Opinion statement
The single agent activity of PARP inhibitors (PARPi) in germline BRCA mutated (gBRCAm) breast and ovarian cancer suggests untapped potential for this new class of drug in breast cancer. The US Food and Drug Administration has approved three PARPi (olaparib, rucaparib, and niraparib) so far to treat certain ovarian cancers, including those with gBRCAm and olaparib for treatment of gBRCAm breast cancers. Several PARPi are now under clinical development for breast cancer in the various treatment settings. Recently, two phase III trials of olaparib (OlympiaD) and talazoparib (EMBRACA) demonstrated 3-month progression-free survival improvement with PARPi compared to physician’s choice single agent chemotherapy in metastatic gBRCAm breast cancer. To date, PARPi seems less efficacious in metastatic breast cancer patients than those with BRCA mutated platinum-sensitive recurrent ovarian cancer, perhaps reflecting the biologic heterogeneity and low somatic BRCA mutation rate in breast cancer. The use of PARPi is gradually evolving, including combination strategies with chemotherapy, targeted agents, radiotherapy, or immunotherapy in women with and without gBRCAm. The role of predictive biomarkers, including molecular signatures and homologous recombination repair deficiency scores based on loss of heterozygosity and other structural genomic aberrations, will be crucial to identify a subgroup of patients who may have benefit from PARPi. An improved understanding of the mechanisms underlying PARPi clinical resistance will also be important to enable the development of new approaches to increase efficacy. This is a field rich in opportunity, and the coming years should see a better understanding of which breast cancer patients we should treat with PARPi and where these agents should come in over the course of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
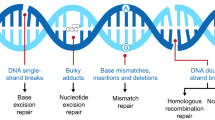
Over the past few decades, significant advances have been made in understanding the genetic causes of familial breast cancers, including cloning of the BRCA1 and BRCA2 genes in the mid-1990s [1]. The essential role of BRCA1 and BRCA2 proteins in homologous recombination repair (HRR), a high-fidelity DNA double-strand break (DSB) repair mechanism, has been extensively documented [2]. Loss of function of the BRCA proteins is thought to contribute to genetic instability, potentially leading to malignancy [3••]. BRCA1 and BRCA2 mutations account for about 10% of all breast cancers and about 30% of hereditary breast cancers [1]. Individuals who harbor germline BRCA1 or BRCA2 mutation (gBRCAm) are at much greater risk of developing breast and/or ovarian cancer over their lifetime: 45–65 and 15–40% for breast and ovarian cancer, respectively [4, 5]. A majority of patients with gBRCA1m that develop breast cancer have tumors that lack estrogen receptor (ER), progesterone receptor (PR), and do not have amplification of human epidermal growth factor 2 (HER2), so called triple negative breast cancer (TNBC). By contrast, only ~ 15% of sporadic breast cancers are TNBC [6•]. Most patients with gBRCA2m who develop breast cancer have tumors that express ER and/or PR in proportions similar to sporadic breast cancer [7, 8].
The seminal advance since the cloning and recognition of the relationship between gBRCAm and breast and ovarian cancers is the identification and application of new important molecular targets, poly-(ADP ribose) polymerase (PARP) family members, and other proteins involved in HRR [9, 10]. Of the 17 PARP family proteins, PARP1 and/or PARP2 are required to repair DNA single-strand breaks (SSBs) and PARP1 also is involved in repair of DSBs and replication fork injury [11]. The PARP-1 enzyme has been implicated in signaling DNA damage through its ability to recognize and rapidly bind to DNA SSBs; it mediates base excision repair by recruiting the scaffolding proteins, e.g., XRCC1, DNA ligase III, and DNA polymerase ß [12]. DNA-bound activated PARP-1 uses nicotinamide adenine dinucleotide (NAD+) to poly-ADPribosylate nuclear target proteins, at the site of DNA damage, including topoisomerases, histones, and PARP-1 itself, to signal the need for both DNA SSB and DSB repair. This observation suggests that inhibition of PARP-1 activity where HRR is compromised would lead to adverse consequences for the tumor cells. PARP inhibitor (PARPi) also traps PARP1 and PARP2 while in complex with damaged DNA, and trapped PARP prevents its participation in DNA repair, resulting in cytotoxic consequences for the cells [13]. This mechanism of action may be important to the clinical activity and toxicity of the PARPi class [13].
The clinical use of PARPi identified the integral role of BRCA1 and BRCA2 in maintaining functional high-fidelity DNA repair through HRR. The single agent PARPi activity in BRCA mutant ovarian cancer treatment suggests untapped potential for this new class in gBRCAm breast cancer. Additionally, there is a potential therapeutic role for PARPi in a wider subgroup of breast cancer that may have defective DNA repair, e.g., mutations in ATM, ATR, PALB2, or CHEK2 [14]. Accumulating evidence suggests that further clinical exploration of PARPi as monotherapy or combinations is warranted in patients not only with gBRCAm-associated breast cancer, but also in breast cancer with HRR dysfunction [14]. Here, we briefly review the preclinical data and clinical development of PARPi and discuss its future development in breast cancer.
