Abstract
Among women, breast cancer accounts for one-third of cancer cases and is the second most frequent cause of death. Improvements in treatment agents and screening procedures have increased the diagnosis of early breast cancer and survival rates. Adjuvant chemotherapy and endocrine treatment decrease the mortality of early breast cancer by approximately 50 %. However, not all early breast cancer patients benefit equally from adjuvant endocrine treatment and/or chemotherapy. Patients at high risk are classically identified based on clinicopathological factors, such as age, tumor size, histopathological grade, nodal status, hormone and HER2 receptor positivity, and menopausal status. However, for patients with early breast cancer, using these standard clinicopathological factors might not thoroughly show the individual risk of disease recurrence and the benefits from adjuvant systemic chemotherapy. Many patients with early breast cancer do not derive benefit from adjuvant systemic chemotherapy. Quality-of-life issues, acute and long-term side effects of systemic chemotherapy, and the cost of unnecessary treatments are the main factors of concern for this group of patients. Quantitative approaches for defining prognoses and for individualizing treatments are required. In recent years, molecular signatures of gene expression have been correlated with breast cancer recurrence risk. Several tests for genomic expression have been developed and validated on specimens from previous phase III studies to improve the prognostication of early breast cancer patients and/or the prediction of adjuvant systemic treatment. The most commonly used genomic expression-based tests used for prognostic information and for the prediction of chemotherapy benefits in early breast cancer are summarized below.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Breast cancer accounts for one-third of cancer cases among women and is the second most frequent cause of death. [1] It consists of heterogeneous subtypes that differ in clinical presentation and disease course. Improvements in treatment agents and screening procedures have increased the diagnosis of early breast cancer and survival rates. Chemotherapy, endocrine treatment (ET), and trastuzumab comprise the main armamentarium for the adjuvant treatment of breast cancer. Adjuvant chemotherapy and ET decrease the mortality of early breast cancer by approximately 50 % [2]. However, early breast cancer patients do not benefit equally from adjuvant ET and/or chemotherapy. The recurrence risk of disease for hormone receptor-positive early breast cancer with tamoxifen after surgery is 15 % in 10 years, and the survival benefit of adjuvant chemotherapy in the same group of patients is 3–10 % [3, 4]. Patients at high risk are classically identified based on clinicopathological factors, such as age, tumor size, histopathological grade, nodal status, hormone and HER2 receptor expression, and menopausal status. However, using these standard clinicopathological factors might not thoroughly show the individual risk of disease recurrence and the benefit from adjuvant systemic chemotherapy for early breast cancer patients. Many early breast cancer patients do not benefit from adjuvant systemic chemotherapy [5]. Quality-of-life issues, acute and long-term side effects of systemic chemotherapy, and cost of the unnecessary treatments are the main concerns for this group of patients. During the past two decades, the level of knowledge regarding the molecular pathways and underlying genetic changes in breast cancer has improved. However, treatment decisions still often rely on classical histopathological and immunohistochemical techniques. Numerous biomarkers have been studied to define the residual risk of recurrence, but none of them have been recommended for routine use [6–11].
Quantitative approaches for defining prognosis and individualization of treatments are thus required. In recent years, molecular signatures of gene expression have been correlated with the risk of breast cancer recurrence [12–15]. Questions of reproducibility and the need for fresh or fresh-frozen tissue have limited their clinical application. A number of genomic expression tests were developed regarding this unmet medical need. Several genomic expression tests have been developed and validated on specimens of previous phase III studies to improve the prognostication of early breast cancer patients and/or the prediction of the utility of adjuvant systemic treatment (Table 11.1) [16]. In this chapter, using satisfactory data from the literature, we review the most commonly used genomic expression-based tests for predicting the prognosis of and chemotherapy benefit for early breast cancer.
Gene Array Tests
Oncotype DX
The Oncotype DX (Genomic Health, Inc., Redwood City, CA) uses real-time reverse-transcriptase chain reaction (RT-PCR) to quantify the expression levels of 21 genes in formalin-fixed, paraffin-embedded (FFPE) breast cancer tissue samples [17]. The test, which is regulated by Clinical Laboratory Improvement Amendments (CLIA) and the College of American Pathologists (CAP), is performed in a central laboratory in the USA. This assay uses a calculation model to generate a risk score ranging between 0 and 100 based on 5 reference and 16 cancer-related genes. The reference genes, GAPDH, ACTB (β-actin), RPLPO, GUS, and TFRC, are used for normalization. The cancer-related genes used in the assay include genes related to proliferation (Ki-67, Survivin, MYBL2, CCNB1 [cyclin B1], and STK15), invasion (CTSL2 [cathepsin L2] and MMP-11 [stromolysin 3]), and hormone receptors (ER, PR, BCL2, and SCUBE2) as well as HER2 (GRB7 and HER2), GSTM1, BAG1, and CD68.
A multistep approach was adopted for developing the assay for expression levels of tumor-related genes by the Genomic Health researchers. Routinely used tumor blocks were used for this purpose and to validate the assay. High-throughput RT-PCR was used to quantify gene expression levels in FFPE tumor tissue sections [18]. In the second phase, they chose 250 candidate genes from the published literature, genomic databases, and experiments based on DNA arrays performed on fresh-frozen tissue [12–14, 19]. In the third phase, data from 447 breast cancer patients from 3 different studies, including patients from the tamoxifen-only arm of the NSABP B-20 trial, were used to test the correlation of 250 candidate genes with the recurrence of breast cancer [20–22]. In the fourth phase, a panel of 16 cancer-related genes and 5 reference genes were selected from the results of three studies based on the strength of their performance in the previous studies and the consistency of the primer and probe performance in the assay. An algorithm was designed based on the expression of these 21 genes for computing a recurrence score (RS) for each tumor sample [17]. The possible RSs ranged between 0 and 100, where a higher recurrence score indicated a higher likelihood of recurrence. The RS was derived from the reference-normalized expression measurements of the 16 cancer-related genes. Reproducibility within and between blocks was also assessed. Based on their RS, patients were divided into 3 risk categories, including low (<18), intermediate (18–30), and high (≥31). The RS was prognostic for estrogen receptor (ER)-positive early breast cancer patients with positive (1–3 lymph nodes involved) and negative lymph node involvement who were treated with tamoxifen. A low RS predicted no likelihood of recurrence in 10 years and little benefit from chemotherapy. Adjuvant chemotherapy showed benefits in high-RS patients but not in patients with a low RS.
Oncotype DX was tested in a community-based population from Northern California [23]. The 21-gene assay was prognostic in ER-positive patients with and without tamoxifen treatment (p = 0.003 and p = 0.03, respectively). There were 220 patients and 570 controls in this study. Archived tumor tissues were tested. Nearly 50 % of the patients were in the low-risk group. The risk for death from breast cancer within 10 years in tamoxifen-treated patients was 2.8 (95 % confidence interval (CI): 1.7–3.9), 10.7 (95 % CI: 6.3–14.9), and 15.5 % (95 % CI: 7.6–22.8) in the low-, intermediate-, and high-risk patients, respectively. The risk of death within 10 years in patients with no tamoxifen treatment was 6.2 (95 % CI: 4.5–7.9), 17.8 (95 % CI: 11.8–23.3), and 19.9 (95 % CI: 14.2–25.2) in low-, intermediate-, and high-risk patients, respectively.
The Oncotype DX assay was further tested in hormone receptor-positive early breast cancer patients from Japan [24]. All patients were treated with tamoxifen. Among the total patients, 280 patients had tumor tissues that were adequate for the assay. Of these patients, 48 % were in the low-risk group, 20 % were in the intermediate-risk group, and 33 % were in the high-risk group. Distant recurrence risks in 10 years were 3.3 (95 % CI: 1.1–10), 0, and 24.8 % (95 % CI: 15.7–37.8) in low-, intermediate-, and high-risk lymph node-negative patients, respectively. Differences between the low-risk and high-risk groups for distant recurrence were significant (log rank p < 0.001). There was also a significant difference in overall survival (OS) between the low-risk and high-risk groups (p = 0.008).
