 2-4 in Raipur might have derived from anthropogenic sources. NH +4 in Raipur was higher than most of the other sites except than in Nanjing, China, whose concentration is two times higher than that at Raipur. The concentration of Na+, K+, Cl-, and Mg2+ were also higher for PM2.5–10 in Raipur than most of the other cities. In PM2.5, all the ions, except Cl- at Cairo, Egypt, showed high concentration in Raipur, indicating that fine-particle pollution is more serious than coarse one. Such high levels of calcium and secondary pollutants (NH +4 , NO -3 , and SO 2-4 ) indicate that the pollution from construction activities and traffic/industrial emissions is a critical issue of the air quality in Raipur. The aerosol collected at the Raipur, India, exhibited the general property of high concentrations of water-soluble inorganic ions.
The acidity of PM2.5–10 and PM2.5 aerosols
pH Variation in PM2.5–10 and PM2.5 aerosols
The pH value ranged from 6.2 to 6.6 for PM2.5–10 and 5.0 to 5.8 for PM2.5, respectively. The mean value was 6.4 ± 0.1 for PM2.5–10 and 5.6 ± 0.2 for PM2.5, with a blank value of 5.8 as shown in Table 3. The pH of the aerosol filtrate was a parameter to directly denote the acidity of the aerosol. The mean value of pH in PM2.5 was less than that in PM2.5–10, suggesting that the acidity of water-soluble fraction in fine particle was stronger than that in coarse particle. The mean value of 5.6 for PM2.5, a little lower than the blank value, indicated the probable acid problem in future. It is well known that the low pH values result from acidic matters, such as sulfate, nitrate, and chloride whereas basic water-soluble matters, such as ammonium, calcium, and magnesium result in the increase of pH value. The difference of pH value between PM2.5–10 and PM2.5 was probably caused by the size distribution of these acidic and basic ions. Compared with the blank value of 5.8, PM2.5 particles might aggravate the acidic precipitation while PM2.5–10 particles might alleviate the tendency of acidification.
Equivalent concentrations of total cations and anions
The equivalent ion balance, expressed by the ratio of total cation equivalents to total anion equivalents, was calculated for each individual samples, and all were found to be larger than 1.0. Table 5 shows the mean ratios and ranges of ratios of total cation equivalents to total anion equivalents. The ion balance expressed by the sum of the equivalent concentration (microequivalents per cubic liter) ratio of cation to anion (C/A) was an indicator to study the acidity of the environment (Wang et al. 2005). The ratio calculated from all the measured ionic species ranged from 2.49 to 4.03 (mean, 3.11; SD 0.46) and 1.27 to 2.62 (mean, 1.84; SD 0.49) for the PM2.5–10 and PM2.5 aerosols, respectively, which is larger than unity and indicates that aerosol particles (PM2.5–10 and PM2.5) were alkaline (Sun et al. 2006). The higher value of C/A were most likely due to the existence of unanalyzed carbonate anion (CO 2-3 ), and these missing carbonates could be largely responsible for the deviation from 1.0 (Mkoma et al. 2009). Carbonate is an important source of mineral dust, in which it is associated mainly with calcium (Dentener et al. 1996). The local soil in Raipur contains a lot of limestone; besides, there are seven large-scale cement plants that are established in the surroundings of sampling site. The alkaline mineral components would provide more reactive sites on their surface for absorbing acidic gases (SO2, NOx, and HCl) in the atmosphere and hence speed-up their neutralizing and scavenging processes and alleviate the acidification of the atmosphere (Wang et al. 2005). The earlier studies from the Indian region (Kulshrestha et al. 1998; Momin et al. 1999; Venkataraman et al. 2002) have also reported cation excess in the ion balance of water extracts, which is largely attributed to the lack of carbonate measurements. Again, when the ion balance is recalculated, with Ca2+ excluded, the new mean C/A becomes to be 0.98 for the coarse size fraction and 1.05 for fine size fraction. These new mean are all fairly close to 1.0 supporting the assumption that missing carbonate is largely responsible for the imbalance of ions in coarse and fine aerosols.
