Abstract
This work analysed the carbon sequestration potential in two species of mangroves (Rhizophora mucronata and Avicennia marina) along with their growth, biomass, sediment characteristics for four seasons of the year 2009–2010, in planted stands of different age (1–17.5 years) in the Vellar-Coleroon estuarine complex, India. The mangroves were recorded to store significant amount of biomass. Avicennia marina performed better to display 75 % higher rate of carbon sequestration than that in Rhizophora mucronata. This could be attributed to growth efficiency and high biomass production. For instance, Avicennia marina exhibited 2.7 fold higher girth, 24 % higher net canopy photosynthesis, 2 fold aboveground biomass (AGB), 40 % more belowground biomass (BGB) and 77.3 % higher total biomass, than R. mucronata did. Seasonally the rate of carbon sequestration was 7.3 fold higher in post-monsoon, 3.4 fold in monsoon, 73 % more in summer than that in pre-monsoon. The rate of carbon sequestration was positively correlated with age of planted site, tree height, tree diameter, net canopy photosynthesis, AGB, BGB, total biomass, carbon stock, growth efficiency, AGB/tree height tree girth, leaf area index, silt content (p < 0.01). The carbon sequestration was negatively corrected with soil temperature and clay content (p < 0.05). Mangroves were found to be a productive system and important sink of carbon in the tropical coastal zone, but increasing soil temperature due to global warming would have a negative impact on carbon sequestration potential of the mangroves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests contribute significantly to the global carbon, although they colonise only 0.7 % of global coastal zone. The mangroves are known to remove CO2 from the atmosphere through photosynthesis. This perhaps reduces the problems that go with the ‘green house gases’ and global warming. They fix greater amounts of CO2 per unit area, than what the phytoplankton do in the tropical oceans (Kathiresan and Bingham 2001). Mangroves also respond well to high CO2. For example, Rhizophora mangle shows greater accumulation of biomass under high CO2 conditions (Farnsworth et al. 1996). The mangroves contribute to 10 % of total net primary production and 25 % of carbon burial in the global coastal zone (Alongi 2007). A recent assessment of global mangrove carbon budget, based on published data, indicates that global mangrove primary production is 218 trillion grams of carbon annually and this contributes carbon to the ocean through the processes of export, sediment burial and mineralization of carbon fixed by net primary production of the mangrove forests. It appears that >50 % of the carbon fixed by mangrove vegetation is unaccounted for (Bouillon et al. 2008).
Because the mangroves fix and store significant amounts of carbon, their loss may have impact on global carbon budget. According to Cebrain (2002), a loss of about 35 % of the world’s mangroves has resulted in a net loss of 3.8 × 1014 g C stored as mangrove biomass. It must be kept in mind that mangroves are among the most productive ecosystems and their carbon stock per unit area can be enormous (Twilley et al. 1992). Rehabilitating degraded mangrove areas will contribute to carbon sequestration to mitigate the global warming threat. It is expected that new counter-measure of global warming is by restoration of lost mangrove forests. This requires much more studies on the potential of such restored sites for carbon sequestration.
It is difficult to study biomass changes of mangrove forests by harvesting the trees from planted or mature forests. In this regard, the allometric method is comparatively better one to estimate the weight of a tree from measurable tree dimension, including trunk diameter and height. This is also a non-destructive method and easy to study temporal changes of biomass (Komiyama et al. 2008). Therefore, allometric equations for mangroves have been developed for several decades to estimate biomass and subsequent growth (Clough et al. 1997; Chave et al. 2005; Komiyama et al. 2005; Dahdouh-Guebas and Koedam 2006). Estimation of the potential of a forest in sequestrating carbon involves calculating the total biomass per hectare, and then applying appropriate conversion factors to get the carbon equivalents.
There are knowledge gaps in mangrove sequestration potential in planted mangroves in relation to growth attributes, age of plantation and seasonal changes and sediment characterizations. In this regard, only dearth of published data is available. Therefore, the present study was made on the carbon sequestration potential in two species of mangroves (Rhizophora mucronata and Avicennia marina) along with their growth, biomass, sediment characteristics for four seasons, in the planted mangrove stands of different ages.