Pivotal Trials of Oncotype DX
NSABP B14 Trial
The data obtained from the large, multicenter NSABP B14 trial and the FFPE tumor tissues were used to validate this 21-gene RT-PCR assay for RS detection in early stage, node-negative, ER-positive breast cancer patients, who had been treated with tamoxifen [17]. The RS was calculated as low, intermediate, or high for each patient, as previously defined. The data from the prospective NSABP B14 trial were used for validation. Cutoff points were determined based on the results of the NSABP B14 study. According to the study results, the rates of recurrence in 10 years were 6.8 (95 % CI: 4–9.6), 14.3 (95 % CI: 8.3–20.3), and 30.5 % (95 % CI: 23.6–38.4) for the low-, intermediate-, and high-risk groups, respectively. The risk of recurrence in the high-risk group was similar to that for the lymph node-positive patients [25]. In this study, 51 % of the patients were in the low-risk group, and 27 % of the patients were in the high-risk group. Age and tumor size are standard factors used for predicting recurrence. However, when RS was added to the multivariate Cox model, recurrence could no longer be predicted based on age or tumor size. Moreover, all patients with tumors smaller than 1 cm (N = 109) were not in the low-risk group. Forty-four of these patients were in the intermediate- or high-risk groups, which means a risk of 15–20 % for recurrence in 10 years. A subgroup of the patients with low-grade tumors also showed high RS and high rates of recurrence in this study. Additionally, concordance of pathologists was moderate for poorly differentiated tumors and low for the well- and intermediate-differentiated tumors. HER2 amplification was detected in 55 of 668 patients (8 %). The 10-year recurrence was 75 % (95 % CI: 63.2–86.9) in HER2-amplified and 86 % (95 % CI: 83.1–88.9) in HER2-nonamplified breast tumors (p = 0.08). In the Cox model including RS and classical factors (estrogen, progesterone receptors, HER2 DNA amplification), any RS was a significant predictor of distant recurrence. The RS provided significant predictive power independent of age or tumor size (p < 0.001). The RS was also prognostic (p < 0.001) and could be used as a continuous function to predict the recurrence in each patient [17]. It is important to note that all patients were treated with tamoxifen; thus, outcomes must be evaluated considering the effects of both tamoxifen and the natural disease course.
TransATAC Trial
ATAC is a phase III trial that included 9366 postmenopausal early breast cancer patients, including both ER-positive and ER-negative patients. This study compared 5 years of adjuvant treatment with tamoxifen alone, anastrozole alone, and tamoxifen in combination with anastrozole [26]. In the translational arm of the ATAC study (TransATAC), the risk of recurrence was evaluated using the Oncotype DX assay in axillary node-negative or node-positive, hormone receptor-positive postmenopausal breast cancer patients who were treated with tamoxifen and anastrozole. RNA was extracted from 1372 tumor blocks from patients in the monotherapy arms of this study. Available scores were obtained from 1231 patients (node positive, N = 306; node negative, N = 872; node status unknown, N = 52). Multivariate analysis showed that RS was significantly associated with time to disease recurrence (p < 0.001 and p = 0.002 for node-negative and node-positive patients, respectively). There was a poor correlation between RS and “Adjuvant! Online” for estimating prognosis (p < 0.001). In node-negative patients, the disease recurrence rate within 9 years was 4, 12, and 25 % for the low-, intermediate-, and high-risk groups, respectively. In node-positive patients, the 9-year disease recurrence rates were 17, 28, and 49 % for the low-, intermediate-, and high-risk groups, respectively. The hazard ratio (HR) for disease recurrence was 2.7 for high-RS and 1.8 for low-RS lymph node-positive patients. Similar results were obtained for OS. For any RS, the risk of distant recurrence was higher in node-positive than node-negative patients and in patients with 4 or more positive nodes than in patients with 1–3 positive nodes. Prognostic value was similar in the tamoxifen and anastrozole groups. In the original study, anastrozole showed a 16 % risk reduction for distant recurrence compared with tamoxifen [27]. However, in this study, HRs for distant recurrence were similar in the tamoxifen and anastrozole treatment arms, and RS did not interact with any treatment arm. Relative risk reductions for anastrozole compared with tamoxifen were similar in all RS groups. A higher risk reduction with anastrozole in patients with high RS might be expected. However, the number of cases was too small to allow for such an analysis.
Studies of Prediction for Chemotherapy
NSABP B20 Trial
In the original NSABP B20 trial, there were 2363 ER-positive, axillary lymph node-negative early breast cancer patients [28]. The aim was to examine the benefit of CMF (cyclophosphamide, methotrexate, 5-fluorouracil) or MF (methotrexate, 5-fluorouracil) chemotherapy followed by 5 years of tamoxifen treatment. The Oncotype DX assay was studied in these tumor blocks, and prospective clinical outcomes were investigated in that study group to examine whether the Oncotype DX assay could predict the benefits of chemotherapy. Tumor blocks with sufficient tumor tissue for the RS assay were obtained in 670 patients and adequate samples were obtained from 651 tumor blocks. Of the total patients, 227 (29.5 %) were treated with tamoxifen, and 424 (27.7 %) were treated with chemotherapy plus tamoxifen. Among the 651 assessable patients, the proportions of patients without distant recurrence in 10 years were 92.2 % in the chemotherapy plus tamoxifen group and 87.8 % in the tamoxifen group. Disease recurrence (locoregional or distant) was observed in 90.1 % of the chemotherapy plus tamoxifen patients and in 83.5 % of the tamoxifen patients. The 10-year survival estimate was 89.5 % in patients treated with chemotherapy plus tamoxifen and 86.4 % in patients treated with only tamoxifen. There were 353 (54.2 %) patients with low RS, 134 (20.6 %) with intermediate RS, and 164 (25.2 %) with high RS.
This study showed that the benefit of chemotherapy was not equivalent across all ER-positive and axillary lymph node-negative early breast cancer patients in the NSABP B20 study. The Oncotype DX assay was shown to predict the chemotherapy (CMF or MF) benefit in this group of patients. The magnitude of the benefit of chemotherapy for distant recurrence was greater for the high-RS group than for the intermediate- and low-RS groups. A Kaplan-Meier estimate indicated that 10 years of freedom from the disease recurrence improved from 60 to 88 % in patients in the high-RS group. There was no demonstrable risk reduction with chemotherapy regarding the 10-year disease recurrence rates in the low-RS group (relative risk, 1.31; CI, 0.46–3.78). A significant risk reduction (27.6 % reduction in absolute risk) was shown with chemotherapy in the high-RS group (relative risk, 0.26; CI, 0.13–0.53). The benefit of chemotherapy was not clear in the intermediate-RS group (relative risk, 0.61; CI, 0.24–1.59). In a multivariate analysis of Cox models containing chemotherapy treatment and RS, the interaction between chemotherapy treatment and RS was significant (p = 0.038).
NSABP B28 Trial
The current standard adjuvant treatment of ER-positive, axillary lymph node-positive breast cancer in pre- or postmenopausal patients is ET plus chemotherapy (ET + CT) [2]. Nevertheless, exploratory analyses show that breast cancer patients with high levels of ER positivity and a lack of HER2 overexpression may not derive substantial benefit, even if those patients show positive axillary nodes [29, 30].
The original phase III NSABP B28 trial included 3060 pre- or postmenopausal ER-positive (N = 2019) and ER-negative/borderline (N = 1041) axillary lymph node-positive, early breast cancer patients [31].
The treatment groups were tamoxifen plus AC (doxorubicin plus cyclophosphamide) and AC plus paclitaxel. In this trial, 2687 patients received concurrent ET. RS analysis was performed in 1065 tumor blocks of ER-positive patients treated with endocrine therapy [32]. In the univariate analysis, RS predicted disease-free survival (DFS), disease recurrence-free interval, and OS (p < 0.001 for each). In a multivariate analysis, RS was identified as an independent prognostic factor for DFS, disease recurrence-free interval, and OS (p < 0.001). DFS was 76 (95 % CI: 71–80), 57 (95 % CI: 52–62), and 48 % (95 % CI: 42–53) for the low-, intermediate-, and high-RS groups, respectively. The disease recurrence-free interval was 81 (95 % CI: 76–85), 65 (95 % CI: 60–70), and 56 % (95 % CI: 50–61) for the low-, intermediate-, and high-RS groups, respectively. OS was 90 % (95 % CI: 86–93), 75 (95 % CI: 70–79), and 63 % (95 % CI: 57–68) for the low-, intermediate-, and high-RS groups, respectively. In patients with low RS, adding paclitaxel to anthracycline-based adjuvant treatment did not provide any additional benefit to the final outcome. Based on these results, aggressive chemotherapy may not be warranted for patients with low RSs [32].