Ratio of water-soluble inorganic species in PM2.5–10 and PM2.5
In order to evaluate the contribution of the various sources (marine, anthropogenic, and natural), usually the mass ratios Cl-/Na+, SO 2-4 /Na+, and K+/Na+ in the samples and in sea water are compared (Table 5).
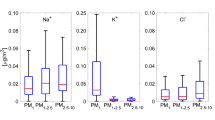
It is noted that Cl-/Na+ < 1.8 indicates a chlorine depletion caused by the accumulation of strong acid anions, such as sulfate, nitrate, and various carboxylic acid anions, while Cl-/Na+ > 1.8 suggests anthropogenic sources for Cl- (e.g., combustion process). The mass ratio of Cl-/Na+ in the PM2.5–10 aerosol (ranges, 1.31–2.20; mean, 1.76) found is close to that reported for sea water ratio (1.8; Prodi et al. 2009). However, in an area with a lot of industrial plants, the major contribution of Na may be attributed to the coal combustion process instead of its marine origin. A closer examination of this ratio, as a function of seasons, reveals mean Cl-/Na+ ratio of 1.99, 1.48, 1.44, and 1.98, during winter, spring, summer, and fall, respectively. For the PM2.5 aerosols, the mass ratio of Cl-/Na+ was very high (ranges, 1.08–7.23; mean, 3.27) clearly indicating their anthropogenic sources (Wang and Shooter 2001).
K+/Na+ ratio is higher than that reported for sea water (0.036; Prodi et al. 2009) for both PM2.5–10 and PM2.5 fractions. Excess K+ in this investigation may be attributed to the combustion of biomass (e.g., wood for domestic heating) or engine exhaust (lubricant additive). In addition, K+ is a major constituent of fertilizers and is generally present in windblown soil. Vegetation is another possible source for K+ (Prodi et al. 2009).
The mass ratios of SO 2-4 /Na+ varied from 1.95 to 5.72 and 3.08 to 25.86, with the mean values of 3.78 and 12.52 for PM2.5–10 and PM2.5, respectively, which is much higher to that reported for sea water ratio (0.25; Prodi et al. 2009). Therefore, sulfate can be considered as prevalently from anthropogenic activities (e.g., SO2 emissions).
We also calculated the ratio of nitrate to sulfate in the PM2.5–10 and PM2.5 aerosols. The mass ratio of NO -3 /SO 2-4 has been used as an indicator of the relative importance of mobile vs. stationary sources of sulfur and nitrogen in the atmosphere (Arimato et al. 1996; Yao et al. 2002; Xiao and Liu 2004). Arimato et al. (1996) ascribed high NO -3 /SO 2-4 mass ratios to the predominance of mobile source over stationary sources of pollutants. It ranged from 0.69 to 1.92 (PM2.5–10: mean, 1.24; SD 0.32) for PM2.5–10 and 0.16 to 0.62 (PM2.5: mean, 0.37; SD 0.10), which is higher than the value in Qingdao (0.35; Hu et al. 2002), Taiwan (0.20; Fang et al. 2002), and much higher than that in Guiyang (0.13; Xiao and Liu 2004). The high value is probably related to both the increase in vehicle and population. The results indicated that with the recent and rapid modernization and motorization of Raipur, the pollutants from vehicle emissions (mobile sources) become severe compared with a few years ago (Deshmukh et al. 2010). However, NO -3 /SO 2-4 was lower than 1 in PM2.5 revealing that stationary emissions are still the important contributors to fine atmospheric particles and particles from vehicular emissions were smaller.
Seasonal variation of water-soluble inorganic ions in PM2.5–10 and PM2.5
The data on seasonal variations of the individual ionic species are presented in Table 3. Generally, for PM2.5–10, all the ions have higher concentrations in winter and spring, and low concentrations in fall. Coefficient of variance (CV), defined as the standard deviation (SD) divided by the average concentration, was used to study the variations of the major ions among seasons. It indicated that the seasonal variations were more obvious for Na+, Ca2+, and Mg2+ than other ions. It was interesting to see that variations in concentrations of these ions in PM2.5–10 were almost similar to those in PM2.5 in all seasons.
