Opinion statement
Breast cancer is a heterogeneous disease. While breast cancer mortality has dropped substantially over the past three decades due to early detection and adjuvant systemic therapy (AST), the risk of recurrence is highly dependent upon numerous factors including tumor size, involvement of regional lymph nodes, histologic grade, expression of hormone receptors (estrogen and progesterone), and human epidermal growth factor receptor 2 (HER2) amplification. We use these factors to determine which early breast cancer (EBC) patients should be treated with AST, including endocrine therapy (ET), chemotherapy, and HER2-directed treatments. While these factors aid in this determination, it remains challenging to identify those patients unlikely to benefit from adjuvant chemotherapy, resulting in over-treatment of patients. Given this dilemma, there has been great interest in the development of prognostic and predictive gene expression profiles. The most extensively studied profile, the 21-gene recurrence score (Oncotype Dx®), estimates 10-year risk of breast cancer recurrence in patients with estrogen receptor (ER)-positive, HER2-negative, node-negative EBC and is likely predictive of chemotherapy benefit. This assay has established analytic validity, clinical validity, and clinical utility for this patient group and, therefore, is indicated in this patient population to help inform decisions regarding administration of adjuvant chemotherapy. Several other assays may have utility in this clinical context or perhaps to identify patients who do not require extended adjuvant ET. These assays include the following: PAM 50 Risk of Recurrence (ROR) Score (Prosigna™), Breast Cancer Index, and EndoPredict®.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant systemic therapy for early-stage breast cancer: a success story
Most patients diagnosed with early breast cancer (EBC) are treated initially with primary surgery and radiation therapy if appropriate. In addition to local treatment, patients may be advised to undergo adjuvant systemic therapy (AST). As a result of the broad implementation of screening programs and delivery of effective AST, we have witnessed a substantial decline in breast cancer mortality over the past 30 years [1].
There are three distinct categories of AST routinely administered to women with EBC: endocrine therapy (ET), human epidermal growth factor receptor 2 (HER2)-directed therapy, and chemotherapy. Current guidelines indicate that all patients with estrogen receptor (ER)/progesterone receptor (PR) positive EBC should receive at least 5 years (or more) of adjuvant ET following local treatment [2]. Similarly, almost all patients with HER2-positive EBC are recommended to undergo adjuvant treatment with trastuzumab in addition to chemotherapy, as the addition of HER2-directed therapy improves disease-free and overall survival [3]. Determining which patients should receive adjuvant chemotherapy is more complex, as serious side effects can occur and many patients may not benefit.
Selection of AST for EBC: should all patients receive chemotherapy?
Several studies have demonstrated that the delivery of adjuvant chemotherapy to women with EBC reduces mortality. In the most recent Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, including data from 123 randomized trials, equal relative risk reduction was observed across all prognostic subgroups [4]. This observation, however, does not imply that all patients warrant treatment with adjuvant chemotherapy. The absolute reduction in recurrence and death is higher in patients with worse prognosis, such as those with positive nodes. Of course, almost 100 % of patients receiving chemotherapy suffer bothersome side effects (i.e., hair loss, fatigue, nausea). More importantly, serious and even life-threatening toxicities (i.e., neutropenic fever, bleeding, transfusion requirement, secondary malignancy, congestive heart failure, and peripheral neuropathy) occur in approximately 1–2 % of patients.
These considerations highlight the importance for the clinician to determine whether the absolute benefit of chemotherapy outweighs the 1–2 % absolute risk of serious toxicity. To do so, the clinician must be aware of the following: (1) odds of a subsequent incurable recurrence in the absence of treatment and (2) the relative effect of the chemotherapy (i.e., an estimate of the sensitivity of the therapy, regardless of the risk of recurrence). These two factors can be used to calculate the absolute benefit of treatment (multiply relative odds of breast cancer recurrence by risk reduction from therapy). For example, if a patient has an estimated risk of recurrence of 50 % and chemotherapy reduces this by one third, then the estimated absolute possible benefit is approximately 16–17 % (50 % × 0.33). This estimate of potential benefit, weighed against inevitable bothersome side effects and risk of serious toxicity, clearly justifies the recommendation for that patient to proceed with chemotherapy.