A subgroup of ER-positive breast cancer patients shows a low risk of disease recurrence even with positive axillary nodes. These patients are unlikely to benefit from adjuvant chemotherapy. Oncotype DX RS was also shown to be prognostic in axillary lymph node-positive, concordant with axillary lymph node-negative tamoxifen-treated breast cancer patients. Oncotype DX RS may also predict patients who will not benefit from the addition of chemotherapy (i.e., patients with low RS).
SWOG 8814 Trial
Recent data from the SWOG 8814 trial showed Oncotype DX to be prognostic and predictive in ER-positive, axillary lymph node-positive breast cancer patients [33]. The SWOG 8814 study, a parent trial, was a phase III trial and showed that adjuvant CAF (cyclophosphamide, doxorubicin, 5-fluorouracil) chemotherapy plus tamoxifen was superior to tamoxifen alone for DFS and OS in postmenopausal, estrogen and/or progesterone receptor (ER/PR)-positive, axillary lymph node-positive breast cancer patients. The Oncotype DX assay was applied to specimens from a tumor bank for RS analysis. That study was performed by Genomic Health, Inc., and the investigators were blinded to patients’ clinical data and outcome. Clinical data and outcome were combined with the Oncotype DX RS results after all assays were completed. Tumor samples were available for 664 of 1477 patients (45 %). The parent trial included three arms: tamoxifen only, concomitant CAF and tamoxifen, and sequential CAF and tamoxifen. In this translational study, the concomitant CAF and tamoxifen arm was omitted due to inferior efficacy. Sufficient RNA was obtained from 149 patients in the tamoxifen arm and 219 patients in the CAF plus tamoxifen arm (367 total patients) for RT-PCR analysis. The 21-gene RS was prognostic in tamoxifen-treated, ER-positive, axillary lymph node-positive patients and predictive for adjuvant chemotherapy with CAF regimen in patients with high RS [33]. The RS was prognostic for DFS in tamoxifen-treated patients (p = 0.006). There was a significant benefit of chemotherapy in the high-RS group (log rank p = 0.03; HR, 0.59; 95 % CI, 0.35–1.01), but no benefit was detected in patients with low RS (log rank p = 0.97; HR, 1.02; 95 % CI, 0.54–1.93) regarding DFS. A low RS identifies patients who may not benefit from adjuvant chemotherapy despite positive nodes. The benefit of adjuvant chemotherapy in the high-RS group was independent from the number of positive nodes. The benefit of chemotherapy on DFS was significant for the first 5 years of follow-up. However, there was no additional prediction of chemotherapy benefit for DFS beyond 5 years, despite the continued presence of the cumulative benefit after 10 years. Similar results were obtained for the prognostic value of the 21-gene RS. Ten-year OS estimates were 77, 68, and 51 % for patients with high, intermediate, and low RS, respectively. Breast cancer-specific survival (BCSS) and OS were significantly better in patients with high RS but not low and intermediate RS when treated with CAF and tamoxifen compared to tamoxifen only. In the exploratory analysis after adjustment for classical risk factors, including age, histopathological grade, race, tumor size, PR status, and HER2 status, both treatment and RS remained significant. The limited number of samples obtained from patients included in the original study was an important caveat of this study. The probability of a chemotherapy effect in low-RS patients cannot be ruled out completely due to the small sample size and broad CI ranges in this study. This study challenged the standard adjuvant chemotherapy treatment of patients with axillary lymph node-positive, ER-positive breast cancer.
ECOG 2197 Trial
In the original ECOG 2197 trial, there were 2185 pre- or postmenopausal ER-positive and lymph node-negative and node-positive (1–3 nodes) breast cancer patients treated with doxorubicin-containing chemotherapy or docetaxel plus ET [34]. The Oncotype DX assay was tested in the doxorubicin-containing chemotherapy arm (N = 465) and was a significant prognostic marker of disease recurrence in this study group. There was a significant correlation between RS and 5-year disease recurrence rates in both lymph node-positive and node-negative patient groups (p < 0.001). Of the lymph node-positive patients, 46 % were in the low-RS group. Patients with zero or one positive node had a 5-year disease recurrence rate under 3 %, and patients with two or three positive nodes had a 5-year disease recurrence rate of 8 %. However, good outcomes may be attributed to low RS, chemotherapy, or both. Ten-year follow-up analysis also showed that RS was still a prognostic marker for disease recurrence for lymph node-positive and lymph node-negative breast cancer patients [35].
Based on all of these studies, Oncotype DX has shown a high level of utility for estimating the risk of distant recurrence in 10 years and the benefit of chemotherapy in ER-positive, HER2-negative early breast cancer patients with up to three metastatic lymph nodes. The Oncotype DX assay has been adopted by the international guidelines of ESMO, ASCO, NCCN, and St. Gallen [36–39].
Impact on Treatment Decision
The Oncotype DX assay changed treatment decisions made on classical clinicopathological factors in several studies. Its impact on decision-making seems to increase with the results of the prospective trials. In a survey study in the USA, oncologists were asked about the treatment recommendation for their most recent ER-positive, lymph node-positive breast cancer patient after getting the Oncotype DX assay result [40]. The vast majority of the patients had one to three positive lymph nodes (92.5 %), and most (96.5 %) had a tumor size smaller than 5 cm. Of the 160 physicians who responded (16 % of the original sample), 86 % made decisions before obtaining the RS. However, 51 % of those oncologists changed their decision after receiving the RS result. Treatment decisions were changed to ET alone from ET + CT in one-third of those cases. Chemotherapy was eliminated in 49 % of the intermediate RS group and in 21 % of the low-RS group of patients. Additional chemotherapy decisions were made in 9 % of the cases.
In a study from Israel, 951 patients with one to three positive nodes received ET with or without chemotherapy [41]. Treatment decisions for 282 patients were made according to Oncotype DX, and there were 669 controls. In the Oncotype DX group, chemotherapy was given to all patients with high RS (100 %), 37 % of patients with intermediate RS, and 7 % of patients with low RS. Chemotherapy was given to 24.5 % of all Oncotype DX patients and 70.4 % of controls (p < 0.001). However, the patients’ clinicopathological features were not balanced between the groups, and patients in the control group had bigger tumor sizes, higher grade, and more positive lymph nodes. After adjustment according to these factors, Oncotype DX was associated with a 65 % decrease in chemotherapy usage.
A study of 50 lymph node-positive breast cancer patients from Australia showed a 26 % change in the treatment decision with Oncotype DX, and the majority of the treatment decisions were to omit chemotherapy [42]. A study of 42 axillary lymph node-positive ER-positive breast cancer patients (22 with macrometastasis) from Spain showed that 73 % of the patients had low RS [43], and the recommendation of chemotherapy decreased from 55 to 17 % with the use of Oncotype DX (p = 0.021).
In a recent meta-analysis of 8 studies (1437 patients) on the impact of Oncotype DX on treatment decisions, the adjuvant therapy recommendation changed in 33.4 % of patients due to Oncotype DX RS versus the decision recommended based on clinicopathological factors [44]. After Oncotype DX, the adjuvant chemotherapy recommendation was 83.4 % in patients with high RS, 37.4 % with intermediate RS, and 5.8 % with low RS. The overall chemotherapy recommendation was 28.2 % after using the Oncotype DX assay.
Prospective Clinical Trials
The phase III SWOG 1007 (RxPONDER, NCT01272037) trial is designed to determine the effect of chemotherapy with adjuvant endocrine therapy in patients with ER-positive, 1–3 axillary lymph node-positive breast cancer and low or intermediate RS (less than or equal to 25) [45]. This study will provide important information about the patients in whom chemotherapy can be omitted in the low- and intermediate-RS groups. It will also address issues of quality of life and long-term side effects such as premature menopause and weight gain.
The prospective TAILORx trial was designed mainly to investigate the benefits of chemotherapy in the intermediate RS group (scores of 11–25) of ER-positive, axillary lymph node-negative early breast cancer patients [46]. The Oncotype DX assay was applied prior to treatment, and patients were divided into three RS groups: low (RS <11), intermediate (RS 11–25), and high (RS >25). Patients in the low-RS group were treated with adjuvant ET, and those in the high-RS group were treated with ET + CT. Enrollment was completed in 2010, and a total of 11,000 patients were recruited in the study. The results will be compiled in 2015.
WSG PLAN B is a phase III trial run by the West German Study Group. In this study, two different chemotherapy regimens were compared in 2549 patients with early breast cancer [47]. Oncotype DX, uPA/PAI-1, Ki67, and histopathological grade were used for comparison of chemotherapy regimens in patients with ER-positive axillary lymph node-positive or high-risk node-negative breast cancer.