As the tracers of soil/dust, Na+, Ca2+, and Mg2+ were frequently observed of higher concentrations in spring and summer, as the dry weather in this season was favorable for the resuspension of soil particles. The concentrations of these crustal ions were low in fall season for both PM2.5–10 and PM2.5, which could be due to large precipitation in this season.
The concentration of Cl- in PM2.5–10 and PM2.5 aerosols was higher in winter than that in spring and summer, and lower in fall. The major source of Cl- in Raipur might be due to coal burning. The seasonal variation of K+ was similar to Cl-, indicating that both Cl- and K+ were primarily related to the burning activities, which were enhanced in the winter season. The seasonal variation of secondary components, i.e., SO 2-4 , NO -3 , and NH +4 were similar (Fig. 5). The high SO 2-4 in winter could be due to the higher concentration of SO2 in this season due to the larger coal burning. The lower temperature in winter would favor the shift from the gas phase of nitric acid to the particle phase of ammonium nitrate (Wang et al. 2006), which could lead to a high concentration of NO -3 in winter. It is well known that an agricultural activity is an important source of ammonia (Intergovernmental Panel on Climate Change IPCC 2007). The higher level of NH +4 in winter is likely due to the much more extensive agricultural activities around the sampling site.
A Spearman correlation analysis
The relationship between PM, water-soluble inorganic ions, and meteorological parameters during the study period were investigated by Spearman correlation analysis. The correlation coefficients (r) between weekly mean PM, ionic species, and meteorological parameters are shown in Table 6. The correlation coefficients were marked in the table to indicate the significant levels (p < 0.01 or p < 0.05). The results showed that the meteorological parameters were all negatively correlated with PM2.5–10 and PM2.5, as observed in other studies (Deshmukh et al. 2010), indicating the prevalence of local sources. Resuspended soil dust under unstable atmospheric conditions (low pressure and high wind speed) during the study period might explain the cause of negative correlations between PM concentrations and vapor pressure (Tsai and Kuo 2005). The relatively low temperature and wind speed in winter season favor the formation of the permanent temperature inversion, stable atmosphere, and low mixed boundary layers, which would then lead to the accumulation of pollutants. The relative humidity and wind speed were two most important factors affecting the concentration of the measured aerosol components. The secondary species (NH +4 , SO 2-4 , and NO -3 ) showed strong positive correlation with relative humidity (p < 0.01) and negative correlation with wind speed (p < 0.01). Besides, other measured species showed relatively weak negative correlations with temperature, clearly indicating that higher temperature would be against the accumulation of pollutants.
Correlation coefficients between water-soluble inorganic species analyzed in PM2.5–10 and PM2.5 aerosols are shown in Table 6. For PM2.5–10, significantly strong paired association were found between species Na+–Mg2+, Na+–Ca2+, NH +4 –K+, NH +4 –Cl-, NH +4 –NO -3 , NH +4 –SO 2-4 , K+–NO -3 , K+–Cl-, K+–SO 2-4 , Cl-–SO 2-4 , Mg2+–Ca2+, and NO -3 –SO 2-4 . However, moderate correlations were observed between pairs of ions such as Na+–NH +4 , Na+–NO -3 , and Ca2+–Cl-. Analysis of these highly correlated species suggests their identical sources of origin. However, those species, which are not significantly correlated with each other, suggest their diverse source of origin. As shown in Table 6, for PM2.5, significant high correlation was observed between species NH +4 –K+, NH +4 –NO -3 , NH +4 –SO 2-4 , K+–Cl-, K+–SO 2-4 , Mg2+–Ca2+, Cl-–SO 2-4 , and NO -3 –SO 2-4 , whereas moderate paired association was observed between species Na+–NH +4 , Na+–Mg2+, and Ca2+–NO -3 . The close relationship among Ca2+ and Mg2+ showed their origin mainly from crustal resuspension. The important fact is that the samples were collected at a location around which there were many cement plants. Therefore, calcium might be largely from cement plants that are established in the surroundings of the sampling location. NO -3 is emitted in enormous concentration by vehicular emissions. The significant and high correlation between K+ and Cl- may indicate the similar anthropogenic origin of these species, which is normally related with coal burning. A poor correlation between Na+ and Cl- for both PM2.5–10 and PM2.5 aerosols confirm the origin of Na+ and Cl- as from non-sea-salt substances.
