Abstract
Present study focuses on the carbon sequestration potential of five dominant mangrove species (Avicenia marina, Avicenia officinalis, Excoecaria agallocha, Rhizophora mucronata and Xylocarpous granatum) in Bhitarkanika and Mahanadi mangrove ecosystem. Water and soil parameters were sampled and analyzed for 10 selected stations along with aboveground biomass (AGB) and aboveground C (AGC) values. AGB value in the study area ranged from 15.00 ± 2.12 to 70.09 ± 6.68 tha−1 for A. marina, 26.13 ± 3.19 tha−1 to 616.94 ± 50.15 tha−1 for A. officinalis, 3.56 ± 0.96 tha−1 to 98.66 ± 5.24 tha−1 for E. agallocha, 7.06 ± 2.21 tha−1 to 224.41 ± 21.20 tha−1 for R. mucronata, and 0.64 ± 0.21 tha−1 to 6.25 ± 1.52 tha−1 for X. granatum, respectively. AGC value ranged from 7.63 ± 1.08 to 35.65 ± 2.63 tha−1 for A. marina, 1.73 ± 0.01 tha−1 to 280.83 ± 21.29 tha−1 for A. officinalis, 1.64 ± 0.41 tha−1 to 44.95 ± 2.53 tha−1 for E. agallocha, 3.44 ± 1.45 tha−1 to 114.05 ± 10.29 tha−1 for R. mucronata and 0.31 ± 0.10 tha−1 to 3.25 ± 0.31 tha−1 for X. granatum, respectively. The average SOC values in tha−1 varied from 3.52 ± 0.12 to 7.71 ± 0.45. The total carbon (AGC + SOC) calculated for the study area varied from 55.20 ± 7.90 to 330.41 ± 111.97 tha−1 with a mean total carbon of 124.11 ± 30.14 which is equivalent to 455.47 ± 110.56 tons of CO2. Considering the total area of Bhitarkanika and Mahanadi mangrove ecosystem (672 + 141,589) to be 142,261 km2, the mean CO2e be 455.47 ± 110.56 tones, it is approx. 64,795,617.67 ≅ 64.80 TgC that were absorbed from the atmosphere, thus reducing the amount of carbon dioxide from the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are distributed between latitude 32°20′ in northern hemisphere in Bermuda to 38°59′ in southern hemisphere in New Zealand (Spalding et al. 2010; Giri et al. 2011). Forest cover and its distribution including mangroves in India were monitored by the Forest Survey of India (FSI) from 1987 (FSI 1987). The mangrove patches are distributed in the nine coastal states including three union territories. The total mangrove cover of India was 4921 km2, which covers 0.15% of India, 3% of world mangrove area, and 8% of Asia (Singh and Odaki 2004; FSI 2017) and all the mangrove patches experienced an increase in area. The top 10 mangrove dominated states in India are West Bengal (2097 km2) > Gujarat (1103 km2) > Andaman and Nicobar Islands (604 km2) > Andhra Pradesh and Telangana (352 km2) > Odisha (213 km2) > Maharashtra (186 km2) > Tamil Nadu (39 km2) > Goa (22 km2) > Kerala (6 km2) > Karnataka (3 km2). In the east coast of India mangroves are concentrated in the Sundarbans region of West Bengal, Subarnarekha, Bhitarkanika and Mahanadi delta of Odisha, Godavari and Krishna delta of Andhra Pradesh, Pichavaram estuary and Cauvery estuary of Tamil Nadu (Mitra 2013). Agarwal et al. (2017) reported that in Odisha, the mangroves spread over an area of 214 km2. Out of the total mangrove area of the state, Mahanadi delta covers an area of 120 km2. The mangrove area in the Mahanadi delta (20°15′ to 20°70′ N latitude and 87° to 87°40′ E longitude) extends from south eastern boundary of Mahanadi river to river mouth of Hansua (a tributary of Brahmani) in the north, from the north eastern end of Mahanadi river up to Jambu river in east. Mahanadi mangrove wetland encompasses eight forest blocks.
Estimation of biomass in mangrove forests typically involves both destructive and non-destructive methods. Destructive method involves cutting down of trees and measuring biomass directly. Although the data are more accurate, but, looking at the conservation aspect, this method is not acceptable. In case of non-destructive method height and DBH, measurements are taken in order to calculate the biomass using mathematical formulae and allometric equations. These results of biomass vary w.r.t the distribution of the species in an area, particularly plantation areas and wild forest (Chave et al. 2005; Komiyama et al. 2005). Mangroves can sequester 3–5 times more atmospheric CO2 than other terrestrial forest (Lee et al. 2014). Including all the economic services, mangrove forest produces an estimated annual economic value of more than US$ 900,000 km−2 (UNEP-WCMC 2006). All the ecological services of mangrove depend on the productivity of the forest. The higher productivity also contributes significantly to global carbon budget. The average productivity of mangrove biomass ranges between 3.07 and 24.1 tha−1 year−1 having turn over time period < 30 year (Twilley et al. 1992; Estrada and Soares 2017). The carbon stock of mangroves in 10 countries vary as per the order Indonesia > Mexico > Malaysia > Bangladesh > Thailand > Philippines > Vietnam > Dominican Republic > Micronesia > Palau, respectively. Carbon values ranged between 441.76 ± 120.76 and 1267.00 ± 872.72 tCha−1 with global mean of carbon stock is 78.0 ± 64.5 tha−1 and carbon sequestration rate of 2.9 ± 2.2 tCha−1 year−1 (Murdiyarso et al. 2015; Estrada and Soares 2017). Globally carbon stored in mangrove biomass (AGB + BGB) is 4.03 Pg C with an annual storage capacity of 0.18 Pg C year−1 (0.16 Pg C year−1 by biomass and 0.02 Pg C year−1 by sediments) (Twilley et al. 1992). The sediment characteristics of mangroves played a pivotal role in carbon sequestration. Optimum physico-chemical composition and condition of sediment increases the rate and potential of carbon sequestration (Banerjee et al. 2018).