WSG ADAPT is another phase II/III trial in pre- and postmenopausal early breast cancer patients [47]. It aims to individualize the adjuvant treatment decision in early breast cancer patients and is using the Oncotype DX assay with conventional prognostic factors (nodal status). Dynamic changes in proliferation rates and apoptosis were checked after a short course of treatment. The aim was to establish early predictive surrogate molecular markers for outcome by assessing the response to a repeated biopsy after 3 weeks of induction treatment.
MammaPrint
MammaPrint is based on DNA microarray technology. Using gene-expression profiling, the Netherlands Cancer Institute™ and its spinoff company Agendia™ developed a 70-gene prognostic signature called MammaPrint™ for axillary lymph node-negative early breast cancer patients. These 70 genes are involved in the cell cycle, invasion, proliferation, angiogenesis, metastasis, and signal transduction. None of these genes are assessed by Oncotype DX. The 70-gene signature was developed for a dichotomous risk classification as low or high risk of disease recurrence in 5 years in a cohort of node-negative breast cancer patients, who were not treated systemically [14]. This gene signature defined the low- and high-risk groups for 5-year disease recurrence [14]. The low-risk group was identified as having a 13 % risk of distant metastasis in 10 years, and the high-risk group was identified as having a 56 % risk of distant metastasis in 10 years without adjuvant treatment. The first validation study was performed with the samples from the tumor bank of the Netherlands Cancer Institute. A total of 295 tumor samples were obtained from breast cancer patients (ER negative and ER positive, lymph node negative and node positive, untreated, treated with chemotherapy and/or endocrine therapy) [15]. Metastasis-free survival and OS were higher in the low-risk group than in the high-risk group in lymph node-negative and node-positive patients and all patients were evaluated using the MammaPrint assay. Of the patients with low-risk profiles, 85 % were disease recurrence-free, and 50.6 % of the patients with high-risk profiles were disease recurrence-free in 10 years. In the multivariate analysis, independent prognostic markers for disease recurrence were high-risk profile with MammaPrint™, tumor size, and absence of chemotherapy. This 70-gene expression profile was developed on microarrays containing 25,000 60-mer oligonucleotides that are not designed for routine clinical practice. This 70-gene prognosis profile, which was translated to a customized microarray (MammaPrint™), contains a reduced set of 1900 probes suitable for high-throughput processing [48]. It allows the use of less RNA and a short processing time of 5 days. To validate its prognostic value, the RNA of 162 patients from two previous studies was used for hybridization with this custom array. Custom microarray results were compared with the original analysis, and they showed an extremely high correlation of prognosis prediction (p < 0.0001). This first study showed that this small, custom microarray can be a reliable diagnostic tool for predicting the outcome of disease in breast cancer patients. MammaPrint™ was intended for use in women under 61 years of age with stage I and II, ER-positive or ER-negative early breast cancer. It was approved by the US FDA in 2007 for determining the risk of distant recurrence at 5 and 10 years but not for predicting the benefit of chemotherapy. Based on the evidence supporting the prognostic value of MammaPrint™, it has been incorporated into the ESMO and St. Gallen guidelines as a prognostic tool [37, 38]. The test was initially developed and validated for fresh or fresh-frozen tumor tissue [15, 49]. A recent study of 211 matched samples showed 91.5 % consistency between using FFPE and using fresh or frozen specimens [50].
Another MammaPrint™ validation study was performed by TRANSBIG researchers in 307 patients with early, axillary lymph node-negative breast cancer, with a median follow-up of 13.6 years, in 5 different European countries [51]. MammaPrint™ results were compared with other clinicopathological risk classification systems. Patients were defined as low risk if their 5-year probability of distant metastasis-free survival was above 90 %. The clinicopathological low-risk group was defined as a 10-year OS probability greater than 88 % (for ER-positive patients) or 92 % (for ER-negative patients). MammaPrint was identified as an independent prognostic marker for distant metastasis-free survival and OS. In the univariate analysis of the 70-gene signature in high- versus low-risk patients, the HR for time to distant metastasis was 2.32 (95 % CI, 1.35–4; p = 0.002), HR for OS was 2.79 (95 % CI, 1.6–4.87; p < 0.001), and the HR for DFS was 1.56 (95 % CI, 1.04–2.16; p = 0.032). MammaPrint was a more powerful prognostic tool for distant metastasis-free survival and OS in comparison with clinicopathological factors defined by Nottingham Prognostic Index, St. Gallen Criteria or Adjuvant! Online. When adjusted for the clinical risk groups, the 70-gene risk score results showed HRs of 2.13 (95 % CI: 1.19–3.82), 2.63 (95 % CI: 1.45–4.79), and 1.36 (95 CI: 0.91–2.03) for time to distant metastasis, OS, and DFS, respectively.
Data for Patients with Positive Lymph Nodes
The 70-gene assay result was also prognostic in lymph node-positive breast cancer patients and predicted the outcome better than the other classical clinicopathological factors. A validation study was conducted with 241 patients from two European institutes, with one to three positive lymph nodes and operable T1–T3 early breast cancer. Adjuvant treatment decisions were made according to national guidelines from the two different countries (the Netherlands and Italy). MammaPrint™ predicted that patients in the low-risk group would have 91 and 96 % distant metastasis-free survival and BCSS, respectively, in 10 years [52]. For the poor prognostic group, the 10-year distant metastasis-free survival and BCSS were 76 and 76 %. The 70-gene risk score was superior to other clinicopathological factors for predicting BCSS. In the multivariate analysis, the 70-gene risk score HR was 7.19 (95 % CI: 1.8–28.43; p = 0.005) for BCSS.
The MammaPrint™ assay was studied using frozen tumors from 173 N2 (with 4–9 axillary lymph node metastatic) breast cancer patients from the Netherlands and Italy [53]. Seventy patients (40 %) were classified as genomic high risk, and 103 (60 %) were classified as genomic low risk. Patients in the genomic high-risk group were more often grade 3 (60 %), hormone receptor negative and HER2 positive (25 %). The 5-year OS was 97 and 76 % in the low- and high-risk groups, respectively (p < 0.01). Distant metastasis-free survival in 5 years was 87 % for low-risk patients and 63 % for high-risk patients (p < 0.01). In the luminal A subgroup, the 70-gene assay result was the only independent risk factor for distant metastasis and breast cancer-specific death in breast cancer patients with 4–9 positive lymph nodes. MammaPrint can be integrated into the selection of treatment strategy in this group of patients.
Data on Adjuvant Treatment Decisions
The performance of the 70-gene signature was assessed in a prospective observational community-based study that included 427 early breast cancer (cT1-3N0M0) patients [54]. Adjuvant systemic treatment decisions were given considering the 70-gene signature, the Dutch CBO 2004 guidelines, and the preferences of the physicians and patients. The median follow-up duration was 62 months. In the 70-gene signature group, 15 % (33/219) of low-risk patients and 81 % (169/208) of high-risk patients received adjuvant chemotherapy. The 5-year probability of disease recurrence-free survival according to the 70-gene score was 97.0 % for low-risk and 91.7 % for high-risk patients. The 5-year probabilities of disease recurrence-free survival for the Adjuvant! Online low- and high-risk groups were 96.7 and 93.4 %, respectively. There were 124 patients in the 70-gene signature low-risk and Adjuvant! Online high-risk groups. Of these patients, 94 (76 %) did not receive adjuvant chemotherapy, and the 5-year disease recurrence-free interval was 93.4 %. The adjuvant chemotherapy decision would decrease by 32 % (94/295) if the 70-gene signature had been used in the Adjuvant! Online high-risk group of patients. The prognostic value of the 70-gene signature was again shown for the 5-year probability of disease recurrence-free survival, showing that in the low-risk 70-gene signature group, omission of chemotherapy did not compromise outcome.