In order to identify patients for whom we can safely recommend withholding adjuvant chemotherapy, it is important to consider both prognostic and predictive factors. Prognostic markers allow us to identify those patients at high risk of metastatic relapse and therefore require treatment that mitigates this risk. Well-established prognostic biomarkers in patients with EBC include tumor stage and grade. Patients with lower stage, lower grade tumors have higher breast cancer-specific survival than those patients with higher stage, higher grade tumors [5, 6]. Predictive markers allow us to estimate which therapies will benefit specific patient groups. ER is the paradigm for a useful predictive factor—approximately 50 % of patients with ER-positive breast cancer benefit from adjuvant ET, while no patients with ER-negative breast cancer will benefit [7]. Many tumor biomarkers are both prognostic and predictive. Again, ER is a good example. In patients who received no AST of any kind, expression of ER is associated with improved overall survival and disease-free survival compared to ER-negative tumors [8, 9]. Similarly, HER-2 over-expression is a marker of poor prognosis when patients are not treated with chemotherapy and HER-2-directed agents, and is predictive of response and benefit from HER-2-directed treatment [3, 10, 11].
Do all breast cancers respond equally to chemotherapy?
Historically, there have been limited predictive biomarkers of chemotherapy benefit. Therefore, the decision to use adjuvant chemotherapy has been based primarily on prognosis. On the basis of prognostic tenets, patients with node-negative, small (<2 cm), grade 1–2, ER-/PR-positive, and HER2-negative tumors were not recommended to receive adjuvant chemotherapy, as the potential benefit was thought to be small [12]. In contrast, chemotherapy was recommended for patients with node-positive, or large, or HER2-positive or ER-/PR-negative tumors.
The Oxford Overview EBCTCG has reported that adjuvant chemotherapy provides an overall proportional reduction in breast cancer recurrence by approximately one third regardless of hormone-receptor status or tumor grade or stage [4]. However, retrospective analyses of prospective trials suggest that response may not be uniform across biological subtypes, particularly for those patients with low-grade, well-differentiated tumors and high expression of hormone receptors [13, 14]. Lippman and colleagues first suggested this relative chemotherapy effect in 1978 when they reported that expression of ER may be a predictor of response to chemotherapy, as those patients with low or absent ER expression had greater objective responses to treatment [15]. These data suggest that a “one size fits all” approach is flawed and that perhaps, we can use information regarding biologic subtypes to inform decisions regarding adjuvant chemotherapy.
Intrinsic subtypes: a short hand for breast cancer biology
Over a decade ago, Perou and colleagues demonstrated that breast cancer could be subdivided into four distinct categories based upon unsupervised patterns of gene expression. They designated these as luminal A, luminal B, HER-2 enriched, and basal like (or claudin-low) [16]. Roughly speaking, these categories correspond to ER-positive tumors with low proliferation (luminal A), ER-positive tumors with high proliferation (luminal B), tumors with HER-2 over-expression (HER-2 enriched), and breast cancers that do not express ER, PR, or HER-2 (basal like, so-called triple negative). This terminology has become a “short-hand” to categorize different breast cancer groups based on biology, much like staging is used to categorize different groups based on anatomy [17]. Given this, there has been great interest in the development of clinically practical assays that approximate these biological intrinsic subtypes, which might then be used to provide both prognostic and predictive information to guide patient care.
Criteria for introduction of tumor biomarker tests into routine clinical practice
If a biomarker assay is utilized to guide clinical care, the clinician must have confidence that the test performs well and that its use is in the best interest of the patient. To address these issues, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative, convened by the Centers for Disease Control, coined three important semantics to provide guidance. These semantics include the following: 1. analytic validity (i.e., how accurately and reliably the assay detects the analyte(s) of interest), 2. clinical validity (i.e., how well the assay can predict the clinical outcome of interest), and 3. clinical utility (i.e., whether there are high levels of evidence demonstrating that the results of the assay provide information that contributes to and improves current optimal management of the patient’s disease) [18]. It should be noted that establishing analytic and clinical validity of gene expression profiles is complex, as it requires simultaneous quantitative measurement of numerous analytes, potentially compromising reproducibility and leading to over-fitting bias [19]. Furthermore, establishing the clinical utility of a biomarker is ideally accomplished through a study in a prospective trial. However, doing so is costly and time-consuming. Simon, Paik, and Hayes have proposed that it is reasonable to utilize a prospective-retrospective design when archival specimens and clinical information from a high-quality dataset, such as a previously conducted prospective trial, are available [20–22].