The alternation and change of an ecosystem, extinction of species and habitat is a continuous natural process of earth (IUCN 2019). Due to multifarious human induced problems like global warming, climate change, oceanic acidification, glaciers melting, sea level rise (SLR), pollution (air, water, soil and spectrum), radiation, etc. the mangroves have been affected directly or indirectly. Since Industrial Revolution, the concentration of CO2 and equivalent gas has increased rapidly in the atmosphere from 280 ppm to > 410 ppm causing global warming (NOAA 2019). The increase in average global temperature to 1.0 ± 0.2 °C from the industrial era is expected to increase by 1.5 °C between 2030 and 2052 (IPCC 2018). CO2 is the major greenhouse gas in the atmosphere that releases ≥ 80% among all other GHG in a year from all sources with a rate of 11.3 ± 0.9 Pg C year−1 in the year 2018 (Le Quere et al. 2018). Hence to mitigate the global problem along with development, sequestration of carbon, increased use of renewable energy and adopting sustainable policies is of utmost importance.
Mangrove forest floor is an important pool of organic matter (OM) and nutrients which are added to the sediment on account of degradation of leaf litter and accumulation of detritus during run-offs hence there is huge amount of nutrient deposition in the mangrove sediment which plays important role in global nutrient cycling. This decomposition rate is basically due to the sulphur reducing bacteria (e.g. Desuifovibrio species), which creates an anoxic environment in the substratum. These generate an acidic character of the soil which gives a blackish colour of the soil (Ferreira et al. 2007a, b). However, there are certain areas which contain higher sand particles in comparison to silt and clay particles (Ferreira et al. 2010). These soils are huge sinks of carbon and hence SOC monitoring has become so important. The huge amount of litter and detritus in mangrove forests along with adjacent land run-off contributes to huge quantities of SOC.
Little information is available on estimation of biomass and carbon stock inside and outside the conservation areas particularly Protected Forests (PF). REDD and REDD + programmes have mainly focused on National Parks, Sanctuaries, and Biosphere Reserves for conservation aspects with quantification on carbon storage potential. Very recently the role of mangroves in carbon sequestration has gained mileage as it stores four times more carbon than any tropical forest including rain forests (Donato et al. 2011). An unprecedented increase in atmospheric CO2 from fossil combustion and land use land cover changes has focused the attention towards mitigation strategies of global warming. The challenges of climate change can be effectively overcome by storage of carbon over long period of time. Carbon storage is a situation where degraded soil is restored through afforestation increased biomass and reduces CO2 concentration generated due to fossil fuel (Panda and Panda 2015). On this background the present research programme has pointed towards estimation of aboveground biomass (AGB), aboveground C (AGC) and soil organic C (SOC) along with other selected water and soil parameters of Bhitarkanika and Mahanadi mangrove ecosystem.
Materials and methods
Study area
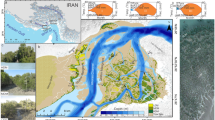
The study area encompasses both Bhitarkanika and Mahanadi mangrove ecosystem of Odisha. These are the two major mangrove chunks of coastal zone of Odisha located in western Bay of Bengal. Geographically the Bhitarkanika mangrove ecosystem is located between the coordinates 20°40′–20°48′ N latitude and 86°45′–87°50′ E longitude bordered and surrounded by river Hansua on the West, Dhamra Port on the North and Bay of Bengal in the eastern and southern side. Politically it is situated in the Rajnagar block of Kendrapara district in the state Odisha under the supervision of Divisional Forest Office, Rajnagar, Kendrapara. The Bhitarkanika mangrove ecosystem was formed by the mighty river system of Brahmani and Baitarani. They join together near Lalitapatia village and formed Dhamra river and formed various small river, creeks and delta. Rivers Khola, Pathasala, Bhitarkanika, Hansua, Kharasrota, Maipura and Hansina are the distributaries of mighty Brahmani–Baitarani, respectively, which are further criss-crossed by numerous creeks, channels and nallahs thus providing peculiar ecological niche for growth and development of mangrove life forms. The Bausagada river is tidal fed river which originates and ends its mouth in Bay of Bengal near Ekakula and Chinchiri, respectively. Five stations namely Stn.1 Dangmal, Stn.2 Bhitarkanika, Stn.3 Gupti, Stn.4 Habalikhati and Stn.5 Ekakula were selected in Bhitarkanika mangrove ecosystem for the present research programme (Fig. 1).
Mahanadi mangrove wetland is located in Kendrapara district between 20°18′ and 20°32′ N latitude and 86°41′–86°48′ E longitudes in Odisha, which is a maritime state in the east coast of Indian sub-continent, being located south of Bhitarkanika Wildlife Sanctuary. The region has dense mangrove, which extend from Hukitola Bay in the north to Mahanadi river mouth near Paradeep port in the south. The ecosystem enjoys tropical monsoon climate. According to the records of Odisha Forest Department, the total mangrove area of the region is about 6651 ha including plantation. Within the Mahanadi mangrove wetland ecosystem, five sites were selected for carrying out the research programme, i.e. Stn.1 Jambu, Stn.2 Kansaridia, Stn.3 Kandarapatia, Stn.4 Kantilo and Stn.5 Bhitar Kharinasi, respectively (Fig. 1).
Estimation of above ground biomass
Simple random sampling method was used to collect the samples. Sample plots were laid along line transects based on tidal variation in the study area. 15 random sampling plots of 10 m × 10 m were selected on the intertidal mudflats. The sampling was carried out during low tide period for continuously 2 years (2017–18 and 2018–19) for three seasons (pre-monsoon, monsoon and post-monsoon) and only the live trees with a diameter at breast height (DBH) ≥ 5 cm were recorded. The DBH was measured at breast height, which is 1.3 m from the ground level of 5 selected mangrove species namely Avicennia marina, Avicennia officinalis, Excoecaria agallocha, Rhizophora mucronata and Xylocarpus granatum. It was measured by using tree calliper and measuring tape. Trees with multiple stems connected near the ground were counted as single individuals and bole circumference was measured separately. Stem height was recorded by using laser-based height measuring instrument (BOSCH DLE 70 Professional model). The methodology and procedures to estimate the stem biomass of the selected true mangrove tree species were carried out step by step as per the VACCIN project manual of CSIR (Mitra and Sundaresan 2016) considering and measuring parameters like DBH, DBR (Diameter of basal region), height of the stem, density of the stem wood and form factor. The population density of each species was also documented to express the value of biomass in tha−1.