PARPi in breast cancer: preclinical evidence
The clinical utility of PARPi as monotherapy in gBRCAm-associated tumors is based on the concept of synthetic lethality, where neither PARP inhibition alone nor BRCA deficiency alone is lethal but the combination is [15]. In a series of pivotal preclinical studies, PARPis were noted to cause selective cytotoxicity for in vitro and in vivo models of BRCA-deficient cells [16, 17]. Bryant et al. were the first to document this finding, showing that the PARPi NU1025 and AG14361 were profoundly cytotoxic in V-C8 (BRCA2-deficient) cells but did not affect V79 (BRCA2-expressing) cells [17]. They observed similar cytotoxic effects of NU1025 in the MCF7 and MDA-MB-231 breast cancer cell lines following siRNA-induced BRCA2 depletion in these cells [17]. Farmer and colleagues also reported that PARPi KU0058684 and KU0058948 exhibited particularly cytotoxic effects in mouse embryonic stem cell lines deficient in either BRCA1 or BRCA2 [16].
The concept of using PARPi as single agents to induce cell death through synthetic lethality represented a novel approach to cancer treatment but may not be the only mechanism by which PARPi could improve cancer therapy. When used in combination therapy, PARPi enhanced the effectiveness of conventional treatments by impairing the repair of damage caused by those agents (e.g., impeding repair of SSB induced by radiotherapy or platinum agents) [18,19,20,21,22,23]. Donawho et al. showed that the PARPi ABT-888 (veliparib) potentiated cytotoxicity of cisplatin and carboplatin and led to tumor regression in BRCA1 and BRCA2 mutated MX-1 breast xenograft model [21]. Other groups have reported similar findings supporting the efficacy of PARPi/platinum therapy in BRCA1 and BRCA2 deficient mammary tumors and in TNBC cell lines [18, 22, 23]. Additionally, other chemotherapeutics such as gemcitabine, temozolomide, and topoisomerase-1 inhibitors have been investigated in combination with PARPis in BRCA-mutated TNBC cell lines, yielding significant reduction in tumor cell replication and increased DNA damage [23,24,25]. Taken together, these preclinical studies have helped the development of clinical trials investigating the benefit of PARPi and platinum agents or other cytotoxic agents.
Recently, targeted agents, e.g., phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitors or cell cycle checkpoint regulators, have been explored preclinically and clinically in combination with PARPi [26, 27]. Combining a PARPi (olaparib or veliparib) with a PI3K inhibitor (NVP-BKM120) has shown synergistic cytotoxicity in both BRCA1-mutated and BRCA wild-type TNBC models [27, 28]. Proteins involved in cell cycle checkpoint pathways, particularly cell cycle checkpoint kinase (CHK)1 or WEE1, also emerged as therapeutic targets as the loss of cell cycle checkpoint control leads to the accumulation of DNA damage and ultimately cell death [29,30,31]. Booth et al. showed that combining any one of four different PARPis (olaparib, veliparib, rucaparib, NU1025) with a CHK1 inhibitor (CHK1i; AZD7762, LY2603618, UCN-01) increased SSBs and DSBs in both BRCA wild-type and BRCA-mutated breast cancer cell lines [24, 31]. Thus, utilizing a PARPi/CHKi strategy may have a broader clinical applicability in breast cancer, independent of gBRCAm status.
Targeting growth factor receptors is also under preclinical and clinical investigation [24]. The epidermal growth factor receptor (EGFR) is mutated in a variety of different cancers, including various subtypes of breast cancer [32]. EGFR-activating mutations often result in receptor amplification, which is targetable via monoclonal antibodies or small molecule tyrosine inhibitors [33, 34]. Sui et al. reported a markedly enhanced antitumor effect of PARPi/EGFR inhibitor therapy (olaparib and erlotinib) compared to each treatment alone in BRCA wild-type EGFR-overexpressing ovarian cancer xenograft models (A2780 cells). These results encourage the expanded use of this therapy to a subgroup of breast cancer containing EGFR amplifications [35]. Furthermore, the insulin-like growth factor type 1 receptor (IGF-1R) is involved in tumorigenesis and shown to exhibit hyper-activation in BRCA1-mutation-associated breast cancers [36,37,38]. Preclinical studies have shown BRCA1-deficient breast and ovarian cancer cell lines to be particularly vulnerable to IGF-1R inhibitors (IGF-1Ri), and PARPi/IGF-1Ri combination therapy resulted in a synergistic cytotoxic effect on these cells [39]. However, despite these promising preclinical results, this approach has yet to be implemented in a clinical setting.
Many of the most significant advances in cancer therapy have recently aimed at stimulating the immune system to participate in tumor cell killing [40]. These approaches have expanded the fundamental role of PARPi in the treatment of cancer, as PARPi has immunomodulatory activity. Huang et al. showed that BMN 673 (talazoparib) significantly increased the number of CD8+ T cells and NK cells in the microenvironment and the production of IFN-gamma and TNF-alpha by lymphocytes in BRCA1-deficient ovarian cancer murine models (BR5FVB1-Akt) [41]. PARPi (olaparib, talazoparib or rucaparib) upregulated PD-L1 expression in breast cancer in vitro and in vivo models, partly due to inactivation of GSK3β [42]. Subsequent blockade of PD-L1 resensitized PARPi-treated cancer cells to T cell killing, yielding greater tumor regression with the combination therapy in breast cancer mouse models [42]. Taken together, these findings highlight the role of PARPi in cellular processes unrelated to DNA damage repair and emphasize the need for further investigation into the immunoregulatory effects of PARPi therapy in breast cancer.