Materials and methods
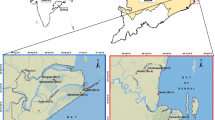
The study area is the Vellar-Coleroon estuarine complex, located along the Bay of Bengal on the southeastern coast of the state of Tamil Nadu, India (Fig. 1). There are four seasons prevailing in the area: post-monsoon (Jan.–Mar.), summer (Apr.–June), Pre-monsoon (July–Sep.) and monsoon (Oct.–Dec.). The tides are semi-diurnal with a range of tidal amplitude from 15 to 100 cm, reaching maximum during monsoon and a minimum in summer. The depth of the water ways ranges from 0.3 to 3 m. The mean annual temperature of this area is 27 °C and annual precipitation is 1465 mm with 52 rainy days (Kathiresan 2000).
In the study area, 17 experimental sites planted with two species of mangroves—Avicenna marina (Forssk.) Vierh and Rhizophora mucronata Poir., of different age from 1 to 17.5 year old stands were fixed. Eleven plots along the Vellar estuary (Lat. 11° 29′ 19.1–25.2″ N; Long. 79° 45′51.9–57.3″ E) and six plots in Pichavaram (Lat. 11° 29′ 21.9–28.3″ N; Long. 79° 46′ 27.2–57.3″ E) were used for experimental study. Although all the experimental sites were located in the same area of similar environmental conditions, there was spatial variation in addition to age of plant growth of the sites. Three healthy plant individuals of R. mucronata and A. marina were selected from each of the experimental plots.
The individual plants were measured for height in meters, girth in cm at 130 cm of breast height, light intensity at above canopy and below canopy using a lux meter. From these measures, leaf area index, net canopy photosynthesis, biomass of aboveground (AGB), belowground (BGB), total and AGB/BGB were calculated using the Internationally recognized scientific techniques recommended by the Australian Institute of Marine Sciences (AIMS) (English and Wilkinson Basker 1997). The measurement was made for four seasons: summer (May 2009), premonsoon (July 2009), monsoon (Dec.2009) and post monsoon (Jan. 2010) in all the 17 plots.
Net canopy production was estimated using the light interception method of Bunt et al. (1979) as modified by Clough (1997). Measurements of light absorption by the forest canopy (100–250 light readings per plot on a sunny day between 1,000 and 1,400 h were used to estimate leaf area index, L (=m2 leaf area m−2 ground area) using the formula.
Where, (I) mean=the mean photosynthetically active radiation (PAR) under the canopy; (I σ ) mean= incident PAR; and k = canopy light extinction coefficient (0.5). L was corrected to a solar zenith angle (θ) for the latitude of the forests.
The leaf area index (L) was then converted to net canopy photosynthesis (PN) using the formula.
Where, d = day length (hrs) and A = average rate of photosynthesis per unit leaf area are 0.3456 for R. apiculata, 0.4752 for A. marina (Clough et al. 2000).
The biomass was estimated using the following allometric equation, proposed by Komiyama et al. (2005).
Where D – Trunk diameter in centimeter at breast height at 30 cm above ground in R. mucronata and at 130 cm above ground in A. marina
- DB :
-
Trunk diameter in centimeter at the lowest living branch
- H:
-
Tree height in meter
- Ρ :
-
Wood density of trunk in ton per m3
The total biomass per tree was converted to carbon biomass per tree by multiplying with a factor 0.42, as the average carbon content is 42 % of total biomass, as found for the present study using in CHN/O analyser (Perkin Elmer-series II 2400). The latter was converted to carbon biomass per year, based on the forest age, as the rate of carbon sequestration in the tree biomass.
Sediment samples were collected from rhizosphere soil of individual trees of each of the sampling plots in four seasons: summer (May 2009), pre-monsoon (July 2009), monsoon (Dec.2009) and post-monsoon (Jan. 2010). The samples were drawn using a 1.5 m long stainless steel corer during low tide and analysed in situ for temperature, hydrogen ion concentration (pH) and redox potential (Eh), and salinity of pore water. Temperature was measured by using a thermometer with 0.5 °C accuracy; pH and redox potential (Eh) by using a millivoltmeter with platinum electrode (pH 315i/SET, Wissenschaftlich Technische Werkstatten, Germany) and pore water salinity by using a hand refractometer (Erma INC, Tokyo). In the laboratory, the sediment samples were analyzed for contents of silt, clay and sand using pipette method (Buchanan 1984).