Estimation of above ground carbon
Direct estimation of percent carbon was done by Vario MACRO elementar CHN analyzer, after grinding and random mixing the oven dried samples from 15 different sampling plots. The estimation was done separately for each species and mean values were expressed as tha−1. The analyses were done from Institute of Forest Biodiversity, Hyderabad.
Analysis of physico-chemical parameters of the ambient media
The soil and water temperature were measured by using digital thermometer (SIGMA). The soil pH was measured by using digital pH meter (Systronics) and water pH was measured through pocket type digital pH meter (Eco Testr). Water samples are from different rivers, creeks whereas the soil samples near the sampling stations are tested for the salinity. The handy portable Refractometer (Atago, Japan) was used for the salinity test. The handy digital EC meter (Model: Eco Testr) was dipped directly in situ in the soil during field sampling and the reading and mean of several reading was taken as final. Several readings were taken from each station for 2 years (2017–18 and 2018–19) and three seasons (viz. pre-monsoon, monsoon and post-monsoon) and the mean of which taken as final.
Soil samples were collected from different forest blocks for the analysis of carbon content in the soil. The carbon in the soil was determined by wet digestion method of Walkley and Black (1934). Soil samples were collected by the help of a cylindrical still ring of known volume. The Bulk density was calculated by dividing the dry soil weight by soil volume and values were expressed in g cm−3.
Soil samples were brought to laboratory from different sites for determining soil texture i.e. sand, silt and clay according to the particle size. Sieve analysis and hydrometric tests were conducted to find out the percentage of sand, silt and clay in soil. Sieve analyses using various sieves of different mesh opening (0.075–4.75 mm) were used to calculate the percentage of sand. The percentage of soil retained on each sieve is calculated on the basis of the total biomass of the soil sample. In addition, soil fraction finer than 0.075 mm were separated out for further hydrometric test. Hydrometric test was carried out for particle size lesser than 0.075 mm to determine the percentage of silt and clay in soil.
Statistical analyses
All data are expressed as mean ± standard deviation. Interrelationships were plotted for all the physico-chemical parameters, AGB and AGC for all the selected species in both Bhitarkanika and Mahanadi mangrove ecosystems through correlation analysis. Multivariate analysis of variance (MANOVA) was performed keeping physico-chemical parameters, AGB and AGC (per species) as dependent variables and stations and seasons as fixed factors in order to pinpoint the variation of parameters between stations and seasons. The analyses were performed using IBM SPSS Statistics-21 software.
Results and discussion
Mangrove forests play an important role in global carbon cycle. Their carbon dynamics are based on long periods of gradual build up of biomass (a sink) altered with short periods of massive biomass loss (source) (Omar et al. 2003). Carbon dioxide is absorbed from the atmosphere by growing trees and other vegetation through a process of photosynthesis. The same CO2 is emitted by the forest through plant respiration and through process of death and decay. Thus, the balance between the two is the net primary productivity (NPP) of the forest which determines the ecosystem to be a sink or source of carbon (Lal 2007).
Aquatic parameters
In the present study the surface water temperature showed pre-monsoon peaks and post-monsoon troughs which ranged from 20.5 ± 1.50 °C at Stn.1 during post-monsoon 2017–18 to 32.4 ± 2.40 °C at Stn.3 during pre-monsoon 2018–19 at Bhitarkanika and 24.65 ± 0.32 °C at Stn.3 during post-monsoon 2017–18 to 33.80 ± 0.46 °C at Stn.5 during pre-monsoon 2018–19 at Mahanadi mangrove ecosystem. The pH values varied from 7.39 ± 0.24 at Stn.3 during monsoon 2018–19 to 8.22 ± 0.56 at Stn.4 during pre-monsoon 2017–18 at Bhitarkanika mangrove ecosystem and 7.75 ± 0.72 at Stn.3 during monsoon 2018–19 to 8.30 ± 0.64 at Stn.5 during pre-monsoon 2017–18 at Mahanadi mangrove ecosystem respectively. In the present study a bimodal pattern of salinity was observed with maximum in pre-monsoon (34.64 ± 8.32 psu at Stn.5 during 2018–19 at Bhitarkanika and 29.21 ± 0.67 psu at Stn.5 during 2018–19) at Mahanadi and minimum in monsoon (10.12 ± 0.23 psu at Stn.1 during 2017–18 at Bhitarkanika and 9.41 ± 0.21 psu at Stn.3 during monsoon 2017–18) at Mahanadi mangrove ecosystem, respectively.
Soil parameters
The overall pH in the study area showed increasing trend with respect to season. The values ranged from 5.32 ± 0.14 at Stn.2 during monsoon 2018–19 to 6.60 ± 0.23 at Stn.5 during pre-monsoon 2017–18 at Bhitarkanika and 5.02 ± 0.21 at Stn.2 during monsoon 2018–19 to 6.74 ± 0.26 at Stn.5 during pre-monsoon 2017–18 at Mahanadi mangrove ecosystem, respectively. Soil salinity expressed in terms of electrical conductivity is an indicator of the amount of river discharge received in the Sanctuary from the major rivers Brahmani, Baitarani and Bausagada and other small distributaries and anthropogenic outfall. The relatively low EC values were observed at Stn.1 and 2 compared to other stations at Bhitarkanika owing to their proximity to riverine discharge of Brahmani and Baitarani, respectively. Similarly, lower EC values at Stn.3 for Mahanadi is also due to the riverine discharge of river Kharinasi. The seasonal EC values ranged from 3.73 ± 0.28 mScm−1 at Stn.2 during monsoon 2017–18 to 10.22 ± 0.24 at Stn.5 during pre-monsoon 2018–19 at Bhitarkanika and 2.15 ± 0.22 mScm−1 at Stn.3 during monsoon 2017–18 to 17.21 ± 2.92 mScm−1 at Stn.5 during pre-monsoon 2018–19 at Mahanadi mangrove ecosystem, respectively.