Clinical development of PARPi in breast cancer
Five PARPis are in clinical development, olaparib, rucaparib, niraparib, talazoparib, and veliparib. The first three listed are the United States (US) Food and Drug Administration (FDA)-approved PARPis for specific indications in ovarian cancer. Several of PARPis are now under clinical development for breast cancer, with some showing clinical activity in gBRCAm breast cancer, and olaparib has recently been approved by the FDA for use in gBRCAm, HER2-negative metastatic breast cancer who have been treated with chemotherapy either in the neoadjuvant, adjuvant, or metastatic setting. Overall, PARPis have been less efficacious in BRCA wild-type patients with breast cancer than in those with ovarian cancer, perhaps reflecting the biological heterogeneity and low somatic BRCA mutantion rate in breast cancer [43]. In gBRCAm recurrent ovarian cancer, PARPi activity correlates with platinum sensitivity [44]; higher response rates (RRs) were reported in platinum-sensitive recurrent gBRCAm ovarian cancer compared with platinum-resistant disease (approximately 48 vs. 28% overall RR) [45]. It is unclear whether platinum sensitivity plays the similar role in breast cancer setting.
PARPi therapy in breast cancer: clinical experiences
A number of clinical trials have reported partial or complete results of PARPi treatment in breast cancer patients, which are summarized in Table 1. PARPis have been studied in monotherapy and in combination with radiotherapy or cytotoxic chemotherapy [24]. The clinical benefit of combining PARPi with cytotoxic chemotherapy or radiotherapy yielded improved efficacy; however, increased adverse events have been a challenge for further development [57, 58, 60]. In phase I/Ib studies of olaparib and carboplatin [58, 59], olaparib schedules had to be changed to interrupted use of the PARPi with carboplatin every 3 weeks, with resumption of continuous daily use of olaparib in the maintenance phase after stopping carboplatin. All other PARPi combination trials showed the increased hematological toxicity in the combination therapies, as well as fatigue and gastrointestinal toxicities [61,62,63,64, 66, 67].
Olaparib
Olaparib is the first US FDA and European Medicines Agency (EMA)-approved PARPi for use in gBRCAm ovarian cancer and now FDA approved for gBRCAm breast cancer [68, 69]. Olaparib was also granted breakthrough therapy designation by the US FDA for treatment of gBRCAm or ATM-mutated metastatic castration-resistant prostate cancer [68]. Olaparib is available in two types of formulations, capsule and tablets [70]. Comparative bioavailability studies demonstrated that 400 mg twice daily capsule formulation is equivalent to 200–250 mg twice daily tablet formulation [59, 71, 72]. Olaparib is rapidly absorbed, with peak plasma concentration of 1–3 h post-ingestion and mean half-life of 6.1 h [46••]. Good reviews have been published recently describing its biology and clinical development in ovarian cancer; therefore, it will not be summarized here [73,74,75]. In earlier studies, the clinical benefit of olaparib was observed in advanced breast cancer patients with gBRCAm [46••]. Olaparib activity was shown to be dose-dependent, with a reported RR of 41% with 400 mg twice daily vs. RR 22% with 100 mg twice daily in gBRCAm carriers with advanced/recurrent triple negative or hormone receptor positive breast cancer [47]. Recently, Robson et al. reported the findings of the randomized, open-label, phase III OlympiAD trial in which they compared olaparib alone with standard chemotherapy in patients with gBRCAm, HER2-negative, metastatic breast cancer [50••]. Two thirds of patients received one or two prior lines of chemotherapy for metastatic disease. They received olaparib (300 mg tablets twice daily) or standard ‘physician’s choice’ chemotherapy (capecitabine, eribulin, or vinorelbine) with 2:1 randomization. Olaparib was clinically superior to the standard therapy with median progression-free survival (PFS; 7.0 vs. 4.2 months; p < 0.001) and RR (59.9 vs. 28.8%) [72]. The impact of prior exposure to platinum agents, whether PARPi induce cross-resistance to the subsequent chemotherapy such as other DNA damaging agents, and the long-term risks and benefits are unclear.