Gene expression profiles for use in EBC: a critical analysis
Prognostic gene expression profiles have been developed primarily to identify those EBC patients with such favorable prognosis that the benefits of adjuvant chemotherapy do not clearly outweigh the risks. There are several assays currently available for clinical use. These include the following: 21-gene recurrence score (Oncotype Dx®), Amsterdam 70-gene signature (MammaPrint®), Predictor Analysis of Microarray 50 Risk of Recurrence (ROR) score (PAM50-ROR, Prosigna™), Rotterdam 76-gene signature, genomic grade index (GGI), breast cancer index (BCI), and EndoPredict®. Throughout this review, we will refer to the EGAPP framework to evaluate these “omics-based” tumor biomarker assays (Table 1).
The 21-gene recurrence score
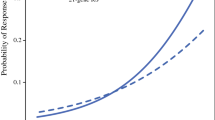
The 21-gene recurrence score (RS) (Oncotype Dx®) was first developed to better extrapolate risk of breast cancer recurrence in patients with ER-positive, HER2-negative, node-negative breast cancer who received 5 years of adjuvant tamoxifen, using reverse-transcriptase (RT)-PCR to measure the messenger RNA (mRNA) levels of genes previously implicated in breast cancer pathogenesis. To identify genes whose expression might predict risk of recurrence, 250 candidate genes were tested across three independent studies involving the group of patients randomized to tamoxifen only in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trial. Expression profiles of genes highly correlated with recurrence across studies were selected for the final gene panel, consisting of 16 cancer-related genes and 5 reference genes. An algorithm is used to generate a RS or quantitative estimate of the 10-year risk of distant recurrence. The RS is reported on a scale of 0 to 100, with result ≤17 indicating low risk of recurrence, 18–30 indicating intermediate risk, and ≥30 indicating high risk of recurrence [23] (Fig. 1). The RS assay has been analytically validated with respect to amplification efficiency, precision, linearity, and dynamic range as well as limits of detection and quantification [24].
Linear fit of the likelihood of distant recurrence as a continuous function of 21-gene RS for the tamoxifen alone (TAM) and tamoxifen plus chemotherapy (TAM + chemo) treatment groups in NSABP B-20. From Paik S et al., “Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer.” J Clin Oncol. 2006:24(23); 3726-3734. Reprinted with permission. © 2006 American Society of Clinical Oncology. All rights reserved.
The 21-gene recurrence score: a prognostic biomarker
Numerous studies have confirmed the clinical validity and utility of the 21-gene RS in node-negative, ER-positive, HER2-negative patients as a prognostic tool. Paik et al. performed the first validation study in a prospective-retrospective fashion, in which RS was determined using fixed, paraffin-embedded tissue from 668 patients enrolled in the tamoxifen-treated arm of the NASBP B-14 trial. In this study, rates of distant recurrence at 10 years in the low-, intermediate-, and high-risk groups were 7, 14, and 31 %, with a statistically significant difference between the low- and high-risk groups [23]. If the proportional risk reduction achieved by administration of adjuvant chemotherapy is approximately one third, as suggested by the most recent EBCTCG study [4], roughly 2 % of patients in the low-risk group may avoid a breast cancer recurrence with chemotherapy treatment (7 % × 0.333 = 2 %). In this scenario, the benefit achieved from chemotherapy is nearly identical to the risk of life-threatening or life-altering side effects, making the use of adjuvant chemotherapy in this prognostic group of questionable value. In contrast, ≥5 % of patients with ER-positive, HER2-negative, node-negative breast cancer whose tumors exhibit a high RS will benefit from chemotherapy, which we believe is sufficiently greater than the 1–2 % of significant toxicity to justify its application. Similar results have been reported in a prospective-retrospective study using archived tissue from the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, indicating that RS can also be utilized as a prognostic tool in patients treated with adjuvant aromatase inhibitor therapy [25, 26]. It remains controversial whether or not patients with an intermediate RS have a sufficiently high risk of recurrence to justify adjuvant chemotherapy. To address this question, the Trial Assigning Individualized Options for Treatment (TAILORx) randomized women with RS of 11–25 to ET alone versus ET plus chemotherapy. The trial has completed accrual, but final results have not been published.