In the present study, low bulk density was observed at all the selected stations being mangrove soil, which are characteristics of more percentage of silt and clay in comparison to sand. However, with respect to season, monsoon has shown a lower bulk density than post-monsoon and pre-monsoon in both mangrove ecosystems, respectively. Bulk density values ranged from 0.71 ± 0.10 g cm−3 at Stn.2 during monsoon 2017–18 to 1.02 ± 0.10 g cm−3 at Stn.5 during pre-monsoon 2018–19 in Bhitarkanika and 0.58 ± 0.05 g cm−3 at Stn.3 during monsoon 2017–18 to 1.29 ± 0.08 g cm−3 at Stn.1 during pre-monsoon 2018–19 in Mahanadi, respectively. The higher values of bulk density during pre-monsoon and lower value in monsoon may be due to the fact that there is more sand, silt and clay deposition in monsoon because of higher precipitation and huge run-off from adjacent landmasses.
The nature of soil texture in the present study is characterized by silt clayey loamy soil in all the stations and in all the months with no much variation among them. The sand percentage varied from 8.21 ± 1.34 at Stn.1 during monsoon 2017–18 to 23.40 ± 3.86 at Stn.5 during pre-monsoon 2018–19 at Bhitarkanika and 2.64 ± 0.25 at Stn.3 during monsoon 2017–18 to 18.72 ± 1.41 at Stn.5 during pre-monsoon 2018–19 at Mahanadi, respectively. The silt percentage varied from 23.41 ± 5.60 at Stn.5 during monsoon 2017–18 to 45.10 ± 2.24 at Stn.2 during post-monsoon 2018–19 at Bhitarkanika and 10.37 ± 1.07 at Stn.5 during monsoon 2017–18 to 30.29 ± 1.24 at Stn.3 during post-monsoon 2018–19 at Mahanadi, respectively. Clay percentage varied from 35.02 ± 1.81 at Stn.5 during monsoon 2017–18 to 55.60 ± 3.51 at Stn.2 during post-monsoon 2018–19 at Bhitarkanika mangrove ecosystem and 28.72 ± 4.31 at Stn.5 during monsoon 2017–18 to 76.29 ± 1.84 at Stn.3 during post-monsoon 2018–19 at Mahanadi mangrove ecosystem, respectively.
Above ground biomass (AGB)
AGB is an important parameter to estimate carbon accumulation of a forest and its current information is required to study the importance of forest distribution on total biomass (Wijaya et al. 2010). AGB has been estimated for years together using different data and approaches namely field observation data (Brown and Lugo 1984, 1992), remote sensing (RS) data (Steininger 2000; Foody 2003; Thenkabail et al. 2004) and GIS (Brown and Gaston 1995). Among the various methods field observation approach is known to be the best and most accurate method although it is costly and time consuming as destructive sampling data is required (Lu 2006). The amount of standing biomass stored in mangrove forest is a function of the system productivity, age and organic matter allocation and exportation strategies (Kasawani et al. 2007).
In the present study the total AGB in Bhitarkanika ranged from 15.00 ± 2.12 tha−1 during monsoon at Stn.3 to 67.69 ± 6.86 tha−1 during pre-monsoon at Stn.5 (2017–18) and 15.17 ± 3.05 tha−1 during monsoon at Stn.3 to 70.09 ± 6.89 tha−1 during pre-monsoon at Stn.5 (2018–19) for A. marina; 3.81 ± 1.19 during monsoon at Stn.5 to 614.51 ± 50.15 tha−1 during pre-monsoon at Stn.2 (2017–18) and 3.91 ± 1.21 tha−1 during monsoon at Stn.5 to 616.94 ± 50.15 tha−1 during pre-monsoon at Stn.2 (2018–19) for A. officinalis; 35.26 ± 2.54 tha−1 during monsoon at Stn.5 to 95.58 ± 5.16 tha−1 during pre-monsoon at Stn.4 (2017–18) and 36.63 ± 2.55 tha−1 during monsoon at Stn.5 to 98.66 ± 5.24 tha−1 during pre-monsoon at Stn.4 (2018–19) for E. agallocha; 8.87 ± 2.12 tha−1 during monsoon at Stn.2 to 111.93 ± 10.79 tha−1 during pre-monsoon at Stn.3 (2017–18) and 8.96 ± 2.13 tha−1 during monsoon at Stn.2 to 113.43 ± 10.65 tha−1 during pre-monsoon at Stn.3 (2018–19) for R. mucronata; 2.66 ± 0.42 tha−1 during monsoon at Stn.5 to 5.84 ± 1.52 tha−1 during pre-monsoon at Stn.2 (2017–18) and 3.06 ± 1.43 tha−1 during monsoon at Stn.5 to 6.25 ± 1.52 tha−1 during pre-monsoon at Stn.2 (2018–19) for X. granatum, respectively (Fig. 2).