There are limited data on combination trials of PARPi and targeted therapies. Michalarea et al. reported preliminary data on the phase I trial of olaparib and an oral AKT inhibitor, AZD5363, in which 16 breast cancer patients were enrolled [61]. Four of eight gBRCAm breast cancer and one of eight sporadic TNBC had RECIST response to the combination therapy. Another phase I study of the PI3K inhibitor BKM120 and olaparib (300 mg tablets twice daily) was reported, in which 24 breast cancer patients (13 TNBC and 11 hormone receptor positive and HER2-negative) were enrolled, including 15 gBRCAm carriers [76]. Of the 18 evaluable patients, five (28%) had partial response and eight (44%) had stable disease. Among 12 gBRCAm carriers of these 18 evaluable patients, four had partial response and five had stable disease. More recently, preliminary results of phase II MEDIOLA study were reported at the 40th San Antonio Breast Cancer Symposium. This single arm, phase II trial evaluated the combination of olaparib and durvalumab, a PD-L1 inhibitor in gBRCAm HER2-negative metastatic breast cancer patients. The combination therapy resulted in 80% (20/25) of disease control rate (defined by CR + partial response + stable disease) at 12 weeks, and 48% (12/25) maintained disease control rate at 28 weeks, with unconfirmed ORR 52% (13/25) [77]. It is unclear how much clinical activity is from PARPi and how much activity is from immune checkpoint inhibition. Future use and clinical trials should take into consideration that immunotherapies may elicit a better immune response if used while the patient is still immunocompetent at earlier stages of the disease course [78].
Talazoparib
Talazoparib is an oral PARPi with a greater PARP-DNA trapping activity compared to other PARPis in preclinical settings [79, 80]. Median peak plasma concentration is 1–2 h post-dose, with mean half-life of 50 h and steady state reached around 2 weeks in most patients taking a recommended phase 2 dose (RP2D) of 1 mg/daily [81]. Early findings from a pilot study of talazoparib demonstrated decrease in tumor volume (median − 78% [range − 30 to − 98%] in all early-stage gBRCAm breast cancer patients (n = 13), treated with talazoparib for 2 months followed by standard neoadjuvant chemotherapy [52]. This study is currently ongoing with a target accrual of 20 patients. More recently, the results of the phase III trial of talazoparib in breast cancer (EMBRACA) were presented at the 40th San Antonio Breast Cancer Symposium. This is the second of four-ongoing phase III clinical trials of PARPis in advanced breast cancer to report findings. gBRCAm carriers with HER2-negative metastatic disease were randomized 2:1 to talazoparib (n = 287) vs. physician’s choice chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine; n = 144). The median PFS was 8.6 months for talazoparib arm vs. 5.6 months for chemotherapy arm (HR = 0.542, p < 0.0001). Overall RR (ORR) was also better in talazoparib arm, with 62.6 vs. 27.2% (HR = 4.99, p < 0.0001) [55]. About 55% of patients in the talazoparib arm experienced grade 3 or 4 hematologic adverse events vs. 38% of those in the physician’s choice chemotherapy arm. It appears that equitoxic doses of high trapping PARPi may result in relatively similar clinical activity to those with less trapping activity [82] and DNA-PARP trapping may also be associated with enhanced toxicity, most often hematologic adverse events.
Veliparib
Veliparib is an oral PARPi-1/2 with a RP2D of 400 mg twice daily when used as single agent [83]. Median peak plasma concentration is 0.5–1.5 h post-dose, with a short half-life, mean of 5 h [84]. Clinical trials of veliparib, either single agent or combinations, are now ongoing for breast cancer in various settings (Table 2). The I-SPY2 trial is a multicenter, phase II trial using Bayesian adaptive randomization as a platform for high-risk patients with stage II/III breast cancer. The patients receive a backbone of standard neoadjuvant therapy, and investigational regimens are added to evaluate pathological complete response (pCR) as a primary endpoint [66]. One of the experimental arms included PARPi, veliparib; patients were randomized to the combination of veliparib and chemotherapy (carboplatin and paclitaxel, followed by doxorubicin plus cyclophosphamide) or standard chemotherapy (paclitaxel alone, followed doxorubicin plus cyclophosphamide) [66]. Patients with HER2-negative breast cancer, with either hormone receptor positive or negative, were enrolled in this part of the I-SPY trial. pCR rates were 51% in veliparib and carboplatin arm, as opposed to 26% in the standard chemotherapy arm in which 17% of patients had deleterious gBRCAm in the experimental arm vs. 5% in the control arm [66]. In a similar way, the phase III BrighTNess study evaluated the addition of carboplatin with and without veliparib to the standard neoadjuvant combination of paclitaxel followed by doxorubicin and cyclophosphamide in 634 TNBC patients. pCR rates increased significantly with the use of carboplatin (53 and 58% in the two arms offering carboplatin vs. 31% without carboplatin), while veliparib added no further benefit to chemotherapy [54].
A phase II trial also enrolled 290 gBRCAm patients with locally advanced or metastatic breast cancer for treatment with the combination of carboplatin and paclitaxel with and without veliparib or a third arm with veliparib and temozolomide [65]. The primary endpoint of PFS was similar between the arms offering carboplatin and paclitaxel (14.1 months with veliparib vs. 12.3 months with placebo, p = 0.227). The ORR was increased by veliparib compared to placebo (77.8 vs. 61.3%, respectively, p = 0.027), without impacting the OS (28.3 vs 25.9 months, respectively, p = 0.156) [65]. Veliparib and temozolomide alone were inferior to the carboplatin and paclitaxel containing arms in ORR, PFS, and OS.