Does the 21-gene recurrence score predict chemotherapy benefit?
In addition to its prognostic value, the RS may also be predictive of relative chemotherapy benefit. In a subsequent analysis of samples from NSABP B-20, RS was retrospectively determined for 651 patients in the tamoxifen-treated and tamoxifen plus chemotherapy-treated arms. The proportional reduction in risk of distant recurrence for patients with high RS treated with chemotherapy was quite high (relative risk = 0.26), whereas risk reduction for patients with low RS was minimal (relative risk = 1.31) [27••] (Fig. 1). These data support the hypothesis first proposed by Lippmann et al.: perhaps, those patients with high RS have a higher proportional reduction in the risk of breast cancer recurrence when treated with chemotherapy than those with low RS. It should be noted, however, that these results may be confounded, as samples from NSABP B-20 were also used to develop the 21-gene RS assay.
Given this apparent predictive role for the 21-gene RS, investigators from SWOG performed a prospective-retrospective analysis of ER-positive, but node-positive, patients enrolled in the SWOG-8814 trial. The results of this study closely resembled those from NSABP B-20, suggesting that low RS was predictive of poor response to adjuvant chemotherapy, in this case cyclophosphamide, doxorubicin, and fluorouracil (CAF) [28]. In contrast, those with high RS experienced substantial benefit from CAF. As this study was small, further studies are needed to confirm the clinical utility of the RS in this context. In the RxPONDER trial (SWOG S1007), which is currently accruing, patients with ER-positive, HER2-negative, non-metastatic disease with one to three involved regional lymph nodes and RS ≤ 25 are randomly assigned to receive ET alone versus ET plus chemotherapy.
In summary, the 21-gene RS has been demonstrated to have analytic validity, clinical validity, and clinical utility as a prognostic tool in patients with ER-positive, HER2-negative, node-negative tumors treated with ET. Given the above, the use of the 21-gene RS in this context has been endorsed by the American Society of Clinical Oncology Recommendations for the Use of Tumor Markers in Breast Cancer as well as the National Comprehensive Cancer Network Clinical Practice Guidelines for Breast Cancer [29, 30]. The assay may also be predictive of benefit from adjuvant chemotherapy (Table 2). However, this concept remains under investigation in a prospective randomized controlled trial being conducted by the Southwest Oncology Group (the Rx PONDER trial), which is designed to determine whether or not chemotherapy can be safely withheld from some patients with involved axillary lymph nodes.
Amsterdam 70-gene assay
The Amsterdam 70-gene signature (MammaPrint®) was developed to determine prognosis in patients with EBC regardless of hormone receptor status or HER2 amplification. The assay was formulated using supervised DNA microarray analysis on frozen tissue from 98 highly selected primary breast tumors to generate a 70-gene signature predictive of short interval to the development of distant metastases [31]. Based upon the results, patients are classified as “good prognosis” or “poor prognosis.” More recently, this 70-gene prognosis profile has been translated into a customized array (MammaPrint®) for use in a high-throughput setting, and has been demonstrated to have reasonable analytic validity [32, 33].
Following the original report by van’t Veer et al. [31], the clinical validity of the 70-gene profile as a prognostic tool has been examined in numerous studies. For example, van de Vijver and colleagues performed a retrospective analysis of frozen tumor samples from patients with T1-T2 node-negative or node-positive breast cancer with 7 years of clinical follow-up data. It should be noted, however, that some of these cases were included in the original study by van’t Veer, and many participants received adjuvant therapy (either hormonal or chemotherapy), potentially confounding the results. Those patients with good prognosis gene signatures had survival rates of approximately 95 %, whereas those patients with poor prognosis gene signatures had survival rates of 55 %, with significantly increased risk of distant metastases at 10 years [34]. In a subsequent retrospective study, the 70-gene assay was more predictive of time to distant metastases and overall survival than clinical factors (using Adjuvant! Online), but not of disease-free survival [35]. Furthermore, the hazard ratios for high- versus low-risk groups were of substantially lower magnitude than previously reported in the original van’t Veer or van de Vijver studies.