In case of Mahanadi mangrove ecosystem, the total AGB ranged from 20.97 ± 1.39 tha−1 during monsoon at station 3 to 41.02 ± 3.19 tha−1 during pre-monsoon at Stn.5 (2017–18) and 22.28 ± 1.41 tha−1 during monsoon at Stn.3 to 43.65 ± 3.31 tha−1 during pre-monsoon at Stn.5 (2018–19) for A. marina; 26.13 ± 3.19 tha−1 during monsoon at Stn.5 to 122.15 ± 12.12 tha−1 during pre-monsoon at Stn.3 (2017–18) and 27.89 ± 3.20 tha−1 during monsoon at Stn.5 to 127.51 ± 12.15 tha−1 during pre-monsoon at Stn.3 (2018–19) for A. officinalis; 3.56 ± 0.96 tha−1 during monsoon at Stn.3 to 31.84 ± 4.10 tha−1 during pre-monsoon at Stn.5 (2017–18) and 4.25 ± 0.95 tha−1 during monsoon at Stn.3 to 39.35 ± 4.11 tha−1 during pre-monsoon at Stn.5 (2018–19) for E. agallocha; 7.06 ± 2.21 tha−1 during monsoon at Stn.5 to 224.41 ± 21.20 tha−1 during pre-monsoon at Stn.4 (2017–18) and 7.49 ± 2.56 tha−1 during monsoon at Stn.5 to 233.96 ± 21.35 tha−1 during pre-monsoon at Stn.4 (2018–19) for R. mucronata; 0.64 ± 0.21 tha−1 during monsoon at Stn.5 to 3.61 ± 0.39 tha−1 during pre-monsoon at Stn.3 (2017–18) and 0.92 ± 0.22 tha−1 during monsoon at Stn.5 to 4.33 ± 0.39 tha−1 during pre-monsoon at Stn.3 (2018–19) for X. granatum, respectively (Fig. 3).
Station wise variation plotted for five selected species at Bhitarkanika showed maximum AGB of A. marina at Stn.5 which is characterized by high saline environment with inland mangroves proving the affinity of A. marina to saline environment. On the contrary, A. officinalis showed maximum growth at Stn.2 owing to lower salinity and continuous fresh water flow of Brahmani and Baitarani rivers, respectively. Excoecaria agallocha and X. granatum has shown an almost similar trend in its growth and distribution in the selected stations proving its acclimatization in thriving in wide range of salinity. Rhizophora mucronata showed high growth at Stn.3 in a moderately saline environment, thus proving its adaptability in tide fed river channels with a constant change in salinity in every tidal action (Fig. 4a). Station wise variation plotted for five selected species at Mahanadi showed maximum AGB of R. mucronata at Stn.4 which is characterized by moderate saline environment and A. marina showed higher AGB at Stn.5 proving the affinity to the saline environment. Avicenia officinalis showed maximum growth at Stn.3 followed by Stns.4 and 2 owing to lower salinity and continuous fresh water flow of river Bhitar kharinasi (Fig. 4b). Avicenia marina, E. agallocha and X. granatum showed similar trend in growth and distribution like Bhitarkanika in the selected stations proving its adaptation in thriving in a wide range of salinity. The overall range of AGB in the present study varied from 2.66 ± 0.42 tha−1 to 616.94 ± 50.15 tha−1 in case of Bhitarkanika and 0.64 ± 0.21 tha−1 to 233.96 ± 21.35 tha−1 in Mahanadi which is comparable with that in the Sundarbans (Roy Choudhuri 1991; Mitra et al. 2009, 2010, 2011; Banerjee et al. 2013; Joshi and Ghose 2014), Japan (Suzuki and Tagawa 1983), Australia (Woodroffe 1985), Senegal (Doyen 1986), Guade-loupe (Imbert and Rollet 1989), Puerto Rico (Golley et al. 1962), Thailand (Christensen 1978), Florida (Lugo and Snedaker 1974) and estuarine complex along Indian Bay of Bengal (Kathiresan et al. 2013), Indonesia (Komiyama et al. 1988), Malaysia (Putz and Chan 1986), Sri Lanka (Amarasinghe and Balasubramaniam 1992), Andaman islands (Mall et al. 1991) and Philippines (Camacho et al. 2011).
In the present study the biomass values showed a wide variation in AGB with respect to season for all the selected species over period of 2 years 2017–18 and 2018–19 (Fig. 5a, b) which is basically due to density and distribution of species in relation to the soil and water parameters particularly water salinity, EC and SOC. In forests with no natural and human disturbances, biomass values can go > 250 tha−1 (Lakyda et al. 2019) as has been observed in Stn.2 in Bhitarkanika mangrove ecosystem and Stn.4 in Mahanadi mangrove ecosystem. Season–wise variation of AGB at Bhitarkanika Wildfile Sanctuary and Mahanadi showed high growth of E. agallocha followed by A. officinalis, A. marina, R. mucronata and X. granatum, respectively. This may be due to adaptability of E. agallocha to wide range of water salinity (9.41 ± 0.21 psu to 34.64 ± 8.32 psu) and soil salinity (2.15 ± 0.22 to 17.21 ± 2.92 mScm−1). Year-wise trend of AGB at Bhitarkanika mangrove ecosystem clearly reveals that there is high growth of E. agallocha > A. officinalis > X. granatum > A. marina > R. mucronata (Fig. 6a). For Mahanadi year-wise trend showed high growth in E. agallocha > A. officinalis > R. mucronata > A. marina > X. granatum, respectively (Fig. 6b). Species-wise average increase in biomass for 2 years at Bhitarkanika mangrove ecosystem (2017–18 and 2018–19) was calculated, where A. marina showed 1.83 ± 0.56 tha−1, A. officinalis showed 1.36 ± 0.31 tha−1, E. agallocha showed 3.24 ± 0.54 tha−1, R. mucronata showed 0.79 ± 0.22 tha−1 and X. granatum showed an increase of 1.05 ± 0.39 tha−1 respectively (Table 1). Species wise average increasing biomass for 2 years at Mahanadi mangrove ecosystem (2017–18 and 2018–19) was calculated where A. marina showed 4.04 ± 0.76 tha−1, A. officinalis showed 6.16 ± 2.17 tha−1, E. agallocha showed 5.99 ± 1.79 tha−1, R. mucronata showed 5.03 ± 2.76 tha−1 and X. granatum showed 0.71 ± 0.25 tha−1 respectively (Table 2).
Mangrove photosynthesis is usually limited by high mid-day leaf temperature (Cheeseman 1994) and thus increases in temperature with declining humidity and rainfall would reduce productivity in mangrove forest by accentuating mid-day depression in photosynthesis. For Bhitarkanika and Mahanadi mangrove ecosystem all the selected species did not show any relationship with temperature which proves that the selected sites are geographically not very distinct from each other being a smaller geographical locale (Tables 3, 4).