Rucaparib
Rucaparib is a second FDA-approved oral PARPi for use in gBRCAm and somatic BRCA-mutated advanced ovarian cancer [85]. The median peak plasma concentration is reached in 1.9 h and mean half-life is 17–19 h after a RP2D of 600 mg twice daily [86]. Additionally, an intravenous (IV) formulation of rucaparib was investigated in breast cancer patients. Drew et al. reported stable disease only in 44% (8/18) of metastatic breast cancer patients with gBRCAm, treated with IV rucaparib at dose of 18 mg/m2 [51]. The phase I trial of IV rucaparib in combination with chemotherapy (carboplatin, paclitaxel and carboplatin, pemetrexed and cisplatin, or epirubicin and cyclophosphamide) resulted in one CR and one partial response out of seven gBRCAm carriers, in a total of 22 metastatic breast cancer patients enrolled. No further details on clinical or histological characteristics were described in this trial which included other solid tumor patients [63]. The single arm, phase II window of opportunity RIO trial also assesses rucaparib efficacy and biomarkers in sporadic TNBC and gBRCAm breast cancer patients prior to commencing primary neoadjuvant treatment. The primary endpoint is Ki67 response defined as ≥ 50% fall from baseline to end of rucaparib treatment [87] and results are awaited.
The Hoosier Oncology BRE09-146 phase II trial randomized 128 TNBC or known gBRCAm patients with residual disease post-neoadjuvant therapy with anthracycline or taxane to cisplatin alone or cisplatin combined with rucaparib [62]. The primary endpoint of 2-year disease-free survival (DFS) was similar between the two arms (58.3% with cisplatin and 63.1% with cisplatin and rucaparib, p = 0.43). The presence of gBRCAm had no impact in those findings which was partly due to the lower dose used than RP2D of rucaparib and the small sample number (n = 22) of gBRCAm patients enrolled in the trial [62].
Niraparib
Niraparib is a recently FDA-approved PARPi for unselected platinum-sensitive recurrent ovarian cancer patients, with a RP2D of 300 mg daily [88]. Median peak plasma concentration is reached around 3 h post-dosage. The mean elimination half-life of niraparib is 36 h, after daily 300-mg doses [88]. In the phase I study evaluating niraparib in solid tumors, 22 of the 100 patients had metastatic breast cancer, and 2 partial responses were seen in 4 breast cancer patients with gBRCAm, no details of histological subtypes were reported for these 22 breast cancer patients [56].
Initial results from phase I part of TOPACIO trial were recently presented, with good tolerability and RP2D for niraparib in combination with pembrolizumab for treatment of patients with metastatic TNBC and ovarian carcinoma [89]. From the 14 patients enrolled in the phase I, 5 had TNBC and the best response in this group was seen in one BRCA wild-type patient with stable disease for 10 months.
Table 2 summarizes ongoing clinical trials using PARPi.
Safety of single agent PARPi
The side effect profile of PARPi monotherapy presents quite uniformly as gastrointestinal (nausea, vomiting, anorexia, diarrhea), hematological (anemia, thrombocytopenia, neutropenia) adverse events and fatigue. Notably, some adverse events are more commonly observed (> 10%) with certain PARPi, e.g., rucaparib (hepatotoxicity) and niraparib (thrombocytopenia) [90, 91]. It is possible that some differences in the “off-target” profile of different PARPis might contribute to adverse side effects [92]. The potential long-term increased risk of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) has been observed in < 1% of patients enrolled in clinical trials so far [46••, 49, 88]. Most patients in those trials were heavily pretreated, making the exact contribution of PARPi in the development of MDS or AML difficult to assess although it is possible that DSBs caused by PARPi may be accumulated in normal tissues, e.g., bone marrow. Careful hematological evaluation and monitoring for second hematological malignancies are warranted.
Future directions
The marked benefit of PARPi in patients with gBRCAm has validated gBRCAm as a predictive biomarker for PARPi response in breast cancer patients. At present, it remains unclear how to best identify breast cancer patients who will respond better to PARPi beyond gBRCAm status. Although tumor phenotypes can provide some predictions, as evidenced by responses of sporadic TNBC to PARPi monotherapy, the RRs are lower than those with gBRCAm breast or ovarian cancer [43, 93]. Other forms of HRR dysfunction, such as mutations in ATM, ATR, PALB2, or CHEK2, also need further clinical investigations for PARPi in breast cancer treatment settings. Another opportunity for PARPi is the treatment of breast cancer patients with brain metastasis. PARPis (olaparib, veliparib, niraparib) have been described as potentially penetrating the blood-brain barrier [94,95,96], which increases their possible clinical utility in brain metastases-prone TNBC.
To date, many studies have been reported describing the mechanisms of action of PARPi, as well as mechanisms of clinical resistance of PARPi, which were not described in detail here. Some of resistance mechanisms are associated with reversion mutations in BRCA1 or BRCA2 gene, as well as inactivation of DNA repair proteins, e.g., 53BP1 and REV7, or increased activity of RAD51, all known to restore HRR function [2, 97, 98]. The combination therapies would be the appropriate next steps to mitigate the resistance by using two distinct treatments and also to potentiate PARPi activity. Among many PARPi combination trials, our phase 2 basket trial of durvalumab and olaparib is now enrolling TNBC patients with and without gBRCAm to examine the role of neoantigen expression and changes in immune microenvironment induced by PARPi (NCT02484404).