Statistical procedures
A suite of statistical analysis (SPSS 11.5) was made to assess the significance for each variable between the plant age groups or mangroves species or season of analysis. Post hoc multiple comparison test (Tukey’s, S-N-K), were also used to identify significance between different combinations. Correlation was made between carbon sequestrations and other variables and significant ones (p < 0.05) are shown in figs.
Results
Plant height varied significantly between species or age of plantation (p < 0.01), but not between season of analysis. The plant height was 8 % higher in Rhizophora mucronata than that in Avicennia marina. It ranged from 0.41 to 8.75 m respectively in 1 year and 16.3 year old plantations (Table 1). Thus, there was 21 fold increase in tree height within 15.3 years of plantation; in other words, the growth was 4.6 cm per month or 55.2 cm per year.
Tree girth at breast height varied significantly between mangrove species or age of plant or seasons of analysis (p < 0.01) (Table 1). Avicennia marina had 2.7 fold higher girth than R. mucronata. Tree diameter at breast height was significantly different between species, age and seasons (p < 0.01) (Table 1). It ranged from 2.61 to 10.22 cm respectively in 7- and 11.1 years of plant growth that was 3.9 fold higher growth within 4.1 years, that was 1.9 cm per year and 0.2 cm per month.
Net canopy photosynthesis varied between mangrove species or age of growth or seasons (Table 1). Avicennia marina exhibited 24 % higher net canopy photosynthesis (65.43 tC ha−1 year−1) than R. mucronata (52.64 tC ha−1 year−1). Leaf area index (LAI) did show variation between seasons or age of growth (p < 0.01; Table 1). It varied from 3.33 to 6.32 in one and 15.3 years of plantations respectively, and thus there was 90 % increase in LAI within 14.3 year; in other words, LAI increased at a rate of 0.2 per year and 0.02 per month. Seasonally, LAI was higher in monsoon (7.36) and post-monsoon (6.5) than that in pre-monsoon (4.22) and summer (3.02) (Table 1); and its value was 2.4 fold higher in monsoon and 2.2 fold in post-monsoon, 40 % higher in pre-monsoon than that in summer.
Aboveground biomass (AGB) exhibited significant difference between mangrove species (p < 0.01) or seasons or age of growth (p < 0.05). AGB was 2 fold higher in A. marina (117.65 t ha−1) than R. mucronata (59.95 t ha−1). Seasonally, AGB was highest in post-monsoon (48.5 t ha−1), followed by monsoon (16.96 t ha−1), summer (6.91 t ha−1) and lowest in pre-monsoon (4.75 t ha−1) (Table 1) and its value was 10.2 fold higher in post-monsoon and 3.6 fold more in monsoon than that in pre-monsoon.
Belowground biomass (BGB) did exhibit significant variation between mangrove species or age of growth or seasons (p < 0.01; Table 1). BGB was 40 % higher in A. marina (43 t ha−1) than R. mucronata (30.65 t ha−1). It ranged from 1.25 to 112.85 t ha−1 respectively in 1 year and 16.8 year-old plantations and thus its value was 90 fold higher within 16.5 years of growth. Seasonally, AGB was highest in post-monsoon (67.2 t ha−1), followed by monsoon (40 t ha−1), summer (22.65 t ha−1) and lowest in pre-monsoon (13.4 t ha−1). BGB was 5 fold higher in post-monsoon, 3 fold more in monsoon and 69 % higher in summer than that in pre-monsoon. The ratio between AGB and BGB displayed variation only between species (p < 0.05). The ratio was 2.73 in A. marina and 1.95 in R. mucronata (Table 1) and it was 40 % higher in the former than in the later.
Total biomass varied significantly between mangrove species or seasons (p < 0.01) or age of growth (p < 0.05) or interactive effects of species x age and species x seasons (p < 0.01) (Table 2). There was 77.3 % higher biomass in A. marina (160.65 t ha−1) than that in R. mucronata (90.6 t ha−1). Total biomass ranged from 2.5 to 407.95 t/ha respectively in 1- and 16.8 years of plantations and thus it was 163 fold higher within 15.8 years of growth, that was 25.7 t/ha/year and 2.1 t/ha/month.