With respect to water pH, A. marina showed significant positive relationship (P < 0.05; P < 0.01) for both Bhitarkanika and Mahanadi mangrove ecosystem. In case of soil pH, it showed significant relationship (P < 0.05) in Bhitarkanika mangrove ecosystem, but insignificant relationship in case of Mahanadi mangrove ecosystem. The relationship of pH with biomass of A. marina in Bhitarkanika proves its affinity to low acidic environment. With respect to water pH, A. officinalis and X. granatum showed significant negative relationship at Mahanadi but insignificant relationship with that of soil pH in both Bhitarkanika and Mahanadi. Rhizophora mucronata showed significant negative relationship with respect to water pH and X. granatum in case of soil pH at Bhitarkanika mangrove ecosystem. For E. agallocha water pH has shown significant positive relationship (P < 0.05) at Mahanadi mangrove ecosystem and insignificant relationship in case of Bhitarkanika mangrove ecosystem which proves that excepting E.agallocha all other species especially A. officinalis and X. granatum are more sensitive to the changing pH (Tables 3, 4).
With respect to salinity, A. marina has shown significant positive relationship in both the blocks of Bhitarkanika and Mahanadi mangrove ecosystem which is quite similar to that of E. agallocha in case of Mahanadi (excepting it has shown insignificant relationship in Bhitarkanika mangrove ecosystem) owing to the affinity of these species to water and soil salinity. In case of R. mucronata it has shown insignificant relationship in both the ecosystem proving that this species is not salinity dependent rather we can say its higher adaptability to tidal inundated areas. However, a contrasting feature was located in case of A. officinalis and X. granatum which has shown significant negative relationship (P < 0.05; P < 0.01) which proves that they are comparatively more acquainted with low saline environment in both Bhitarkanika and Mahanadi mangrove ecosystem as has also been stated by Kathiresan et al. (1996) (Tables 3, 4). Lower total AGB is observed generally in higher saline areas as has been observed at Stn.5 (128.64 ± 15.81 tha−1) in Bhitarkanika and (120.78 ± 13.14 tha−1) in Mahanadi mangrove ecosystem in contrast to Stn.2 in Bhitarkanika (706.75 ± 68.96 tha−1) and Stn.4 (399.82 ± 37.43 tha−1). This has also been proved by earlier workers (Mitra et al. 2010, 2011; Banerjee et al. 2013).
With respect to soil bulk density significant positive correlation was observed for A. marina (P < 0.01) and significant negative relationship for A. officinalis and X. granatum (P < 0.05; P < 0.01) proving the variation in sediment composition for the growth of the species in Bhitarkanika and Mahanadi mangrove ecosystem. On contrary E. agallocha and R. mucronata did not show any relationship with bulk density in case of Bhitarkanika mangrove ecosystem but significant positive relationship in case of Mahanadi mangrove ecosystem (only for E. agallocha, P < 0.01) proving that these species are adapted to more silt and clayey soil with higher bulk density. Such type of studies for effect of BD on mangrove forests has also been studied by Hossain and Nuruddin (2016) (Tables 3, 4).
With respect to soil organic carbon AGB of A. marina showed significant negative relationship (P < 0.01) and for A. officinalis and X. granatum it showed significant positive relationship (P < 0.01) proving that with increasing carbon load in the soil the growth of A. marina decreases and A. officinalis and X. granatum increases. This is in contrast with soil pH which has been explained earlier. This is true both for Bhitarkanika and Mahanadi mangrove ecosystem. For E. agallocha and R. mucronata no relationship was observed for SOC in Bhitarkanika and Mahanadi mangrove ecosystem (excepting E. agallocha at Mahanadi which has shown significant negative relationship like A. marina, P < 0.05) during the study period which justifies that the minimum amount of SOC is sufficient for the growth of these two species. This might be the probable cause for a wide spread distribution of these two species in all the stations respectively (Tables 3, 4). Increasing SOC along with biomass growth has also been demonstrated by Ren et al. (2010).
Soil texture relationship has been studied with respect to sand, silt and clay composition respectively. With respect to sand, A. marina showed significant positive relationship (P < 0.01), with silt and clay, it showed significant negative relationship (P < 0.05; P < 0.01) both for Bhitarkanika and Mahanadi mangrove ecosystem. The reverse trend has been followed for A. officinalis and X. granatum in all the selected stations (P < 0.05; P < 0.01). For R. mucronata, there is no relationship with respect to soil texture. However, for E. agallocha although there is no relationship in Bhitarkanika but it has shown significant positive relationship with sand (P < 0.01) and significant negative relationship (P < 0.05; P < 0.01) with respect to silt and clay in Mahanadi mangrove ecosystem which is proved by the distribution of both the species almost evenly in Mahanadi mangrove ecosystem (Tables 3, 4). Soil texture in the study area has shown higher percentage of silt and clay compared to sand particles, proving that the present study area has finer clay than sand and have greater ability to trap nutrients (Nguyen et al. 2013).
MANOVA computed for biomass of A. marina showed significant variation between stations (P < 0.05) both in case of Bhitarkanika and Mahanadi mangrove ecosystem, but insignificant variation between season as well as station vs season respectively. Similar variation has also been observed for A. officinalis, E. agallocha, R. mucronata and X. granatum, respectively.