Lastly, it would be critical to design and interpret clinical trials based on the biological hypothesis and robust preclinical data. Understanding more about the molecular abnormalities involved in HRR-deficient tumors, exploring novel therapeutic trial strategies and drug combinations, and defining potential predictive biomarkers, is necessary to rapidly advancing the field of PARPi therapy for breast cancer. This is a field rich in opportunity, and the coming years should see a better understanding of which breast cancer patients we should treat with PARPi and where these agents should come in over the course of treatment.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Economopoulou P, Dimitriadis G, Psyrri A. Beyond BRCA: new hereditary breast cancer susceptibility genes. Cancer Treat Rev. 2015;41(1):1–8.
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–90.
•• Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–8. Recent concise review on the concept of PARPi use in clinic.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast Cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–16.
Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25(43):5885–97.
• Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast Cancer. Clin Cancer Res. 2011;17(5):1082–9. Incidence and relation TNBC and BRCA mutation in breast cancer.
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomark Prev. 2012;21(1):134–47.
Antoniou AC, Kuchenbaecker KB, Soucy P, Beesley J, Chen X, McGuffog L, et al. Common variants at 12p11, 12q24, 9p21, 9q31.2 and in ZNF365 are associated with breast cancer risk for BRCA1 and/or BRCA2 mutation carriers. Breast Cancer Res. 2012;14(1):R33.
Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin Cancer Res. 2007;13(5):1383–8.
Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8(4):363–9.
Pommier Y, O'Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362ps17.
Chaudhuri AR, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610–21.
Murai J, Huang SYN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588–99.
McCabe N, Turner NC, Lord CJ, Kluzek K, Białkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–15.
Lord CJ, Tutt AN, Ashworth A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med. 2015;66:455–70.
Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7.
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AOH, Zander SAL, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105(44):17079–84.
Xu K, Chen Z, Cui Y, Qin C, He Y, Song X. Combined olaparib and oxaliplatin inhibits tumor proliferation and induces G2/M arrest and γ-H2AX foci formation in colorectal cancer. Onco Targets Ther. 2015;8:3047–54.
Nguewa PA, Fuertes MA, Cepeda V, Alonso C, Quevedo C, Soto M, et al. Poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide enhances apoptosis induction by platinum complexes in cisplatin-resistant tumor cells. Med Chem. 2006;2(1):47–53.
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13(9):2728–37.
Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PWB, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14(12):3916–25.
Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70(20):7970–80.
Dréan A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, Curtin NJ, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6(7):2860–7.
Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–56.
Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2(11):1048–63.
Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2(11):1036–47.
Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115.
Lee JM, Ledermann JA, Kohn EC. PARP inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25(1):32–40.
Booth L, Cruickshanks N, Ridder T, Dai Y, Grant S, Dent P. PARP and CHK inhibitors interact to cause DNA damage and cell death in mammary carcinoma cells. Cancer Biol Ther. 2013;14(5):458–65.
Teng YH, et al. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast Cancer Res. 2011;13(2):R35.
Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD, Morse D, et al. United States Food and Drug Administration drug approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10(4):1212–8.
Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin Ther. 2005;27(6):684–94.
Sui H, et al. Combination of erlotinib and a PARP inhibitor inhibits growth of A2780 tumor xenografts due to increased autophagy. Drug Des Devel Ther. 2015;9:3183–90.
Farabaugh SM, Boone DN, Lee AV. Role of IGF1R in breast Cancer subtypes, Stemness, and lineage differentiation. Front Endocrinol (Lausanne). 2015;6:59.
Maor S, Yosepovich A, Papa MZ, Yarden RI, Mayer D, Friedman E, et al. Elevated insulin-like growth factor-I receptor (IGF-IR) levels in primary breast tumors associated with BRCA1 mutations. Cancer Lett. 2007;257(2):236–43.
Hudelist G, Wagner T, Rosner M, Fink-Retter A, Gschwantler-Kaulich D, Czerwenka K, et al. Intratumoral IGF-I protein expression is selectively upregulated in breast cancer patients with BRCA1/2 mutations. Endocr Relat Cancer. 2007;14(4):1053–62.
Amin O, Beauchamp MC, Nader PA, Laskov I, Iqbal S, Philip CA, et al. Suppression of homologous recombination by insulin-like growth factor-1 inhibition sensitizes cancer cells to PARP inhibitors. BMC Cancer. 2015;15:817.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Huang J, Wang L, Cong Z, Amoozgar Z, Kiner E, Xing D, et al. The PARP1 inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(−/−) murine model of ovarian cancer. Biochem Biophys Res Commun. 2015;463(4):551–6.
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances Cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–20.
Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–406.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian Cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9.
Matulonis UA, Penson RT, Domchek SM, Kaufman B, Shapira-Frommer R, Audeh MW, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol. 2016;27(6):1013–9.