While the above data are suggestive that the 70-gene signature may yield reliable prognostic information, the clinical utility of this assay has never been tested in a prospective or an adequate prospective-retrospective fashion; therefore, more studies are required before utilizing this assay in routine clinical practice. Indeed, an international study, the Microarray in Node-Negative Disease May Avoid Chemotherapy trial (MINDACT), has now completed accrual and will help to determine if the 70-gene assay should be used in this context. In this study, those patients with discordant clinical (using Adjuvant! Online) and genomic predictions were randomly assigned to receive or not to receive adjuvant chemotherapy. Until the results of this study are available, the 70-gene assay should not be utilized to inform patient management unless used in the context of a clinical trial.
Predictor analysis of microarray 50 risk of recurrence score
The PAM50 Breast Cancer Intrinsic Classifier was developed to catalog tumors according to “intrinsic subtype” (i.e., luminal A, luminal B, HER2 enriched, basal like, and normal like). The original intrinsic subtypes were established via microarray gene expression patterns across the entire genome [16], a logistically challenging platform. In an effort to make this concept clinically applicable, Parker and colleagues developed an intrinsic subtype classifier based upon the expression patterns of 50 genes to classify patients into these same categories [36]. They also reported the generation of a risk of recurrence (ROR) score (Prosigna™), which uses an algorithm incorporating gene signature, intrinsic subtype, and tumor size to place patients into high-, intermediate-, and low-risk groups [36]. The analytic validity of the ROR score was recently demonstrated in a study that confirmed the analytic precision as well as reproducibility of the assay across three different laboratories [37].
The clinical validity and clinical utility of this assay as a prognostic tool has also been well established in numerous prospective-retrospective studies. For example, in an analysis of patients enrolled in the NCIC Clinical Trials Group MA.12 study, RNA was isolated from formalin-fixed paraffin-embedded (FFPE) tumor samples of premenopausal women with stages I–III primary breast cancer previously randomized to 5 years of tamoxifen therapy (regardless of ER status) versus placebo after receiving adjuvant chemotherapy. In this study, the PAM50 ROR assay was prognostic for both disease-free (p < 0.0002) and overall survival (p < 0.0003) [38]. In a separate prospective-retrospective study of the ATAC trial by Dowsett et al., ROR was determined from approximately 1000 post-menopausal patients treated with adjuvant tamoxifen or anastrazole. The ROR score had a continuous relationship with risk of distant recurrence at 10 years in node-negative and node-positive patients across breast cancer subtypes [39••]. In this same study, the ROR score was also compared with the 21-gene RS; results indicated that the ROR score classified more ER-positive, HER2-negative, node-negative patients into the high-risk group and fewer patients into the intermediate-risk group than the RS [39••]. Finally, Gnant and colleagues reported that in 1400 postmenopausal patients with ER-positive EBC treated with tamoxifen alone or tamoxifen followed by anastrazole (ABCSG-8 trial), the luminal A cohort had a significantly higher proportion of patients free of distant recurrence than the luminal B cohort at 10 years (94 versus 82 %, p < 0.0001) [40]. In a subsequent analysis using samples from this same trial, ROR score risk groups were also demonstrated to be predictive of late distant recurrence (5–15 years from original diagnosis) [41•].
Numerous prospective-retrospective studies have established the PAM50 ROR score as a clinically valid and useful prognostic tool in ER-positive, HER2-negative, node-negative breast cancer. Although there are no studies directly correlating ROR score with benefit from adjuvant chemotherapy, it has been compared in a rigorous fashion to the 21-gene RS [39••], indicating that ROR score may also be useful in this capacity.