Above ground carbon (AGC)
Mangrove forest contributes a significant proportion to global carbon cycle although they comprise 0.7% of the global coastal zone (Kathiresan et al. 2013). Since mangroves play a major role in reducing greenhouse gases and problems of global warming through the process of photosynthesis, they are excellent store house of carbon. Carbon storage in mangroves is a function of the quantity of biomass which varies with respect to age and growth efficiency. In the present study we observed significantly higher stored carbon in above ground structures of species thriving in Stn.2 and Stn.4 in Bhitarkanika and Mahanadi mangrove ecosystem in comparison to other stations. In case of Bhitarkanika mangrove ecosystem for A. marina AGC values varied from 7.63 ± 1.08 tha−1 during monsoon at Stn.3 to 34.42 ± 2.03 tha−1 during pre-monsoon 2017–18 at Stn.5 and 7.72 ± 0.81 tha−1 during monsoon at Stn.3 to 35.65 ± 2.63 tha−1 during pre-monsoon 2018–19 at Stn.5; for A. officinalis the AGC varied from 1.73 ± 0.01 tha−1 during monsoon at Stn.5 to 279.72 ± 22.01 tha−1 during pre-monsoon 2017–18 at Stn.2 and 1.78 ± 0.02 tha−1 during monsoon at Stn.5 to 280.83 ± 21.29 tha−1 during pre-monsoon 2018–19 at Stn.2; for E. agallocha AGC varied from 16.06 ± 1.05 tha−1 during monsoon at Stn.5 to 43.54 ± 2.89 tha−1 during pre-monsoon 2017–18 at Stn.4 and 16.69 ± 1.06 tha−1 during monsoon at Stn.5 to 44.95 ± 2.53 tha−1 during pre-monsoon 2018–19 at Stn.4; for R. mucronata the values ranged from 4.88 ± 1.05 tha−1 during monsoon at Stn.2 to 61.56 ± 4.95 tha−1 during pre-monsoon at Stn.3 2017–18 and 4.93 ± 1.06 tha−1 during monsoon at Stn.2 to 62.39 ± 4.95 during pre-monsoon 2018–19 at Stn.3; For X. granatum AGC values ranged from 1.38 ± 0.10 tha−1 during monsoon at Stn.5 to 3.04 ± 0.32 tha−1 during pre-monsoon at Stn.2 (2017–18) and 1.59 ± 0.11 tha−1 during monsoon at Stn.5 to 3.25 ± 0.31 tha−1 during pre-monsoon at Stn.2 (2018–19), respectively (Fig. 7). In case of Mahanadi, for A. marina AGC values varied from 9.14 ± 0.72 tha−1 during monsoon at Stn.3 to 17.76 ± 1.57 tha−1 during pre-monsoon at Stn.5 (2017–18) and 9.70 ± 0.69 tha−1 during monsoon at Stn.3 to 18.89 ± 1.99 tha−1 during pre-monsoon at Stn.5 (2018–19); for A. officinalis AGC values varied from 11.72 ± 1.41 tha−1 during monsoon at Stn.5 to 55.25 ± 4.26 tha−1 during pre-monsoon at Stn.3 and 12.48 ± 1.43 tha−1 during monsoon at Stn.5 to 57.63 ± 4.52 tha−1 during pre-monsoon at Stn.3 (2018–19); for E. agallocha AGC values varied from 1.64 ± 0.41 tha−1 during monsoon at Stn.3 to 14.08 ± 2.16 tha−1 during pre-monsoon at Stn.5 (2017–18) and 1.94 ± 0.43 tha−1 during monsoon at Stn.3 to 17.40 ± 2.21 tha−1 during pre-monsoon at Stn.5 (2018–19); for R. mucronata the AGC values ranged from 3.44 ± 1.45 tha−1 during monsoon (2017–18) at Stn.5 to 109.40 ± 10.25 tha−1 during pre-monsoon (2017–18) at Stn.4 and 3.64 ± 1.52 tha−1 during monsoon (2018–19) at Stn.5 to 114.05 ± 10.29 tha−1 during pre-monsoon (2018–19) at Stn.4 and for X. granatum the AGC values ranged from 0.31 ± 0.10 tha−1 during monsoon at Stn.5 to 1.82 ± 0.19 tha−1 during pre-monsoon (2017–18) at Stn.3 and 0.44 ± 0.11 tha−1 during monsoon at Stn.5 to 2.17 ± 0.19 tha−1 during pre-monsoon (2018–19) at Stn.3, respectively (Fig. 8).
Station-wise trend of AGC was replicate of AGB with highest value of A. officinalis at Stn.2 for Bhitarkanika mangrove ecosystem (Fig. 9) and Stn.3 for Mahanadi mangrove ecosystem (Fig. 10) owing to lower salinity of these stations due to discharge from Brahmani and Baitarani at Bhitarkanika and river Kharinasi at Mahanadi mangrove ecosystem. Another highest peak was recorded at Stn.4 in Mahanadi for R. mucronata which is due to the fact that this area is basically a plantation site of R. mucronata by Forest Department in collaboration with M.S. Swaminathan Research Foundation.
Correlation coefficient computed for AGC along with water and soil parameters has shown similar relationship with that of AGB (Tables 3, 4). However significant variation within stations (P < 0.05) have proved variation in carbon storage with respect to stations (Tables 3, 4). Species-wise average of carbon (tha−1) in Bhitarkanika mangrove ecosystem was E. agallocha (1.48 ± 0.28) > A. marina (0.93 ± 0.29) > A. officinalis (0.62 ± 0.18) > X. granatum (0.55 ± 0.21) > R. mucronata (0.43 ± 0.13) respectively (Tables 5, 6). In case of Mahanadi species-wise average carbon (tha−1) was E. agallocha (2.50 ± 1.79) > R. mucronata (2.45 ± 1.15) > A. marina (1.74 ± 0.32) > A. officinalis (1.13 ± 0.96) > X. granatum (0.34 ± 0.12), respectively.
Total carbon (AGC + SOC) calculated for the study area varied from 66.31 ± 13.39 tha−1 at Stn.5 to 330.41 ± 111.97 tha−1 at Stn.2 in case of Bhitarkanika and 55.20 ± 7.90 tha−1 at Stn.5 to 187.89 ± 43.81 tha−1 at Stn.4 in Mahanadi, respectively (Tables 5, 6) with a mean total carbon of 149.07 ± 38.32 tha−1 at Bhitarkanika mangrove ecosystem and 99.14 ± 21.93 tha−1 for Mahanadi mangrove ecosystem, respectively. Carbon dioxide equivalent (CO2e) calculated station-wise varied from 243.37 ± 49.14 tons at Stn.5 to 1212.59 ± 410.92 tons at Stn.2 for Bhitarkanika mangrove ecosystem with a mean CO2e of 547.08 ± 140.62 tons for Bhitarkanika mangrove ecosystem. In case of Mahanadi mangrove ecosystem, the CO2e varied from 202.60 ± 29.00 tons at Stn.5 to 689.55 ± 160.77 tons at Stn.4 with mean CO2e of 363.85 ± 80.50 tons, respectively (Tables 5, 6).