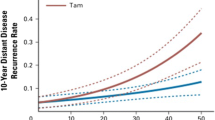
The 70-gene assay was studied for the predictive value of chemotherapy in 541 patients whose tumor samples were obtained from 7 previously reported studies conducted between 1984 and 2006 with known adjuvant treatment data [55]. There were 315 patients in the ET group and 226 patients in the ET + CT group. According to the 70-gene assay, 252 (47 %) patients were in the low-risk group, and 289 (53 %) patients were in the high-risk group. BCSS at 5 years was 97 % in patients treated with ET and 99 % in patients treated with ET + CT in the low-risk group (HR, 0.58; 95 % CI, 0.07–4.98, p = 0.20). In the high-risk group, BCSS was 81 % in patients treated with ET and 94 % in patients treated with ET + CT (HR, 0.21; 95 % CI 0.07–0.59, p < 0.01). Distant disease-free survival in the low-risk group was 93 % in patients treated with ET and 93 % in patients treated with ET + CT (HR, 0.26; 95 % CI, 0.03–2.02, p = 0.2). In the high-risk group, distant disease-free survival was 76 % and 88 % in the patients treated with ET and ET + CT, respectively (HR, 0.35; 95 % CI, 0.17–0.71, p < 0.01). There was a significant benefit of adding CT to ET for the group identified as high risk according to the 70-gene signature (Fig. 11.1). This benefit was not significant in patients with low risk according to the 70-gene signature. High-risk ER-positive breast cancer patients may be treated with more intensive treatment (i.e., chemotherapy) strategies in addition to adjuvant endocrine therapies.
Panel (a), five-year breast cancer-specific survival by treatment within the 70-gene signature groups (70-gene low risk on the left, high risk on the right). Panel (b), five-year distant disease-free survival by treatment within the 70-gene signature groups (70-gene; low risk on the left, high risk on the right). Abbreviations: BCSS breast cancer-specific survival, DDFS distant disease-free survival, n number, ET endocrine therapy, ET + CT endocrine + chemotherapy, HR univariate hazard ratio (Reproduced from Reference [55] with kind permission of Springer Science + Business Media)
MammaPrint can affect the adjuvant chemotherapy recommendation. Using the MammaPrint™ assay may decrease the variability of adjuvant treatment decisions. A cohort of 194 patients from 4 different countries in Europe was used to measure the impact of MammaPrint™ on adjuvant treatment decisions [56]. Patients’ clinicopathological data were sent to different multidisciplinary teams with and without the MammaPrint™ assay result, and adjuvant treatment decisions were provided for each patient. Treatment decisions in ER-positive and HER2-negative patients were changed in 37, 24, 28, and 35 % of cases by the Dutch, Belgian, Italian, and Spanish teams, respectively. MammaPrint™ increased the interinstitutional agreement in treatment advice (i.e., whether to utilize chemotherapy) from 51 to 75 %.
Prospective Trials
The MINDACT (Microarray In Node-negative and 1–3 node-positive Disease may Avoid ChemoTherapy) trial was designed to investigate the clinical utility of MammaPrint™ (70-gene profile) with clinicopathological criteria for the selection of ER-positive early breast cancer patients with one to three positive nodes for adjuvant chemotherapy. The clinicopathological risk definition was made using a modified version of Adjuvant! Online. Patients were also defined as low or high risk by MammaPrint™. The MINDACT trial hypothesizes that using the MammaPrint™ assay will outperform the clinicopathological classification through the better classification of patients as high or low risk and by reducing chemotherapy usage by 10–20 % without impairing the outcome. Patients who were defined as low risk by both Adjuvant! Online and MammaPrint™ were given ET, and adjuvant ET + CT were given to patients characterized as high risk by both Adjuvant! Online and MammaPrint™. Patients were randomized to the ET or chemotherapy arms if the risk groups differed between Adjuvant! Online and MammaPrint™. The study was designed to address whether a non-anthracycline-containing regimen (docetaxel plus capecitabine) may be used instead of an anthracycline-containing regimen. There were two arms for ET: letrozole only and tamoxifen followed by letrozole.
The pilot phase of the study included 800 patients [57]. Of those patients, 386 (48 %) were in the low-risk group based on both Adjuvant! Online and MammaPrint, and 198 (24.8 %) patients were in the high-risk group based on both Adjuvant! Online and MammaPrint™. There were 216 (27 %) discordant patients, of whom 75 (9.4 %) were in the low-risk group from Adjuvant! Online and the high-risk group from MammaPrint™ and 141 were in the high-risk group from Adjuvant! Online and the low-risk group from MammaPrint™. There was an 8.25 % difference (95 % CI: 4.7–11.8 %; p < 0.001) between the high-risk groups defined by Adjuvant! Online (42 %) and MammaPrint™ (34 %). There was a high consistency among the treatments in both groups (>92 %).
Prosigna
The NanoString Prosigna assay uses the expression of 50 target genes and eight constitutively expressed normalization genes. The test can be performed using qRT-PCR, but Prosigna™ relies on the NanoString nCounter Analysis System, which delivers direct, multiplexed measurement of gene expression. The assay is highly sensitive and precise. It uses 250 ng of RNA from FFPE tumor tissue. Assay controls are included along with test samples, and the process meets the pre-defined quality criteria. The PAM50 test was generated as a second-generation multigene expression assay and was developed to define the intrinsic breast cancer subtypes as luminal A/B, HER2 enriched, and basal-like from FFPE tissue [58]. PAM50 is more effective than classical immunohistochemical methods and clinicopathological factors for subtyping breast cancer. It uses a set of 50 genes and 5 control genes for analysis. In addition to classifying the tumor’s intrinsic subtype, PAM50 gives a numeric score for the patient’s distant recurrence probability by calculating the molecular subtype correlations, a subset of proliferation genes and pathological tumor size. The PAM50 test was adopted for performance by nCounter Analysis in a local molecular pathology lab (Prosigna™ Breast Cancer Gene Signature Assay, NanoString Technologies, Seattle). Training is required for the operators to demonstrate proficiency in studying this locally used test. The embedded software automatically applies all of the quality thresholds to the data. A clinically validated algorithm is used for expressing the risk of recurrence and the intrinsic subtype, both of which are prognostic indicators of the risk of disease recurrence for breast cancer. This test works with either frozen or FFPE samples and uses multiplexed gene-specific fluorescently labeled probe pairs to measure gene expression.
PAM50 reflects the underlying biology associated with the ER and HER2 pathways, and it also includes proliferation genes and markers of basal phenotype. Luminal A and B breast cancers are the most frequent subtypes. Luminal A tumors are characterized by lower expression levels of proliferation genes and ERBB2 and by lower recurrence rates compared with luminal B tumors, and these characteristics can be shown by the PAM50 risk of recurrence score.
RNA is extracted from tumor tissue, and the samples are hybridized without reverse transcription or amplification for both capture and reporter probes for the measured genes and assay controls. After hybridization, the target-probe complexes are processed on the nCounter Analysis System. A minimum threshold of expression for normalization genes must be met by the test sample data to ensure that the assay signal is high enough to produce precise results.
The test was prognostic in untreated (no systemic treatment) or tamoxifen-treated early breast cancer patients [58, 59]. It was validated in 786 stage I and II ER- and/or PR-positive postmenopausal patients (in this validation study, only 40 of 789 patients were premenopausal) and provided accurate information for subtyping and prognosis by risk of recurrence [59]. This risk of recurrence score gave the estimated 10-year recurrence probability in postmenopausal early breast cancer patients with ET. Despite clinical ER positivity, 10 of the cases were assigned to nonluminal subtypes by PAM50 in this study. PAM50 expression for intrinsic subtyping provided more prognostic information than standard clinical factors and IHC.
PAM50 was validated on over 2400 patients from two large retrospective studies [60, 61]. Using the mRNA of 1017 ER-positive early breast cancer patients from the ATAC trial, the PAM50 risk of recurrence score was studied and compared with Oncotype DX and IHC4 results [60]. IHC4 is a distant recurrence index derived from immunohistochemical analysis of estrogen and progesterone receptors, HER2 and Ki67. Additional prognostic information beyond that in the clinical treatment score, which integrates the prognostic information from nodal status, tumor size, histopathological grade, age, and treatment, was greater in the PAM50 risk of recurrence than the Oncotype DX RS for the overall population and for each subgroup (lymph node-negative and node-positive and HER2-negative/lymph node-negative subgroups). In the node-negative/HER2-negative subgroup, prognostic information obtained using the PAM50 risk of recurrence score was more accurate than the Oncotype DX RS. The correlation between risk of recurrence and clinical treatment score was similar to what was previously found in the TransATAC study between Oncotype DX RS and Adjuvant! Online. The risk of recurrence includes more clinicopathological information than the other factors. The PAM50 risk of recurrence score can be applied in the context of clinicopathological factors involved in the clinical treatment score.