Rotterdam 76-gene signature
The Rotterdam signature was developed using microarray data from approximately 100 frozen archived patient samples, all with node-negative EBC, whom had not received AST. Of note, both ER-positive and ER-negative samples were included. From the analysis, a 76-gene set was identified that separated patients into “good signature” and “poor signature” groups [42]. The assay was retrospectively clinically validated in a separate data set including 171 patients. The signature was highly predictive of patients that would develop distant metastases within 5 years (HR = 5.67, 95 % CI = 2.59–12.4), even when corrected for traditional prognostic factors [42]. The Rotterdam signature has also been retrospectively validated in two additional data sets (each containing approximately 200 patient samples), from node-negative patients who did not receive AST. These studies yielded similar results, noting that 10-year distant metastasis-free survival rates were above 90 % for the good profile groups and approximately 70 % for the poor profile groups [43, 44].
While these data imply that the 76-gene assay may have clinical validity as a prognostic tool, the analytic validity and clinical utility of this assay have not been confirmed. The above studies do suggest that similar prognostic results can be obtained in different laboratories using different data sets; however, no studies have addressed the issue of reproducibility within the same tumor samples. In addition, there have been no prospective randomized or prospective-retrospective studies (using samples from a previously conducted clinical trial) to substantiate the observations from retrospective analyses.
Genomic grade index
Although the histologic grade of breast carcinomas is known to provide prognostic information (i.e., high grade portending a worse prognosis), use of this data has historically been challenging to interpret for two primary reasons: (1) there are often inconsistencies in the determination of histologic grade between pathologists and institutions and (2) it is unclear how to determine the prognosis of patients with grade 2 tumors, representing the majority of breast cancers. Given this, GGI was developed to grade tumors more accurately. To develop the assay, samples from 64 ER-positive tumors ranging in histologic grade from 1 to 3 were retrospectively analyzed to detect differentially expressed genes. The resultant 97-gene assay was validated using data from nearly 600 samples to determine if an association existed between GGI and relapse-free survival. Sotiriou and colleagues found that among patients with histologic grade 2 tumors, a high GGI score was associated with a higher risk of recurrence than a low GGI score (HR = 3.61, 95 % CI = 2.25–5.78) [45]. In a subsequent study of untreated or tamoxifen-treated ER-positive patients, Loi et al. reported that GGI distinguished two prognostic molecular subtypes and also strongly correlated with the 21-gene RS algorithm [46]. Furthermore, in a prospective-retrospective study of 204 patient samples from the PACS01 trial, Bertucci and colleagues found that GGI was more indicative of prognosis than standard histologic grade, Ki67 mRNA expression, and IHC as well as mitotic activity index [47].
GGI may also be predictive of chemotherapy responsiveness. In a study published by Liedtke et al., gene expression data was obtained prospectively from 229 fine-needle aspirate samples prior to patients receiving neoadjuvant chemotherapy. The authors observed high correlation between high GGI and pathologic response to chemotherapy among patients with both ER-positive and ER-negative tumors. High GGI score was also associated with worse distant relapse-free survival in patients with ER-positive disease [48].
While these data suggest clinical utility for GGI as both a prognostic and predictive tool, the prospective-retrospective studies reported to date have been small. Furthermore, there have been no studies directly correlating GGI with benefit of adjuvant chemotherapy or directly assessing the analytic validity of the assay. Larger prospective-retrospective studies are needed before GGI should be routinely used to determine prognosis or guide adjuvant chemotherapy decisions in clinical practice.
Breast cancer index
The BCI was developed to identify those ER-positive EBC patients who are at highest risk of distant recurrence despite adjuvant ET. Gene expression profiles were generated retrospectively for a group of 60 patients treated with adjuvant tamoxifen. The gene expression signature that resulted was further reduced to a two-gene ratio, HOXB13 versus IL17BR, found to be highly predictive of distant recurrence in this patient population [49]. This ratio was further investigated in a larger retrospective study of 1252 ER-positive frozen primary tumor samples from patients treated with adjuvant tamoxifen. Jansen and colleagues reported that a high HOXB13-to-IL17BR ratio correlated with both tumor aggressiveness and tamoxifen therapy failure [50]. The BCI has since been modified to also incorporate the 5-gene molecular grade index (MGI).