•• Fong PC, et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. New England J Med. 2009;361(2):123–34. Proof of concept trial on PARPi and BRCA mutation, with ovarian and breast cancer patients.
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced Cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
•• Robson M, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523–33. First phase III trial of PARPi demonstrating PFS benefit in gBRCAm breast cancer.
Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114(7):723–30.
Litton JK, Scoggins M, Ramirez DL, Murthy RK, Whitman GJ, Hess KR, et al. A pilot study of neoadjuvant talazoparib for early-stage breast cancer patients with a BRCA mutation. Ann Oncol. 2016;27
Turner, N.C., et al., Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO). J Clin Oncol, 2017. 35.
Loibl, S., O'Shaughnessy J., Untch M., Sikov W.M., Rugo H.S., McKee M.D., Huober J., Golshan M., von Minckwitz G., Maag D., Sullivan D., Wolmark N., McIntyre K., Ponce Lorenzo J.J., Metzger Filho O., Rastogi P., Symmans W.F., Liu X., Geyer Jr C.E., Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol, 2018.
• Litton, J., H Ettl, J Hurvitz, S Gonçalves, A Lee, K-H Fehrenbacher, L Yerushalmi, R Mina, L Martin, M Roché, H Im, Y-H Quek, R Tudor, I Hannah, A Eiermann, W Blum, A phase 3 trial comparing talazoparib, an oral PARP inhibitor, to physicians choice of therapy in patients with advanced breast cnacer and a germline BRCA mutation., in San Antonio Breast Cancer Symposium. 2017: San Antonio, TX. Early data of Phase III trial of talazoparib, PARPi showing PFS benefit in gBRCAm breast cancer.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–92.
Dent RA, Lindeman GJ, Clemons M, Wildiers H, Chan A, McCarthy NJ, et al. Phase I trial of the oral PARP inhibitor olaparib in combination with paclitaxel for first-or second-line treatment of patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2013;15(5):R88.
Lee J-M, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of Olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian Cancer with biomarker analyses. Jnci-J Nat Cancer Instit. 2014;106(6)
Lee J-M, Peer CJ, Yu M, Amable L, Gordon N, Annunziata CM, et al. Sequence-specific pharmacokinetic and Pharmacodynamic phase I/Ib study of Olaparib tablets and carboplatin in Women's Cancer. Clin Cancer Res. 2017;23(6):1397–406.
Balmana J, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25(8):1656–63.
Michalarea V, Roda D, Drew Y, Carreira S, O’Carrigan BS, Shaw H, et al. Phase I trial combining the PARP inhibitor olaparib (01a) and AKT inhibitor AZD5363 (AZD) in germline (g)BRCA and non-BRCA mutant (m) advanced cancer patients (pts) incorporating noninvasive monitoring of cancer mutations. Cancer Res. 2016;76:CT010.
Miller K, et al. Cisplatin with or without rucaparib after preoperative chemotherapy in patients with triple negative breast cancer: final efficacy results of Hoosier oncology group BRE09-146. J Clin Oncol. 2015;33(15)
Wilson RH, Evans TRJ, Middleton MR, Molife LR, Spicer J, Dieras V, et al. A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br J Cancer. 2017;116(7):884–92.
Somlo G, et al. Efficacy of the PARP inhibitor (PI) ABT-888 (veliparib vel ) either with carboplatin (carb) or as a single agent followed by post-progression therapy in combination with carb in patients (pts) with BRCA1- or BRCA2-(BRCA)-associated metastatic breast cancer (MBC). J Clin Oncol. 2015;33(15)
Han HS, Diéras V, Robson M, Palácová M, Marcom PK, Jager A, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29(1):154–61.
Rugo HS, Olopade OI, DeMichele A, Yau C, van 't Veer L, Buxton MB, et al. Adaptive randomization of Veliparib-carboplatin treatment in breast Cancer. N Engl J Med. 2016;375(1):23–34.
Han HS, Diéras V, Robson ME, Palácová M, Marcom PK, Jager A, et al. Efficacy and tolerability of veliparib (V; ABT-888) in combination with carboplatin (C) and paclitaxel (P) vs placebo (plc) plus C/P in patients (pts) with BRCA1 or BRCA2 mutations and metastatic breast cancer: a randomized, phase 2 study. Cancer Res. 2017;77:S2–05.
FDA. Approved Drugs. 2017 December 3rd, 2017.].
EMA. Lynparza / Olaparib. 2015 December 3rd, 2017.].
FDA. Olaparib Label. [Cited 2017 December 3rd, 2017].
Molife LR, et al. Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations. J Clin Oncol. 2012;30(15)
Molife LR, Forster MD, Krebs M, Pwint T, Middleton MR, Kaye SB, et al. A phase I study to determine the comparative bioavailability of two different oral formulations of the PARP inhibitor, olaparib (AZD2281), in patients with advanced solid tumors. J Clin Oncol. 2010;28(15):2599.
Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58.
Ledermann JA. PARP inhibitors in ovarian cancer. Ann Oncol. 2016;27:40–4.