One of the other large validation studies was on the patients from the ABCSG-8 trial. PAM50 was evaluated for obtaining the risk of recurrence and defining subtypes from the FFPE tumor tissues of 1478 patients from the ABCSG-8 trial [61]. The original study was a phase III prospective design of 3901 patients to test tamoxifen versus tamoxifen followed by anastrozole in the adjuvant treatment of ER-positive early breast cancer patients. Both risk of recurrence and intrinsic subtypes (luminal A/B, basal-like, and HER2 enriched) were defined using PAM50. There were 1004 (67.9 %) patients in the luminal A, 418 (28.3 %) patients in the luminal B, 48 (3.3 %) patients in the HER2-enriched, and 8 (0.5 %) patients in the basal-like subgroups according to the PAM50 test. The aim was to test whether the risk of recurrence score adds prognostic value in predicting disease recurrence beyond standard clinical factors. For 10-year disease recurrence risk, lower than 10 % was defined as low risk, and higher than 20 % was defined as high risk (Fig. 11.2). The risk of recurrence score added prognostic information to the clinical predictors in all subgroups (p < 0.0001). The luminal A subgroup had a lower risk of recurrence at 10 years compared with the luminal B subgroup (p < 0.0001). Ten-year distant recurrence-free survival rates were higher in the luminal A subgroup than in the luminal B (HR, 2.85; 95 % CI:, 2.04–4; p < 0.0001) (Fig. 11.3). Low-risk and high-risk groups were discriminated using the risk of recurrence score in all subgroups of patients. As a result, the PAM50 test was validated in this study for predicting disease recurrence in ER-positive postmenopausal early breast cancer patients. A 10-year disease recurrence under 3.5 % in the risk of recurrence low category makes it unlikely that additional chemotherapy would improve the outcome.
Prognosis based on PAM50 classifier for DRFS (Reproduced from Reference [61] with permission of Oxford University Press on behalf of the European Society for Medical Oncology. © The author (Michael Gnant) 2013)
Distant recurrence-free survival: Kaplan–Meier plot of luminal (A, B) subtypes with 95 % CI (Reproduced from Reference [61] with permission of Oxford University Press on behalf of the European Society for Medical Oncology. © The author (Michael Gnant) 2013)
The combined analysis of the TransATAC and ABCSG-8 trials with 2137 patients showed that the risk of recurrence adds significant prognostic information for late recurrences (>5 years) in women with hormone receptor-positive early-stage breast cancer [62]. Median follow-up time was 10 years for that analysis. Predefined risk stratification showed significant differences between risk groups based on the risk of recurrence for 10-year distant recurrence rates. Patients with recurrence in the first 5 years were excluded from this analysis. The risk of recurrence score for the patients with recurrence (53.7 ± 20.4) in the first 5 years was higher than the patients with no recurrence (41.89 ± 19.5) (p < 0.001). There were more late recurrences and more patients with poor differentiation, larger tumor size, and >3 positive lymph nodes in the TransATAC study than the ABCSG-8 trial. Of the 2137 patients analyzed, 1530 (73.8 %) women had luminal A and 542 (26.2 %) women had luminal B breast cancer. Patients in the luminal B subgroup had a 2.9-fold higher risk of distant recurrence (HR, 2.9; 95 % CI, 2.07–4.02; p = 0.001). Patients were divided according to the 10-year recurrence probability as having a low, intermediate, and high risk of recurrence (lower than 10, 10–20 %, and higher than 20 %, respectively). The risk of distant recurrence in 5–10 years was 2.4 % (95 % CI: 1.6–3.5), 8.3 % (95 % CI: 6.1–11.2), and 16.6 % (95 % CI: 13.1– 20.9) in patients in the low, intermediate, and high risk of recurrence groups, respectively. The risk of late recurrence was 6.9 (HR, 6.9; 95 % CI, 4.54–10.47) times higher in the high-risk group than in the intermediate-risk group and 3.3 (HR, 3.26; 95 % CI, 2.07–5.13) times higher in the intermediate-risk group than the low-risk group. Based on the risk of recurrence score, patients in the high-risk group may be separated for extended therapy.
Tissue samples from the MA.12 NCIC CTG (National Cancer Institute of Canada Clinical Trials Group) prospective trial, which compared tamoxifen and placebo in early premenopausal breast cancer patients, were used for PAM50 risk of recurrence evaluation. The aim was to evaluate intrinsic breast cancer subtypes and prognosis with PAM50 and IHC [63], and 395 patients were evaluated. PAM50 gave significant prognostic information for both DFS and OS, but IHC analysis could not. The 5-year DFS and OS rates were higher in the luminal A group and lower in the HER2-enriched group according to PAM50 (p = 0.0003). Classification of the patients into intrinsic subtypes using PAM50 was also more effective than using IHC. This study also concluded that PAM50 was predictive for adjuvant tamoxifen treatment in node-negative and node-positive premenopausal breast cancer patients. There was significant interaction with 10-year DFS probability and the risk of recurrence score in lymph node-negative and node-positive early breast cancer patients treated with tamoxifen.
The phase III GEICAM/9906 study population (N = 814) was evaluated for PAM50 breast cancer subtyping and clinical standard markers [64]. The standard IHC panel for breast cancer (ER, PR, and HER2) could not adequately define the PAM50 expression subtypes in this study. There was high agreement between biomarker scoring by protein IHC and gene expression, but gene expression determinants for ESR1 and ERBB2 status were more prognostic.
In a population-based study, 1319 women with breast cancer from the LACE and Pathways cohort were tested for intrinsic subtyping [65]. According to PAM50 subtyping, 53.1 % of the patients were luminal A, 20.5 % were luminal B, 13 % were HER2 enriched, 9.8 % were basal-like, and 3.6 % were normal-like. Among low-risk hormone receptor-positive patients with classical clinicopathological tests, only 76.5 % were categorized as luminal A by PAM50. In this population-based cohort, African-American women were more likely to have basal-like tumors (OR: 4.4; 2.3–8.4), and Asian and Pacific Islander women had reduced odds of the basal-like subtype (OR: 0.5; 0.3–0.9). In another population-based cohort of 1691 patients, early (<5 years) and late (>5 years) risks of recurrence were determined based on the PAM50 risk of recurrence score according to intrinsic subtypes of breast cancer [66]. The risks of disease recurrence and death were lower in patients with luminal A tumors compared with luminal B, HER2-enriched, and basal-like subgroups of breast cancer at 2, 5, and 10 years. In that study, PAM50 better defined the patients with lower risk from the higher risk of recurrence tumors than the standard immunohistochemistry or tumor grade.
Prediction of Response to Treatment
In a study of 104 postmenopausal ER-positive breast cancer patients, tumor biopsies were taken before and 2 weeks after the beginning of treatment with anastrozole [67]. The risk of recurrence was calculated by PAM50 for luminal A and B tumors. Among the pretreatment samples, all intrinsic subtypes were present, but the luminal subgroups were most highly represented. The decrease in Ki67 levels was evaluated between subgroups according to PAM50, and there was a similar proportionate decrease in Ki67 levels in the luminal A and B subgroups (mean suppression: 75 % for both), which suggests that patients in the luminal A and B subgroups derive similar benefit from anastrozole treatment. However, in the subgroups of basal-like (15 %) and HER2-enriched (50 %) cancers, Ki67 reductions were low under treatment with anastrozole. The normal breast-like subgroup showed the greatest reduction of Ki67 (83 %) with anastrozole treatment. Residual Ki67 staining remained high after 2 weeks of anastrozole treatment in the luminal B subgroup. The PAM50 risk of recurrence score was significantly associated with clinical outcome (p = 0.03) and antiproliferative response to anastrozole treatment (p = 0.0019). These data show that the short-term response to anastrozole treatment may be similar between luminal subgroups and that higher residual Ki67 levels might show poor response to anastrozole treatment in the luminal B subgroup.
Another validation study was the MA.5 NCIC CTG trial, which randomized early premenopausal, lymph node-positive breast cancer patients into CMF versus CEF (cyclophosphamide, epirubicin, and 5-fluorouracil) chemotherapy. PAM50 classified 467 patients into intrinsic subtypes, and the HER2-enriched subtype strongly predicted anthracycline sensitivity [68].
A subset of patients from the GEICAM/9906 phase III trial who were identified by PAM50 as having low proliferation status derived a larger benefit from weekly paclitaxel [49]. The original GEICAM/9916 study tested FEC versus FEC followed by weekly paclitaxel in node-positive early breast cancer patients. The PAM50 risk of recurrence was studied in 820 patients, and the median follow-up was 8.7 years. The median OS was higher in the FEC plus weekly paclitaxel arm compared with the FEC arm (HR, 0.693; p = 0.013). A benefit from weekly paclitaxel treatment was achieved only in patients with a low PAM50 risk of recurrence score (HR, 0.23; p < 0.001).