Principal component analysis
In order to study the origin of these aerosols and the components dominating it, factor analysis was performed. Factor loading for each variable was calculated by principal component analysis. In general, factor loading 0.70 are considered significant in source apportionment studies. The factor loadings with the variance accounted by the various factors for Eigen value >1 for PM2.5–10 and PM2.5 aerosol data set are presented in Table 7. Only factor loading >0.70 was included in Table 7; smaller loading (<0.70) was considered to be insignificant.
The variables of chemical species in the coarse fraction may be explained on the basis of three common factors accounted for 94.0% of the total variance in the entire set of the data. Factor 1 has high loading of Na+, NH +4 , K+, Cl-, and SO 2-4 and explains 50.7% of the total variance. This factor can be attributed to regional pollution arising from coal-burning-like activities (Na+, K+, Cl-, SO 2-4 ) and agricultural practices (NH +4 ) in this region. Factor 2 explains 25.3% of the total variance in the system and can be attributed to airborne road dust, since it is highly loaded with Mg2+ and Ca2+. However, as discussed above, it is most likely that Mg2+ and Ca2+ are dominated by resuspension of local materials and influenced by contaminated soil from the cement production industries at Raipur. Industrial source of calcium emissions include cement manufacturing, iron and steel manufacturing, and power production plants (Deshmukh et al. 2010). Finally, Factor 3 explains an additional 18.0% of the total variance and could be identified as traffic emissions since it is highly loaded with NO -3 . The station was located close to a highway. Although fuel contains sulfur, sulfate production from SO2 takes several hours and favored only under light conditions. This is the reason that NO -3 was separated from SO 2-4 , as NO -3 production is much faster than SO 2-4 .
The results of factor analysis for the PM2.5 mode are also presented in Table 7. The variables of chemical species in the fine fraction may be explained on the basis of three common factors, elucidating 83.2% of the total variance of the system. Factor 1, consisting of high loading of Na+, NH +4 , K+, SO 2-4 , and Cl-, explains 33.2% of the total variance, and it may be attributed to regional pollution. Factor 2 has high loading of Mg2+ and Ca2+, and accounts for 31.6% of the total variance of the system and hence may be attributed to airborne road dust. Finally, factor 3 clarifies further 18.4% of the total variance. This factor may be ascribed to traffic emissions as it has high loading for NO -3 .
Conclusions
This study was sought to measure the concentration and seasonal variation and to investigate the possible origin of the inorganic ions like Na+, NH +4 , K+, Mg2+, Ca2+, Cl-, NO -3 , and SO 2-4 in particles collected at Raipur, eastern central India, during the period of October 2008 to September 2009. The annual average concentration of PM2.5–10 and PM2.5 were 200.7 and 185.9 μg/m3, respectively. Frequencies of PM2.5 atmospheric aerosol particulate matter concentration exceeding 65 μg/m3 for PM2.5 at Raipur were 100% in all seasons. PM2.5 particles were the major part of PM10 particles, as the ratios of PM2.5/PM10 were 0.51, 0.48, 0.44, and 0.46 in winter, spring, summer, and fall, respectively.
Ca2+ is the dominant species followed by NO -3 and SO 2-4 in PM2.5–10 aerosols. In the PM2.5 aerosols, SO 2-4 is the dominant species followed by Ca2+ and NO -3 . As the tracers of soil/dust, Ca2+ and Mg2+ were frequently observed of higher concentrations in spring and summer, since the dry weather in this season was favorable for the resuspension of soil particles. The concentrations of Cl- and K+ in PM2.5–10 and PM2.5 aerosols were higher in winter, indicating their primary relation to heating activities, which were enhanced in the cold season. The secondary components NH +4 , NO -3 , and SO 2-4 show high concentrations in winter and low concentrations in fall for both PM2.5–10 and PM2.5 aerosols. For both PM2.5–10 and PM2.5 aerosols, all the ions show low concentrations in monsoon due to large precipitation in this season.