Carbon stock of the total biomass did exhibit variation between age of growth or mangrove species (p < 0.05) or seasons (p < 0.01) (Table 2). The carbon stock was 77 % higher in A. marina (67.47 t ha−1) than that in R. mucronata (38.05 t ha−1). Carbon stock ranged from 1.05 to 171.33 t/ha in one and 16.8 years of plantations respectively and there was 163 fold higher biomass within 15.8 years of growth, that was 10.3 t/ha/year or 0.86 t/ha/month. Carbon stock was highest in post-monsoon (114.36 t ha−1) followed by monsoon (52.58 t ha−1), summer (24.02 t ha−1) and lowest in pre-monsoon (15.6 t ha−1) and there was 7.3 fold higher value in pre-monsoon, 3.4 fold in monsoon, 54 % more in summer than in pre-monsoon.
Rate of carbon sequestration varied between mangrove species or age of growth or seasons (p < 0.01) or between the interactive effects of species x seasons (p < 0.05) (Table 2). The rate of carbon sequestration was 75 % higher in A. marina (4.8 t ha−1 year−1) than that in R. mucronata (2.75 t ha−1 year−1). Seasonally the rate of carbon sequestration was the highest in post-monsoon (8 t/ha/year), followed by monsoon (3.75 t ha−1 year−1), summer (1.9 t ha−1 year−1) and the least in pre-monsoon (1.1 t ha−1 year−1) and thus the value was 7.3 fold higher in post-monsoon, 3.4 fold in monsoon, 73 % more in summer than that in pre-monsoon.
Growth efficiency varied between age of growth or seasons or their interactive effects (p < 0.01), but not between mangrove species (Table 2). It ranged from 0.02 to 1.32 t ha−1 year−1 in 2 and 14.9-years of plantations respectively and thus there was 66 fold increase in growth efficiency within 12.9 years of growth. The growth efficiency was highest in summer (0.53 t ha−1 year−1) followed by post-monsoon (0.38 t ha−1 year−1), monsoon (0.15 t ha−1 year−1) and lowest in pre-monsoon (0.13 t ha−1 year−1) and there was 4 fold increase in summer, 2.9 fold in post-monsoon, 15 % more in monsoon than that in pre-monsoon.
Leaf longevity varied between mangrove species or age of growth or seasons, (p < 0.05); it was the highest (6.9 months) in post-monsoon, followed by monsoon (3.58 months), summer (3.39 months) and least (2.92 months) in pre-monsoon, and thus leaf longevity was high by 2.4 fold, 23 % and 16 % respectively, as compared to pre-monsoon. Leaf turnover rate exhibited significant variation between mangrove species, age of growth or seasons (p < 0.05). The leaf turnover rate was 2.6 fold higher in R. mucronata than that in A. marina. It ranged from 0.64 per year in 11.1 year of plant growth and 7.8 per year in 15.2 year of growth with 12 times increase of leaf turnover rate within 4.1 years. The leaf turn over rate also varied seasonally with 2 times greater value in pre-monsoon, 85 % higher in monsoon, and 81 % more in summer than that in post-monsoon. The ratio between AGB and tree height varied only between mangrove species (p < 0.01) or seasons (p < 0.05) (Table 2). The ratio was 2 fold higher in A. marina (27.42 t ha−1 m−1) than that in R. mucronata (12.92 t ha−1 m−1) and it was 7.8 fold higher in post-monsoon (43.44 t ha−1 m−1), 3.6-fold in monsoon (19.95 t ha−1 m−1) and 50 % more in summer (8.36 t ha−1 m−1) than that in pre-monsoon (5.58 t ha−1 m−1).