Ang YLE, Tan DSP. Development of PARP inhibitors in gynecological malignancies. Curr Probl Cancer. 2017;41(4):273–86.
Matulonis UA, Wulf GM, Barry WT, Birrer M, Westin SN, Farooq S, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol. 2017;28(3):512–8.
Domchek SM, Postel-Vinay S, Bang YJ, Park YH, Alexandre J, Italiano A. Delord JP, You B, Bastian S, Krebs MG, Wang D, Waqar S, Angell HK, and Chung S, Learoyd M, Gresty C, Herbolsheimer P, Kaufman B, An open-label, multitumor, Phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCAm) HER2-negative metastatic breast cancer (MBC), in San Antonio Breast Cancer Symposium. 2017: San Antonio, TX.
Lee JM, Gulley JL. Checkpoint and PARP inhibitors, for whom and when. Oncotarget. 2017;8(56):95036–7.
Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19(18):5003–15.
Murai J, Huang SYN, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with Olaparib and Rucaparib. Mol Cancer Ther. 2014;13(2):433–43.
de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor Talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discovery. 2017;7(6):620–9.
Kohn EC, Lee J-M, Ivy SP. The HRD decision--which PARP inhibitor to use for whom and when. Clin Cancer Res : Off J Am Assoc Cancer Res. 2017;23:7155–7.
Puhalla S, et al. Final results of a phase 1 study of single-agent veliparib (V) in patients (pis) with either BRCA1/2-mutated cancer (BRCA plus ), platinum-refractory ovarian, or basal-like breast cancer (BRCA-wt). J Clin Oncol. 2014;32(15)
Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, et al. A phase I study of Veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18(6):1726–34.
Balasubramaniam S, Beaver JA, Horton S, Fernandes LL, Tang S, Horne HN, et al. FDA approval summary: Rucaparib for the treatment of patients with deleterious BRCA mutation-associated advanced ovarian Cancer. Clin Cancer Res : Off J Am Assoc Cancer Res. 2017;23:7165–70.
Syed YY. Rucaparib: first global approval. Drugs. 2017;77(5):585–92.
Toms C, Chopra N, Houlton L, Jarman K, Kilburn L, Bliss J, et al. Window study of the PARP inhibitor rucaparib in patients with primary triple negative or BRCA1/2 related breast cancer (RIO). Ann Oncol. 2016;27
Scott LJ. Niraparib: First Global Approval. Drugs. 2017;77(9):1029–34.
Konstantinopoulos, P.A., Sachdev J.C., Schwartzberg L., Matulonis U.A., Sun P., Wang J.Y., Guo W., Bobilev D., Aktan G., Karantza V., Dezube B., Vinayak S., Dose-finding combination study of niraparib and pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC) or recurrent platinum-resistant epithelial ovarian cancer (OC) (TOPACIO/Keynote-162). Ann Oncol, 2017. 28.
Coleman RL, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial (vol 390, pg 1949, 2017). Lancet. 2017;390(10106):1948–8.
Mirza, M.R., et al., A randomized, double-blind phase 3 trial of maintenance therapy with niraparib vs placebo in patients with platinum-sensitive recurrent ovarian cancer (ENGOT-OV16/NOVA trial). Ann Oncol, 2016. 27.
Knezevic CE, Wright G, Remsing Rix LL, Kim W, Kuenzi BM, Luo Y, et al. Proteome-wide profiling of clinical PARP inhibitors reveals compound-specific secondary targets. Cell Chem Biol. 2016;23(12):1490–503.
Lee J-M, Hays JL, Chiou VL, Annunziata CM, Swisher EM, Harrell MI, et al. Phase I/Ib study of olaparib and carboplatin in women with triple negative breast cancer. Oncotarget. 2017;8(45):79175–87.
Halford SER, et al. Results of the OPARATIC trial: a phase I dose escalation study of olaparib in combination with temozolomide (TMZ) in patients with relapsed glioblastoma (GBM). J Clin Oncol. 2017;35
Mehta MP, Wang D, Wang F, Kleinberg L, Brade A, Robins HI, et al. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase 1 study. J Neuro-Oncol. 2015;122(2):409–17.
Chornenkyy Y, Agnihotri S, Yu M, Buczkowicz P, Rakopoulos P, Golbourn B, et al. Poly-ADP-ribose polymerase as a therapeutic target in pediatric diffuse intrinsic pontine glioma and pediatric high-grade astrocytoma. Mol Cancer Ther. 2015;14(11):2560–8.
Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422–9.
Montoni, A., Robu M., Pouliot É., Shah G.M., Resistance to PARP-inhibitors in cancer therapy. Front Pharmacol, 2013. 4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alexandra S. Zimmer, Mitchell Gillard, Stanley Lipkowitz, and Jung-Min Lee declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Zimmer, A.S., Gillard, M., Lipkowitz, S. et al. Update on PARP Inhibitors in Breast Cancer. Curr. Treat. Options in Oncol. 19, 21 (2018). https://doi.org/10.1007/s11864-018-0540-2
Published:
DOI: https://doi.org/10.1007/s11864-018-0540-2