The NCIC CTG M1.21 trial was a phase III study of 2104 patients who were ≤61 years old and had high-risk node-negative or node-positive disease [69]. It tested different anthracycline and taxane combinations for the adjuvant treatment of breast cancer. Randomization was performed among doxorubicin plus cyclophosphamide and paclitaxel (AC/T), dose-dense CEF, and dose-dense, intense epirubicin, cyclophosphamide, and paclitaxel (EC/T) groups. Intrinsic subtyping was performed in 1094 available patients with the PAM50 assay [70]. Of these patients, 27 % were in the luminal A subgroup, 23 % were in the luminal B subgroup, 18 % were in the HER2-enriched subgroup, and 32 % were in the basal-like subgroup. Dose-dense CEF and dose-dense, intense EC/T treatments were superior to AC/T treatment (p = 0.01). In the multivariate analysis, a high risk of recurrence was associated with worse RFS (p = 0.03). However, categorical risk of recurrence was neither prognostic nor predictive for any treatment. In the multivariate analysis, intrinsic subtyping with PAM50 had a significant prognostic effect on RFS (p = 0.002). Compared with luminal A, the hazard ratios were 1.48 (95 % CI: 0.92–2.37) for luminal B, 2.68 (95 % CI: 1.6–4.48) for HER2 enriched, and 1.97 (95 % CI: 1.1–3.53) for basal-like. Intrinsic subtypes were not predictive of treatment benefit (AC/T vs. EC/T-CEF). However, subgroup analysis showed that the nonluminal subtype was predictive for taxane benefit compared with the luminal subtype (p = 0.05).
Based on the results of these trials, PAM50 provides valuable information for intrinsic subtyping and also distant relapse-free survival and likelihood of recurrence at 10 years, at least for ER-positive and tamoxifen-treated breast cancer patients. PAM50 subtype classification is superior to IHC for both prognosis and predicting the benefit of tamoxifen. The risk of recurrence score gives an individual risk assessment in early stage, hormone receptor-positive, pre- and postmenopausal breast cancer patients and allows subtype classification. The risk of recurrence score estimates the probability of disease recurrence in pre- and postmenopausal, hormone receptor-positive early breast cancer patients treated with ET. The HER2-enriched subtype, as defined by PAM50, was a strong predictor of adjuvant anthracycline treatment. In light of these data, PAM50 was approved in Europe in 2012 and by the US FDA in 2013.
Other Genomic Tests
BluePrint® is an 80-gene microarray that was specifically designed for molecular subclassification in early-stage (stage I and II), lymph node-negative or node-positive and ER-positive or ER-negative breast cancer patients [71]. In a neoadjuvant chemotherapy study of 426 breast cancer patients, the BluePrint® assay reassigned 22 % of the previously classified patients with IHC/FISH [72]. Of 211 patients classified as hormone receptor-positive/HER2-negative according to IHC/FISH, 37 were reclassified into the basal (N = 35) and HER2 (N = 2) subtypes by the BluePrint® assay. The pathological complete response rate was higher in the HER2 subgroup defined by BluePrint® (53 %) than in the HER2 subgroup defined by IHC/FISH (38 %) (p = 0.047).
Endopredict® (Sividon Diagnostics GmbH, Germany) is an 11-gene assay that was recently introduced to predict disease recurrence risk in ER-positive and HER2-negative early breast cancers treated with adjuvant ET alone [73]. Eight selected genes and three additional control genes were used for quantification of mRNA levels by RT-PCR. FFPE can be used for Endopredict® analysis. The assay result is expressed as the Endopredict® (EP) score and was used in combination with nodal status and tumor size (EPclin) for a comprehensive risk estimation. EPclin was used in two validation studies of 378 and 1324 patients from the ABCSG-6 and ABCSG-8 studies, respectively [73, 74]. The 11-gene EP risk score was an independent predictor of disease recurrence in a multivariate analysis, and the EPclin score outperformed conventional clinicopathological risk factors. This test is used in laboratories in several centers in Germany, Austria, and Switzerland.
MapQuantDx® (Genomic Grade, Ipsogen, France) is a 97-gene histologic grade predictor that was developed in recent years. It is a microarray-based assay that calculates the Genomic Grade Index (GGI). It provides prognostic information in addition to standard clinicopathological variables and had significant impact on treatment decisions in several retrospective studies [75, 76]. Fresh or frozen tissue is needed for this 97-gene signature. However, a 6-gene PCR genomic grade was developed from the initial 97 genes for FFPE tissue. A high correlation was shown between the microarray and RT-PCR assays studied in frozen and FFPE tissues. The prognostic value of PCR-GGI was confirmed on FFPE samples [77].
The Rotterdam 76-gene signature was developed at the Erasmus University Cancer Center in Rotterdam (the Netherlands) [78, 79]. It was introduced to the market by Veridex (Raritan, USA). This 76-gene assay includes proliferation genes, none of which overlap with MammaPrint® or Oncotype DX®. Fresh or frozen tissue is needed for this assay. Validation was performed in patients with node-negative breast cancer that was either positive or negative for hormone receptors. The 76-gene assay was validated in a group of 198 node-negative, systemically untreated breast cancer patients, and a high-risk group was defined for early disease recurrence in this study [80]. A very low-risk subgroup was also defined in another validation study in breast cancer patients treated with adjuvant ET [81].
Conclusion
Numerous multigene assays have been developed based on multigene profiling assays to avoid over- and undertreatment and to better define the prognosis and predictive markers in early-stage breast cancer. Some of these assays provide subclassifications based on gene expression profiling. The main limitation for many gene profiling assays is the lack of “level of evidence I” due to the need for crucial prospective data and sufficient numbers of robust retrospective studies. Very few assays are suitable for use in common clinical practice due to technical and clinical validity. Clinicians must carefully consider the indications of these gene array-based assays regarding differences in technical prerequisites, reproducibility, clinical validity, underlying evidence, and clinical impact on the special patient populations. Among the most widely used multigene assays are Oncotype DX™, MammaPrint™, and PAM50™. Less expensive and more feasible assays are needed for decision-making about adjuvant or neoadjuvant treatment of breast cancer. Avoiding chemotherapy for a group of patients who would not benefit is very important for acute and long-term toxicities, quality-of-life issues, and cost. Based on numerous retrospective studies, ongoing prospective studies will provide important information about the treatment prediction in early breast cancer. Technical ease is a major concern for the general implementation of these tests in clinics. The availability of the test in a local lab is an advantage. However, the need for special equipment and trained personnel is critical for the local facility. Several other assays are undergoing validation studies or are otherwise in the process of development.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930–42.
Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–67.
Bedard PL, Cardoso F. Can some patients avoid adjuvant chemotherapy for early-stage breast cancer? Nature reviews. Clin Oncol. 2011;8:272–9.
Bryant J, Fisher B, Gunduz N, Costantino JP, Emir B. S-phase fraction combined with other patient and tumor characteristics for the prognosis of node-negative, estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 1998;51:239–53.
Henderson IC, Patek AJ. The relationship between prognostic and predictive factors in the management of breast cancer. Breast Cancer Res Treat. 1998;52:261–88.
Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78.
Bast Jr RC, Ravdin P, Hayes DF, Bates S, Fritsche Jr H, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78.
Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cance. J Clin Oncol. 2001;19:3817–27.
Eifel P, Axelson JA, Costa J, Crowley J, Curran Jr WJ, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–89.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–7.
van ’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Harbeck N, Sotlar K, Wuerstlein R, Doisneau-Sixou S. Molecular and protein markers for clinical decision making in breast cancer: today and tomorrow. Cancer Treat Rev. 2014;40:434–44.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Cronin M, Pho M, Dutta D, Stephans JC, Shak S, Kiefer MC, et al. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 2004;164:35–42.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74.
Esteva FJ, Sahin AA, Coombes K, Baker J, Cronin M, Walker M, Watson D, Cristofanilli M, Shak S, Hortobagyi GN. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients – M.D. Anderson Clinical Validation Study [abstract]. Breast Cancer Res Treat. 2003;82:A17. doi:10.1023/B:BREA.0000003871.38587.8b. http://www.sabcs.org.