Soil temperature showed significant difference between mangrove species or seasons (p < 0.05) (Table 3) and it was 5.8 % higher in R. mucronata than that in A. marina. The soil temperature ranged from 24.24 °C in monsoon to 31.52 °C in summer with an increase of 30 % in the latter. Soil pH and did not show any significant difference between mangrove species or seasons or age of growth. Soil salinity varied seasonally (p < 0.05) from 20.61 ppt in monsoon to 36.91 ppt in summer. Silt content of soil also showed variation between season (p < 0.01) ranging from 17.29 % in pre-monsoon to 24.91 % in monsoon with 14 % increase between the two seasons. Clay content varied between mangrove species (p < 0.01) or seasons (p < 0.05) and it was 30 % more in R. mucronata than that in A. marina. It ranged from 42.41 % in summer to 53.52 % in monsoon with 26 % increase between the seasons. Sand content showed significant difference between age of growth (p < 0.05) or seasons (p < 0.05) and it was 48.75 % in 2 year old plantation and 12 % in 11.1 year-old plantations with a 4 fold decline of sand as the plantation aged. Sand ranged from 19.32 % in monsoon to 41.58 % in pre-monsoon with 2.2 fold increase between the seasons.
Rate of carbon sequestration was positively correlated with age of plant growth (Fig. 2a), tree height (Fig. 2b), tree girth (Fig. 2c), tree diameter (Fig. 2d) (p < 0.01), leaf area index (Fig. 2e; p < 0.05), net canopy photosynthesis (Fig. 2f), AGB (Fig. 2g), BGB (Fig. 2h), total biomass (Fig. 2i), carbon stock (Fig. 2j), growth efficiency (Fig. 2k), AGB/tree height (Fig. 2 l) (p < 0.01), silt (Fig. 2n). The rate of carbon sequestration was negatively corrected with soil temperature (Fig. 2 m), and clay (Fig. 2 0) (p < 0.05).
Regression between rate of carbon sequestration in mangrove biomass and forest age (a), tree height (b), gbh (c), dbh (d), leaf area index (e), net canopy photosynthesis (f), AGB (g), BGB (h), total biomass (i), carbon stock (j), growth efficiency (k), AGB/tree height (l), soil temperature (m), silt (n), and clay (o)
Discussion
The rate of carbon sequestration was 75 % higher in Avicennia marina than that in Rhizophora mucronata (Table 1). This could be attributed to higher growth efficiency and biomass production of A. marina. For instance, the growth efficiency was 43 % higher in A. marina than that in R. mucronata (Table 2). Similarly the biomass density was 2.1 fold greater in A. marina than in R. mucronata (Table 2). In most mangroves, the biomass density appears to be higher than in terrestrial forests (Teas 1979).
In the present study, above ground biomass (AGB) was higher in Avicennia marina (117.65 t.ha−1) than that in Rhizophora mucronata (59.95 t.ha−1), which was similar to the trend of carbon sequestration in the mangrove species (Table 1). However, AGB values vary with the mangrove forest: 281 t ha−1 in Rhizophora forest (Tamai et al. 1986), 357 t ha−1 in Sonneratia forest (Komiyama et al. 1987) and 315 t ha−1 in Avicennia germinans (Fromard et al. 1998), 94.8 t ha−1 in a secondary mangrove forest of R. mucronata and Bruguiera gymnorrhiza (Suzuki and Tagawa 1983) and 62.9 t ha−1 in a R. mangle forest (Golley et al. 1962). The AGB are reportedly more than 300 t.ha−1 in Malaysia (Putz and Chen 1986), Indonesia (Komiyama et al. 1988) and French Guiana (Fromard et al. 1998). The AGB is also reportedly less than 100 t ha−1 in most secondary forests and primary forests of high latitude areas (>24°23′N or S) (Mackey 1993). The lowest AGB reported is 7.9 t ha−1 for a Rhizophora mangle forest in Florida, USA (Lugo and Snedaker 1974).
In the present study, belowground biomass (BGB) was higher in A. marina (43 t ha−1) than that in R. mucronata (30.65 t ha−1), similar to the trend of carbon sequestration in the mangrove species (Table 1). The values are closer to 32.4 t ha−1 in the Sonneratia forest (Komiyama et al. 1987). However, the values are much lower than that of other mangrove forests: 106–173 t ha−1 for Bruguiera, 187–273 t ha−1 for Rhizophora (Komiyama et al. 1987) and 87.5 t ha−1 in the Ceriops tagal (Komiyama et al. 2000).