The application of factor analysis gave a clear indication of the origin of inorganic ions from both anthropogenic and natural sources such as industrial process, incomplete combustion of hydrocarbons and automobile exhaust. Factor analysis has identified three components/sources in the PM2.5–10 and PM2.5 modes, namely, regional pollution, airborne dust, and traffic emissions. Photochemical and traffic sources become more significant with the motorization in recent year. The data on mass concentration and ionic composition for coarse and fine aerosols were higher than those reported in some selected sites in the world, as in Table 4, due to the heavy traffic and other local anthropogenic activities around the sampling site. This indicated that aerosol pollution is a much serious issue in Raipur, India.
References
Akyuz A, Cabuk H (2009) Meteorological variations of PM2.5/PM10 concentrations and particle associated polycyclic aromatic hydrocarbons in the atmospheric environment of Zonguldak, Turkey. J Hazard Mater 70:13–21
Alastuey A, Querol X, Rodriguez S, Plana F, Lopez-Soler A, Ruiz C, Mantilla E (2004) Monitoring of atmospheric particulate matter around sources of secondary inorganic aerosol. Atmos Environ 38:4979–4992
Arimato R, Duce RA, Savoie DL, Prospero JM, Talbot R, Cullen JD, Tomza U, Lewis NF, Ray BJ (1996) Relationship among aerosol constituents from Asia and the North pacific during PEM-WEST A. J Geophys Res 101:2011–2023
Bai N, Khazaei M, Van Eedenc SF, Laher I (2007) The pharmacology of particulate matter air pollution induced cardiovascular dysfunction. Pharmacol Ther 113:16–29
Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F (2009) Hospital admission and chemical composition of fine particle air pollution. American J Respir Crit Care Med 179:1115–1120
Bem H, Gallorini M, Rizzio E, Krzeminska M (2003) Comparative studies on the concentrations of some elements in the urban air particulate matter in Lodz City of Poland and Milan, Italy. Environ Inter 29:423–428
Dentener FJ, Carmichael GR, Zhang Y, Lelieveld J, Crutzen PJ (1996) Role of mineral aerosol as a reactive surface in the global troposphere. J Geophys Res 101:22869–22889
Deshmukh DK, Deb MK, Verma SK (2010) Distribution patters of coarse, fine and ultrafine atmospheric aerosol particulate matters in major cities of Chhattisgarh. Indian J Environ Prot 30:184–197
Fang G, Chang C, Wu Y, Fu PP, Yang C, Chen C, Chang S (2002) Ambient suspended particular matters and related chemical species study in Taiwan, Taichung during 1998–2001. Atmos Environ 36:1921–1928
Franklin M, Koutrakis P, Schwartz P (2008) The role of particle composition on the Association between PM2.5 and Mortality. Epidemiology 19:680–689
Harrison RM, Yin J (2000) Particulatematter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ 249:85–101
Hien PD, Bac VT, Thinh NTH (2004) PMF receptor modeling of fine and coarse PM10 in air masses governing monsoon conditions in Hanoi, Northern Vietnam. Atmos Environ 38:189–201
Ho KF, Lee SC, Chan CK, Yu JC, Chow JC, Yao XH (2003) Characterization of chemical species in PM2.5 and PM10 aerosols in Hong Kong. Atmos Environ 37:31–39
Hu M, He L, Zhang Y, Wang M, Kim YP, Moon KC (2002) Seasonal variation of ionic species in fine particles at Qingdao. China, Atmos Environ 36:5853–5859
Intergovernmental Panel on Climate Change (IPCC) (2007) IPCC fourth assessment report 2007. Cambridge University Press, London
Kim K, Lee M, Lee G, Kim Y, Youn Y, Oh J (2002) Observation of aerosol-bound ionic composition at Cheju Island, Korea. Chemos 48:317–327