In a study published by Sgroi et al., the prognostic ability of the BCI was compared to the 21-gene RS and IHC4, an immunohistochemistry analysis of four standard markers (ER, PR, HER2, Ki67 index). In this prospective-retrospective analysis, 665 ER-positive, node-negative archival tumor blocks from the ATAC trial were obtained for analysis. The study determined that while BCI was predictive of late (10-year) distant recurrence in this patient population (HR = 1.95, 95 % CI = 1.22–3.14), RS and IHC4 were not, potentially identifying a population of patients that may benefit from extended ET [51•]. In addition, two prospective-retrospective studies involving samples from approximately 600 ER-positive, node-negative patients enrolled in the Stockholm TAM trial also found that BCI was predictive of late recurrence [52, 53].
The prospective-retrospective analyses described above suggest the analytic and clinical validity, and perhaps clinical utility, of the BCI as a prognostic tool. It is of particular interest in regard to identification of those ER-positive patients who may not require extended ET beyond 5 years.
Endopredict
The endopredict (EP) assay was developed for use in ER-positive, HER2-negative patients with EBC, with the goal of identifying those patients who have a low risk of recurrence without adjuvant chemotherapy. EP measures expression levels of 11 genes via RT-PCR. The analytic validity of this assay has been confirmed in two studies, where EP scores were highly correlative across multiple laboratories and matched samples [54, 55]. The EP assay has been clinically validated in over 1000 patient samples from two large randomized trials, ABCSG-6 and ABCSG-8 [56]. In a subsequent analysis, EPclin scores (combining EP result with tumor size and nodal status) identified a subgroup of patients who did not receive ET after 5 years of treatment but who had an excellent long-term prognosis, suggesting as for the BCI assay described above, that they might not need extended ET [57•]. In a recent prospective-retrospective study conducted by Martin and colleagues using 1246 samples from patients with ER-positive, HER2-negative breast cancer treated with adjuvant chemotherapy (either fluorouracil, epirubicin, and cyclophosphamide (FEC) or FEC followed by 8-weekly treatments of paclitaxel), EP scores were highly predictive of metastasis-free survival in both low-and high-risk groups [58•].
The EP assay has also been compared directly to the 21-gene RS in a small study, where the authors noted a major discrepancy between the EP and RS results in six cases. In each case, those patients categorized as low risk by 21-gene RS were deemed high risk by the EP assay [59]. To date, there have been no direct comparisons between the EP assay and other gene expression profiles.
EP has been demonstrated to have analytic validity, clinical validity, and clinical utility as a prognostic tool and, therefore, can be utilized for this purpose in clinical practice.
Conclusion
Gene expression profiling in EBC can provide valuable information beyond standard clinical and histopathologic factors. At the present time, 21-gene RS is the only assay with proven clinical validity and utility as a prognostic tool and as a predictor of benefit from adjuvant chemotherapy. The PAM50 ROR score, breast cancer index, and EndoPredict® assays also have established clinical validity and utility in determining prognosis, which can also inform decisions regarding administration of adjuvant therapy. Given this, these tests are indicated in patients with ER-/PR-positive, HER2-negative, node-negative EBC. Clinical trials are underway to determine if the 21-gene RS or other assays of intrinsic subtype may also be used to identify those women with ER-positive, HER2-negative breast cancer with positive axillary lymph nodes who may not benefit from adjuvant chemotherapy. Finally, several studies have begun to assess whether one or more of these assays can identify patients who have received 5 years of adjuvant ET and do not require further, extended therapy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92.
Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69.
Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4, CD006243.
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44.
Newman LA. Epidemiology of locally advanced breast cancer. Semin Radiat Oncol. 2009;19:195–203.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84.
Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Bartlett JM, Brookes CL, Robson T, et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol. 2011;29:1531–8.
Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–704.
Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049–56.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–68.
Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–67.
Lippman ME, Allegra JC, Thompson EB, et al. The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med. 1978;298:1223–8.
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Hayes DF. Targeting adjuvant chemotherapy: a good idea that needs to be proven! J Clin Oncol. 2012;30:1264–7.
Teutsch SM, Bradley LA, Palomaki GE, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3–14.
Institute of Medicine: Evolution of translational omics: lessons learned and the path forward. Washington, D.C., The National Academies Press, 2012.
Hayes DF, Bast RC, Desch CE, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88:1456–66.
Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52.
Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100:1432–8.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–91.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–34.
Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–83.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. In this prospective-retrospective analysis of patients enrolled in NSABP-B20, patients with high 21-gene RS had a higher proportional reduction in risk of distant recurrence when administered adjuvant chemotherapy than those patients with low RS. This study supports the hypothesis that benefit from chemotherapy may be impacted by tumor biology.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Version 3.2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed January 19, 2015.
van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Ach RA, Floore A, Curry B, et al. Robust interlaboratory reproducibility of a gene expression signature measurement consistent with the needs of a new generation of diagnostic tools. BMC Genomics. 2007;8:148.
Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278.
van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Buyse M, Loi S, van’t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–92.
Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
Nielsen T, Wallden B, Schaper C, et al. Analytical validation of the PAM50-based Prosigna Breast Cancer Prognostic Gene Signature Assay and nCounter Analysis System using formalin-fixed paraffin-embedded breast tumor specimens. BMC Cancer. 2014;14:177.
Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18:4465–72.
Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90. Clinical validity and prognostic utility of the ROR score were demonstrated in this study, where ROR score was found to have a continuous relationship with risk of distant recurrence at 10 years.
Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–45.
Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res. 2014;20:1298–305. Prospective-retrospective analysis of patients with ER-positive EBC treated with adjuvant tamoxifen or adjuvant tamoxifen followed by anastrazole (ABCSG-8 trial). ROR score risk group was predictive of late (5–15 years) distant recurrence in these patients.
Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9.
Desmedt C, Piette F, Loi S, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007;13:3207–14.
Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–71.
Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–72.
Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–46.
Bertucci F, Finetti P, Roche H, et al. Comparison of the prognostic value of genomic grade index, Ki67 expression and mitotic activity index in early node-positive breast cancer patients. Ann Oncol. 2013;24:625–32.
Liedtke C, Hatzis C, Symmans WF, et al. Genomic grade index is associated with response to chemotherapy in patients with breast cancer. J Clin Oncol. 2009;27:3185–91.
Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–16.
Jansen MP, Sieuwerts AM, Look MP, et al. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: a retrospective study. J Clin Oncol. 2007;25:662–8.
Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067–76. Prospective-retrospective analysis of patients with ER-positive, node negative EBC enrolled in the ATAC trial, comparing BCI to 21-gene RS and IHC4. BCI was predictive of late recurrence in this study, whereas 21-gene RS and IHC4 were not, potentially identifying a population of patients that may not benefit from extended adjuvant ET.
Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res. 2013;19:4196–205.
Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer. 2011;104:1762–9.
Denkert C, Kronenwett R, Schlake W, et al. Decentral gene expression analysis for ER+/Her2− breast cancer: results of a proficiency testing program for the EndoPredict assay. Virchows Arch. 2012;460:251–9.
Muller BM, Brase JC, Haufe F, et al. Comparison of the RNA-based EndoPredict multigene test between core biopsies and corresponding surgical breast cancer sections. J Clin Pathol. 2012;65:660–2.
Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–20.
Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–64. In this study, EPclin scores identified a subgroup of patients with an excellent long-term prognosis with 5 years of adjuvant ET, potentially identifying a population of patients that may not benefit from extended adjuvant ET.
Martin M, Brase JC, Calvo L, et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res. 2014;16:R38. Prospective-retrospective study including over 1,200 samples from patients with ER-positive, HER2-negative EBC treated with adjuvant chemotherapy, demonstrating that EP scores were highly predictive of metastasis-free survival in both high and low risk groups.
Varga Z, Sinn P, Fritzsche F, et al. Comparison of EndoPredict and Oncotype DX test results in hormone receptor positive invasive breast cancer. PLoS One. 2013;8, e58483.
Compliance with Ethics Guidelines
Conflict of Interest
Erin F. Cobain declares that she has no conflict of interest.
Daniel F. Hayes declares that he has no conflicts relative to this manuscript. However, he has received research funding from Janssen; he has served as a paid consultant to Eli Lilly and Pfizer; and the University of Michigan holds and has licensed a patent in his name regarding use of circulating tumor cells.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Cobain, E.F., Hayes, D.F. Indications for Prognostic Gene Expression Profiling in Early Breast Cancer. Curr. Treat. Options in Oncol. 16, 23 (2015). https://doi.org/10.1007/s11864-015-0340-x
Published:
DOI: https://doi.org/10.1007/s11864-015-0340-x