Generally mangroves show relatively high amount of root biomass than other forms of forests (Saintilan 1997a, b; Komiyama et al. 2000). It is noteworthy that the large biomass allocated to the underground roots in the mangroves as revealed by the low ratio in the present study (Table 1). Mangroves are usually coping with the stresses of high water tables, salty soil and less mechanical support due to soft muddy substrate. Mangroves are unable to mechanically support their above-ground weight without a heavy root system. Therefore, a large allocation of the net production into roots is necessary. In addition, soil moisture may cause increased allocation of biomass to the roots with enhanced cambial activity induced by ethylene production under submerged conditions (Yamamoto et al. 1995). Most mangrove species are highly sensitive to variation in nutrient availability (Boto and Wellington 1988; Feller 1995; Koch 1997; Feller et al. 2007; Lovelock et al. 2005, 2007; Naidoo 2006). Enhanced allocation to root biomass relative to shoot biomass is a common adaptation to low nutrient availability. Species of Rhizophoraceae are more tolerant to low nutrient conditions than other mangrove species (Komiyama et al. 2000; Krauss et al. 2008), as was substantiated in the present study by low ratio of AGB/BGB in R. mucronata, as compared to A. marina (Table 1). This ratio between AGB and BGB was 2.73 for A. marina and 1.95 for R. mucronata in the present study (Table 1). The ratio is much higher than other reports: 1.12 in Kandelia obovata (Khan et al. 2009), 1.05 in Ceriops tagal (Komiyama et al. 2000), 1.72 in A. marina (Mackey 1993), but is closer to 2.27 in a Rhizophora forest (Tamai et al. 1986). In tropical inland forests the ratio which ranges from 5.1 to 10.7 is higher than that in mangroves (Ogawa et al. 1965; Hozumi et al. 1969).
In the present study, the growth variables exhibited significant variation largely between mangrove species rather than mangrove sites. This finds the support of Komiyama et al. (2005) who have suggested that the allometric equations of mangrove species are highly species-specific but less site-specific. For example, the present study recorded leaf area index in a range of 3.33–6.32 (Table 1). A similar range of leaf area index is reported in other mangrove areas: 3.3 to 4.9 in R. apiculata in Mekong Delta, Vietnam (Clough et al. 2000) and 1.6 to 5.1 in Sawi Bay, Southern Thailand (Along and Dixon 2000). The present study registered net canopy photosynthesis in a range of 37.27 to 75.44 tC ha−1 year−1 (Table 1). A similar range of the net canopy photosynthesis has been recorded in other mangrove areas: from 24.5 tC ha−1 year−1 in 5 year old forest to 76.6 tC ha−1 year−1 in 25 year old forest in Sawi Bay (Along and Dixon 2000).
The rate of carbon sequestration recorded in present study was in a range between 1.1 and 8 t C ha−1 year−1 (Table 2). This value is lower than previous report: 13.57 t C ha−1 year−1 in 20-year old stand of Rhizophora apiculata in Peninsular Malaysia (Ong et al. 1995). In the Matang of Malaysia, total standing biomass was 114 t C ha−1 for R. apiculata (Ong et al. 1995) as against 90.6 t C ha−1 in R. mucronata in the present study (Table 2). Similarly, the 20 year old stand of Rhizophora apiculata in Matang of Malaysia shows the girth at breast height (gbh) of 30 cm and the average height of 21 m with biomass ranging from 10 to 510 kg with a mean biomass of 122 kg (Ong et al. 1995). However in the present study, all these values were low for R. mucronata, showing the mean gbh value of 11.87 cm, tree height of 4.64 m with a mean biomass of 8.53 kg (Table 1). The reason for these low values is that the present site is experiencing high salinity, high wind action, lack of freshwater, and man-made pressures (Kathiresan 2000; Alongi et al. 2005a, 2005b), whereas the Malaysian mangrove forest has been well-managed and it has moderate salinity and all other environmental conditions, favourable for growth of mangrove species (Ong et al. 1995).
The organic carbon burial rate in mangrove sediment is estimated at 1.39 t C ha−1 year−1 (Duarte et al. 2005). This value is lower than the carbon sequestered in the form of mangrove standing biomass, as evident in the present study (Table 2). Relatively high primary production of tree biomass is considered to bring about unusual carbon dynamics (Komiyama 2006). Therefore, mangroves forest is a highly efficient carbon sink in the tropics. However, the global warming may hit the efficiency of carbon sequestration by mangroves, as evident by a negative correlation between carbon sequestration and soil temperature (Fig. 2m).