Kocak M, Mihalopoulos N, Kubilay N (2007) Contributions of natural sources to high PM10 and PM2.5 events in the eastern Mediterranean. Atmos Environ 41:3806–3818
Kouyoumdjian H, Saliba NA (2006) Mass concentration and ion composition of coarse and fine particles in an urban area in Beirut: effect of calcium carbonate on the absorption of nitric and sulfuric acids and the depletion of chloride. Atmos Chem Phys 6:1865–1877
Kulmatov R, Hojamberdiev M (2010) Distribution of heavy metals in atmospheric air of the arid zones in Central Asia. Air Qual Atmos Health 3:183–194
Kulshrestha A, Bisht DS, Masih J, Massey D, Tiwari S, Taneja A (2009) Chemical characterization of water-soluble aerosols in different residential environments of semi arid region of India. J Atmos Chem 62:121–138
Kulshrestha UC, Saxena A, Kumar N, Kumari KM, Srivastava SS (1998) Chemical composition and association of size differentiated aerosols at a suburban site in a semi-arid tract of India. J Atmos Chem 29:109–118
Kumar R, Srivastava SS, Kumari KM (2007) Characteristics of aerosols over urban and suburban site of semiarid region in India: seasonal and spatial variations. Aerosol Air Qual Res 7:531–549
Lin M, Stieb DM, Chen Y (2005) Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics 116:E235–E240
Mahmoud A, Alan W, Douglas H (2002) A preliminary apportionment of the sources of ambient PM10, PM2.5 and VOCs in Cairo. Atmos Environ 36:5549–5557
Mkoma SL, Wang W, Maenhaut W (2009) Seasonal variation of water-soluble inorganic species in the coarse and fine atmospheric aerosols at Dar Es Salaam, Tanzania. Nuclear Inst Method in Phys Res B 267:2897–2902
Momin GA, Rao PSP, Safai PD, Ali K, Naik MS, Pillai AG (1999) Atmospheric aerosol characteristics studies at Pune and Thiruvananthpuram during INDOEX programme-1998. Current Sci 76:985–989
Moorthy KK, Nair PR, Krishnamurthy BV (1991) Size distribution of coastal aerosols: effects of local sources and sinks. J App Meteorol 30:844–852
Mori I, Nishikawa M, Tanimura T, Quan H (2003) Change in the size distribution and chemical composition of Kosa (Asian dust) aerosol during long-range transport. Atmos Environ 37:4253–4263
Pakkanen TA, Hillamo RE, Aurela M, Andersen HV, Grundahl L, Ferm M, Persson K, Karlsson V, Reissell A, Royset O, Floisand I, Oyola O, Ganko T (1999) Nordic intercomparison for measurement of major atmospheric nitrogen species. J Aerosol Sci 30:247–263
Parmar RS, Satsangi GS, Kumari M, Lakhani A, Srivastav SS, Prakash S (2001) Study of size-distribution of atmospheric aerosols at Agra. Atmos Environ 35:693–702
Parmeshwaran K, Rajan R, Vijaykumar G, Rajeev K, Moorthy KK, Nair PR, Satheesh SK (1998) Seasonal and long-term variations of aerosol content in the atmosphere mixing region at a tropical station on the Arabian sea coast. J Atmos Solar Terr Phys 60:17–25
Pilli PS, Basu SS, Moorthy KK (2002) A study of PM, PM10 and PM2.5 concentration at a tropical coastal station. Atmos Res 61:149–167
Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K (2002) Lung cancer, cardiopulmonary mortality and long-term exposure to fine particulate air pollution. J Am Med Assoc 287:1132–1141
Prodi F, Belosi E, Contini D, Santachiara G, Matteo LD, Gambaro A, Donateo A, Cesari D (2009) Aerosol fine fractions in the Venice Lagoon: particle composition and sources. Atmos Res 92:141–150
Querol X, Alastuey A, Rodriguez S, Plana F, Mantilla E, Ruiz CR (2001) Monitoring of PM10 and PM2.5 around primary particulate anthropogenic sources. Atmos Environ 35:845–858
Rastogi N, Sarin MM (2005) Chemical characterization of individual rain events from a semi-arid region in India: three-year study. Atmos Environ 39:3313–3323