Cobleigh MA, Bitterman P, Baker J, Cronin M, Liu M-L, Borchik R, Tabesh B, Mosquera J-M, Walker MG, Shak S. Tumor gene expression predicts distant disease-free survival (DDFS) in breast cancer patients with 10 or more positive nodes: high throughput RT-PCR assay of paraffin-embedded tumor tissues [abstract]. Proc Am Soc Clin Oncol. 2003;22:A3415.
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner R, Walker M, Watson D, Park T, et al. Multi-gene RT-PCR assay for predicting recurrence in node negative breast cancer patients – NSABP studies B-20 and B-14 [abstract]. Breast Cancer Res Treat. 2003;82:A16. http://www.sabcs.org.
Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8(3):R25. doi:10.1186/bcr1412.
Toi M, Iwata H, Yamanaka T, Masuda N, Ohno S, Nakamura S, et al. Clinical significance of the 21-gene signature (Oncotype DX) in hormone receptor-positive early stage primary breast cancer in the Japanese population. Cancer. 2010;116:3112–8.
Fisher B, Redmond C, Legault-Poisson S, Dimitrov NV, Brown AM, Wickerham DL, et al. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the National Surgical Adjuvant Breast and Bowel Project B-16. J Clin Oncol. 1990;8:1005–18.
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9.
Arimidex TAoiCTG, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53.
Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–82.
Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–67.
Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–506.
Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96.
Mamounas E, Tang G, Paik S, et al. Association between the 21-gene recurrence score (RS) and benefit from adjuvant paclitaxel (Pac) in node-positive (N+), ER-positive breast cancer patients (pts): results from NSABP B-28 [Abstract]. Cancer Res. 2012;72(24 Suppl):S1–10.
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–71.
Sparano JA, O’Neill A, Gray RJ, et al. 10-year update of E2197: phase III doxorubicin/docetaxel (AT) versus doxorubicin/cyclophosphamide (AC) adjuvant treatment of LN+ and high-risk LN- breast cancer and the comparison of the prognostic utility of the 21-gene Recurrence Score (RS) with clinicopathologic features. J Clin Oncol. 2012;30(Suppl 15) [abstract 1021].
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi7–23.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed: 1 Jan 2015.
Oratz R, Kim B, Chao C, Skrzypczak S, Ory C, Bugarini R, et al. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. J Oncol Pract. 2011;7:94–9.
Klang S, Liebermann N, Rizel S, et al. The recurrence score and chemotherapy treatment in node-positive, ER+ early-stage breast cancer patients in Israel. J Clin Oncol. 2010;28(15 Suppl):[Abstract] 6075.
de Boer RH, Baker C, Speakman D, Chao CY, Yoshizawa C, Mann GB. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust. 2013;199:205–8.
Estevez LG, Calvo I, Abad MF, et al. A retrospective study in the Spanish population with Oncotype dx recurrence score (RS) in breast cancer patients with positive and negative-lymph nodes. J Clin Oncol. 2013;31(Suppl; [abstract] e11531).
Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:13–22.
Goncalves R, Bose R. Using multigene tests to select treatment for early-stage breast cancer. J Natl Compr Cancer Network. 2013;11:174–82; quiz 82.
Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx). Clin Breast Cancer. 2006;7:347–50.
Gluz O, Kreipe H, Dehenhardt T, Christgen M, Kates R, Liedtke C, et al. Prospective comparison of risk assessment tools in early breast cancer (recurrence score, uPA/PAI-1, central grade, and luminal subtypes): final correlation analysis from the phase III WSG plan B trial. In: San Antonio Breast Cancer symposium; 2011. [Abstract] S4–3.
Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278.
Martin M, Prat A, Rodriguez-Lescure A, Caballero R, Ebbert MT, Munarriz B, et al. PAM50 proliferation score as a predictor of weekly paclitaxel benefit in breast cancer. Breast Cancer Res Treat. 2013;138:457–66.
Sapino A, Roepman P, Linn SC, Snel MH, Delahaye LJ, van den Akker J, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 2014;16:190–7.
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–92.
Mook S, Schmidt MK, Viale G, Pruneri G, Eekhout I, Floore A, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat. 2009;116:295–302.
Saghatchian M, Mook S, Pruneri G, Viale G, Glas AM, Guerin S, et al. Additional prognostic value of the 70-gene signature (MammaPrint((R))) among breast cancer patients with 4–9 positive lymph nodes. Breast. 2013;22:682–90.
Drukker CA, Bueno-de-Mesquita JM, Retel VP, van Harten WH, van Tinteren H, Wesseling J, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer. 2013;133:929–36.
Knauer M, Mook S, Rutgers EJ, Bender RA, Hauptmann M, van de Vijver MJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat. 2010;120:655–61.
Cusumano PG, Generali D, Ciruelos E, Manso L, Ghanem I, Lifrange E, et al. European inter-institutional impact study of MammaPrint. Breast. 2014;23:423–8.
Rutgers E, Piccart-Gebhart MJ, Bogaerts J, Delaloge S, Veer LV, Rubio IT, et al. The EORTC 10041/BIG 03–04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;47:2742–9.
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32.
Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–90.
Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–45.
Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2014.
Chia SK, Bramwell VH, Tu D, Shepherd LE, Jiang S, Vickery T, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18:4465–72.
Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44.
Sweeney C, Bernard PS, Factor RE, Kwan ML, Habel LA, Quesenberry Jr CP, et al. Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer Epidemiol Biomarkers Prev. 2014;23:714–24.
Caan BJ, Sweeney C, Habel LA, Kwan ML, Kroenke CH, Weltzien EK, et al. Intrinsic subtypes from the PAM50 gene expression assay in a population-based breast cancer survivor cohort: prognostication of short- and long-term outcomes. Cancer Epidemiol Biomarkers Prev. 2014;23:725–34.
Dunbier AK, Anderson H, Ghazoui Z, Salter J, Parker JS, Perou CM, et al. Association between breast cancer subtypes and response to neoadjuvant anastrozole. Steroids. 2011;76:736–40.
Cheang MC, Voduc KD, Tu D, Jiang S, Leung S, Chia SK, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012;18:2402–12.
Burnell M, Levine MN, Chapman JA, Bramwell V, Gelmon K, Walley B, et al. Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by Paclitaxel versus Doxorubicin and cyclophosphamide followed by Paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010;28:77–82.
Liu S, Chapman JA, Burnell MJ, Levine MN, Pritchard KI, Whelan TJ, et al. Prognostic and predictive investigation of PAM50 intrinsic subtypes in the NCIC CTG MA.21 phase III chemotherapy trial. Breast Cancer Res Treat. 2015;149:439–48.
Krijgsman O, Roepman P, Zwart W, Carroll JS, Tian S, de Snoo FA, et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat. 2012;133:37–47.
Whitworth P, Stork-Sloots L, de Snoo FA, Richards P, Rotkis M, Beatty J, et al. Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol. 2014;21:3261–7.
Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–20.
Dubsky P, Filipits M, Jakesz R, Rudas M, Singer CF, Greil R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24:640–7.
Reyal F, Bollet MA, Caly M, Gentien D, Carpentier S, Peyro-Saint-Paul H, et al. Respective prognostic value of genomic grade and histological proliferation markers in early stage (pN0) breast carcinoma. PLoS One. 2012;7:e35184.
Metzger-Filho O, Michiels S, Bertucci F, Catteau A, Salgado R, Galant C, et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann Oncol. 2013;24:377–84.
Toussaint J, Sieuwerts AM, Haibe-Kains B, Desmedt C, Rouas G, Harris AL, et al. Improvement of the clinical applicability of the Genomic Grade Index through a qRT-PCR test performed on frozen and formalin-fixed paraffin-embedded tissues. BMC Genomics. 2009;10:424.
Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9.
Foekens JA, Atkins D, Zhang Y, Sweep FC, Harbeck N, Paradiso A, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer. J Clin Oncol. 2006;24:1665–71.
Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007;13:3207–14.
Zhang Y, Sieuwerts AM, McGreevy M, Casey G, Cufer T, Paradiso A, et al. The 76-gene signature defines high-risk patients that benefit from adjuvant tamoxifen therapy. Breast Cancer Res Treat. 2009;116:303–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Arslan, C., Altundag, M.K., Ozisik, Y.Y. (2016). Gene Arrays, Prognosis, and Therapeutic Interventions. In: Aydiner, A., İğci, A., Soran, A. (eds) Breast Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-22843-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-22843-3_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22842-6
Online ISBN: 978-3-319-22843-3
eBook Packages: MedicineMedicine (R0)