References
Along DM, Dixon P (2000) Mangrove primary production and above—and below ground biomass in Sawi Bay, southern Thailand. Phuket Mar Biol Cent Spec Publ 22:31–38
Alongi DM (2007) The contribution of mangrove ecosystems to global carbon cycling and greenhouse gas emissions. In: Tateda Y, Upstill-Goddard R, Goreau T, Alongi D, Nose A, Kristensen E, Wattayakorn G (eds) Greenhouse gas and carbon balances in mangrove coastal ecosystems. Maruzen, Tokyo, pp 1–10
Alongi DM, Ramanathan AL, Kannan L, Tirendi F, Trott LA, Balakrishna Prasad M (2005a) Influence of human-induced disturbance on benthic microbial metabolism in the Pichavaram mangroves, Vellar-Coleroon estuarine complex, India. Mar Biol 147:1033–1044
Alongi DM, Pfitzner J, Trott LA, Tirendif Dixon P, Klumpp DW (2005b) Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang Estuary, China. Estuar Coast Shelf Sci 63:605–618
Boto KG, Wellington JT (1988) Seasonal variations in concentrations and fluxes of dissolved organic and inorganic materials in a tropical, tidally-dominated, mangrove waterway. Mar Ecol Prog Ser 50:151–160
Bouillon SR, Connolly, Lee SY (2008) Carbon exchange and cycling in mangrove ecosystems: a synthesis of recent insights based on stable isotope studies. J Sea Res 59:44–58
Buchanan JB (1984) Sediment analysis. In: Holme NA, McIntyre AD (eds) Methods for the study of Marine Benthos. Blackwell Scientific Publictaions, London, pp 41–65
Bunt JS, Boto KG, Boto G (1979) A survey method for estimating potential levels of mangrove forest primary production. Mar Biol 52:123–128
Cebrain J (2002) Variability and control of carbon consumption, export, and accumulation in marine communities. Limnol Oceanogr 47:11–22
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Clough BF (1997) Mangrove ecosystems. In: English S, Wilkinson C, Baker V (eds) Survy manual for tropical marine resources, 2nd edn. Australian Institute of Marine Science Townsville pp 119–196
Clough BF, Dixon P, Dalhaus O (1997) Allometric relationships for estimating biomass in multi-stemmed mangrove trees. Aust J Bot 45:1023–1031
Clough BF, Patanaporpaiboon P, Poungparn S (2000) Forest structure and carbon fixation by mangroves at Chumphon, Southern Thailand. In: Alongi DM, Ayukai T (eds) Carbon Fixation and storage in coastal ecosystems-phase 2. Final Report to the Kansai Electric Power Co., Japan, Australian Institute of Marine Science, Townsville pp 10–16
Dahdouh-Guebas F, Koedam N (2006) Empirical estimate of the reliability of the use of the Point-Centred Quarter Method (PCQM): Solutions to ambiguous field situations and description of the PCQM + protocol. Forest Ecol Manage 228:1–18
Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2:1–8
English SC, Wilkinson Basker V (1997) Survey manual for tropical marine resources, 2nd edn. Australian Institute of Mar Sci Townsville pp 119–195
Farnsworth EJ, Ellison AM, Gong WK (1996) Elevated CO2 alters anatomy, physiology, growth and reproduction of red mangrove (Rhizophora mangle L.). Oecologia 108:599–609
Feller IC (1995) Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecol Monogr 65:477–505
Feller IC, Lovelock CE, McKee KL (2007) Nutrient addition differentially affects ecological processes of Avicennia germinans in nitrogen vs. phosphorus limited mangrove. Ecosystems 10:347–359
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L (1998) Structure above-ground biomass and dynamics of mangrove ecosystems: new data from French Guiana. Oecologia 115:39–53
Golley F, Odum HT, Wilson R (1962) The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 43:9–19
Hozumi K, Yoda K, Kokawa S, Kira T (1969) Production ecology of tropical rain forest in southern Cambodia I. Plant Biomass. Nat Life SE Asia 6:1–51
Kathiresan K (2000) A review of studies on Pichavaram mangroves, southeast India. Hydrobiologia 430:185–205