Rinaldi M, Emblico L, Decesari D, Fuzzi S, Facchini MC, Librando V (2007) Chemical characterization and source apportionment of size-segregated aerosols collected at an urban site in Sicily. Water Air soil Pollut 185:311–321
Rodriguez S, Querol X, Alastuey A, Kallos G, Kakaliagou O (2001) Saharan dust contributions to PM10 and TSP levels in Southern and Eastern Spain. Atmos Environ 35:2433–2447
Ruhling A, Tyler G (2004) Changes in the atmospheric deposition of minor and trace elements between 1975–2000 in south Sweden, as measured by moss analysis. Environ Poll 131:417–423
Salam A, Hossain T, Siddhique MNA, Alam AMS (2008) Characteristics of atmospheric trace gases, particulate matter and heavy metal pollution in Dhaka, Bangladesh. Air Qual Atmos Health 1:101–109
Sharma M, Maloo S (2005) Assessment of ambient air PM10 and PM2.5 and characterization of PM10 in the city of Kanpur, India. Atmos Environ 39:6015–6026
Shi JP, Evans DE, Khan AA, Harrison RM (2001) Source and concentration of nanoparticles (<10 nm diameter) in the urban atmosphere. Atmos Environ 35:1193–1202
Sun Y, Zhuang G, Tang A, Wang Y, Gan Z (2006) Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environ Sci Technol 40:3148–3155
Sun Y, Zhuang G, Wang Y, Han L, Guo J, Dan M, Zhang W, Wang Z, Hao Z (2004) The air-born particulate pollution in Beijing—concentration, composition, distribution and sources. Atmos Environ 38:5991–6004
Tiwari S, Srivastava AK, Bisht DS, Bano T, Singh S, Behura S, Srivastava MK, Chate DM, Padmanabhamurty B (2009) Black carbon and chemical characteristics of PM10 and PM2.5 at an urban site of north India. J Atmos Chem 62:193–209
Tsai YI, Kuo SC (2005) PM2.5 aerosol water content and chemical composition in a metropolitan and a coastal area in Southern Taiwan. Atmos Environ 39:4827–4839
Venkataraman C, Reddy CK, Josson S, Reddy MS (2002) Aerosol size and chemical characteristics at Mumbai, India, during the INDOEX-IFP (1999). Atmos Environ 36:1979–1991
Wang G, Haung L, Gao S, Wang L (2002) Characterization of water soluble aerosols in urban area in Nanjing, China. Atmos Environ 36:1299–1307
Wang H, Shooter D (2001) Coarse-fine and day-night differences of water-soluble ions in atmospheric aerosols collected in Christchurch and Auckland, New Zealand. Atmos Environ 36:3519–3529
Wang H, Wang G, Gao S, Wang L (2003) Characteristics of atmospheric particulate pollution in spring in Nanjing City. China Environ Sci 23:55–59
Wang Y, Zhuang G, Tang A, Yuan H, Sun Y, Chen S, Zheng A (2005) The ion chemistry and source of PM2.5 aerosol in Beijing. Atmos Environ 39:3771–3784
Wang Y, Zhuang G, Zhang X, Huang K, Xu C, Tang A, Chen J, An Z (2006) The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos Environ 40:2935–2952
Xiao H, Liu C (2004) Chemical characteristics of water soluble components in TSP over Guiyang, SW China, 2003. Atmos Environ 38:6297–6306
Yao X, Chan CK, Fang M, Cadle S, Chan T, Mulawa P, He K (2002) The water soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos Environ 36:4223–4234
Zhuang H, Chan CK, Fang M, Wexler AS (1999) Formation of nitrate and non-sea salt sulfate on coarse particles. Atmos Environ 33:4223–4233
Acknowledgment
We are thankful to Head, Pt. Ravishankar Shukla University, Raipur, India, for providing necessary laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deshmukh, D.K., Deb, M.K., Suzuki, Y. et al. Water-soluble ionic composition of PM2.5–10 and PM2.5 aerosols in the lower troposphere of an industrial city Raipur, the eastern central India. Air Qual Atmos Health 6, 95–110 (2013). https://doi.org/10.1007/s11869-011-0149-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-011-0149-0