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Khan MNI, Suwa R, Hagihara A (2009) Biomass and above-ground net primary production in a subtropical mangrove stand of Kandelia obovata (S.L.) Yong at Manko Wetland, Okinawa, Japan. Wetl Ecol Manag 17:585–599
Koch MS (1997) Rhizophora mangle L. seedling development into the sapling stage across resource and stress gradients in subtropical Florida. Biotropica 29:427–439
Komiyama A (2006) What is required for scientists towards the mangrove management? In: Kyoto Symposium on mangrove management 1–17
Komiyama A, Ogino K, Aksornkoae S, Sabhasri S (1987) Root biomass of a mangrove forest in southern Thailand. J Trop Ecol 3:97–108
Komiyama A, Moriya H, Prawiroatmodjo S, Toma T, Ogino K (1988) Forest primary productivity. In: Ogino K, Chihara M (eds) Biological system of mangrove. Ehime University pp 97–117
Komiyama A, Havanond S, Srisawatt W, Mochida Y, Fujimoto K, Ohnishi T, Ishihara S, Miyagi T (2000) Top/root biomass ratio of a secondary mangrove (Ceriops tagal (Perr.)C.B.Rob) forest. Forest Ecol Manage 139:127–134
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21:471–477
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Krauss KW, Lovelock CE, Mckee KL, Lopez-Hoffman L, Ewe SML, Sousa WP (2008) Environmental drivers in mangrove establishment and early development: a review. Aquat Bot 89:105–127
Lovelock CE, Feller IC, Mckee KL, Thompson R (2005) Variation in mangrove forest structure and sediment characteristics in Bocas del Toro Republic of Panama. Caribb J Sci 41:456–464
Lovelock CE, Feller IC, Ellis J, Hancock N, Schwarz AM, Sorrell B (2007) Mangrove growth in New Zealand estuaries: the role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 153:633–641
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Ann Rev Ecol Evol Syst 5:39–64
Mackey AP (1993) Biomass of the mangrove Avicennia marina (Forsk.)Vierh. near Brisbane, south eastern Queensland. Aust J Mar Freshwat Res 44:721–725
Naidoo G (2006) Factors contributing to dwarfing in the mangrove Avicennia marina. Ann Bot 97:1095–1101
Ogawa H, Yoda K, Ogino K, Kira T (1965) Comparative ecological studies on three main type of forest vegetation in Thailand. Nat Life SE Asia 4:49–80
Ong JE, Gong WK, Clough CH (1995) Structure and productivity of a 20-year old stand of Rhizophora apiculata Bl. Mangrove forests. J Biogeogr 22:417–427
Putz F, Chen HT (1986) Tree growth dynamics and productivity in a mature mangrove forest in Malaysia. Forest Ecol Manage 17:211–230
Saintilan N (1997a) Above- and below- ground biomass of two species of mangrove in a sub-tropical estuary. Mar Freshwat Res 48:601–604
Saintilan N (1997b) Above and below ground biomass of two specie of mangrove on the Hawkesbury River estuary, New South Wales. Mar Freshwat Res 48:47–152
Suzuki E, Tagawa E (1983) Biomass of a mangrove forest and a sedge marsh on Ishigaki Island, south Japan. J Ecol 33:231–234
Tamai S, Nakasuga T, Tabuchi R, Ogino K (1986) Standing biomass of mangrove forests in southern Thailand. J Jpn For Soc 68:384–388
Teas HJ (1979) Silviculture with saline water. In: Hollaender A, Aller JC, Epstein E, Pietro AS, Zaborsky OR (eds) The bio saline concept. Plenum Press, New York, pp 117–161
Twilley RR, Chen RH, Hargis T (1992) Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut 64:265–288
Yamamoto F, Sakata T, Terazawa K (1995) Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol 15:713–719
Acknowledgments
Authors are thankful to the authorities of Annamalai University for providing facilities and to the Ministry of Earth Sciences, Govt. of India for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kathiresan, K., Anburaj, R., Gomathi, V. et al. Carbon sequestration potential of Rhizophora mucronata and Avicennia marina as influenced by age, season, growth and sediment characteristics in southeast coast of India. J Coast Conserv 17, 397–408 (2013). https://doi.org/10.1007/s11852-013-0236-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11852-013-0236-5