The carbon sequestration rate for the selected species was calculated as per the order A. officinalis (197.26 tha−1 year−1) > E. agallocha (74.89 tha−1 year−1) > R. mucronata (40.10 tha−1 year−1) > A. marina (37.53 tha−1 year−1) > X. granatum (6.10 tha−1 year−1) > P. coarctata (3.14 tha−1 year−1) for Bhitarkanika mangrove ecosystem. In case of Mahanadi mangrove ecosystem the carbon sequestration rate varied from A. officinalis (92.29 tha−1 year−1) > R. mucronata (85.43 tha−1 year−1) > A. marina (33.86 tha−1 year−1) > E. agallocha (16.55 tha−1 year−1) > X. granatum (2.85 tha−1 year−1) > P. coarctata (2.06 tha−1 year−1), respectively.
Conclusion
Estimation of C-stocks revealed a very high potential of mangroves in sequestering carbon. Assessment of biomass and carbon stocks in mangroves can increase their perceived conservation value through quantification of carbon storage potential that would attract investment for its protection and/or restoration under international emerging mechanism such as REDD + programme. Hence, financial incentives available for mangrove conservation based on carbon Payments for Ecosystem Services (PES) should be introduced and possible approaches for increasing forest biomass programmes should be undertaken. The present research suggests participatory plantation and restoration of mangrove forests for climate change mitigation measure.
References
Agarwal S, Banerjee K, Pal N, Mallik K, Bal G, Pramanick P, Mitra A (2017) Carbon sequestration by mangrove vegetations: a case study from Mahanadi mangrove wetland. J Environ Sci Comput Sci Eng Technol 7(1):016–029
Amarasinghe MD, Balasubramaniam S (1992) Net primary productivity of two mangrove forest stands on the northwest coast of Sri Lanka. Hydrobiologia 247:37–47
Banerjee K, Sengupta K, Raha AK, Mitra A (2013) Salinity based allometric equations for biomass estimation of Sundarban mangrove. Biomass Bioenergy 56:382–391
Banerjee K, Bal G, Mitra A (2018) How soil texture affects the organic carbon load in the mangrove ecosystem? A case study from Bhitarkanika, Odisha. In: Singh VP, Yadav S, Yadava RM (eds) Environmental pollution. Springer Nature Singapore Pvt Ltd, Singapore, pp 329–341
Brown S, Gaston G (1995) Use of forest inventories and geographic information systems to estimate biomass density of tropical forests: application to tropical Africa. Environ Monit Assess 38:157–168
Brown S, Lugo AE (1984) Biomass of tropical forests: a new estimate based on forest volume. Science 223:1290–1293
Brown S, Lugo AE (1992) Aboveground biomass estimations for tropical moist forests of the Brazilian Amazon. Interciencia 17:8–18
Camacho LD, Gevaña DT, Carandang AP, Camacho SC, Combalicer EA, Rebugio LL, Youn YC (2011) Tree biomass and carbon stock of a community managed mangrove forest in Bohol, Philippines. For Sci Technol 7:161–167
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Cheeseman J (1994) Depression of photosynthesis in mangrove canopies. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis in mangrove canopies: from molecular mechanisms to the field. Bios, Oxford, pp 377–389
Christensen B (1978) Biomass and productivity of Rhizophora apiculata B1 in a mangrove in southern Thailand. Aquat Bot 4:43–52
Donato CD, Kauffman JB, Murdiarso D, Kurni–Anto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297
Doyen (1986) La mangrove a usage multiple de I’estuarine Saloum (Senegal). In: Dost H (ed) Selected Papers of the Dakar Symposium on Acid Sulphate Soils, (Publication no. 44). International Institute for Land Reclamation and Improvement, Wageningen, pp 176–201
Estrada GCD, Soares MG (2017) Global patterns of aboveground carbon stock and sequestration in mangroves. An Acad Bras Ciênc 89(2):973–989
Ferreira TO, Otero XL, Vidal-Torrado P, Macias F (2007a) Redox processes in mangrove soils under Rhizophora mangle in relation to different environmental conditions. Soil Sci Soc Am J 71:484–491
Ferreira TO, Vidal-Torrado P, Otero XL, Macias F (2007b) Are mangrove forest substrates sediments or soils? A case study in southeastern Brazil. CATENA 70:79–91
Ferreira TO, Otero XL, de Souza Jr VS, Vidal-Torrado P, Macias F, Firme LP (2010) Spatial patterns of soil attributes and components in a mangrove system in Southeast Brazil (Sao Paulo). J Soil Sediment 10:995–1006
Foody GM (2003) Remote sensing of tropical forest environments: towards the monitoring of environmental resources for sustainable development. Int J Remote Sens 24:4035–4046
FSI (1987) The state forest report, Government of India, Ministry of Environment and Forest
FSI (2017) Ministry of Environment and Forests, State Forest Report, Dehradun
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, Masek Z, Duke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Glob Ecol Biogeogr 20:154–159
Golley F, Odum HT, Wilson R (1962) The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 43:9–19
Hossain MD, Nuruddin AA (2016) Soil and mangrove: a review. J Environ Sci Technol 9:198–207
Imbert D, Rollet B (1989) Phytomass eaerienneet production primairedans la mangrove du Grand Cul–de–Sac Maria (Guadeloupe, Antilles francaises). Bull Ecol 20:27–39
IPCC (2018) Special Report on Global Warming of 1.5 °C
IUCN (2019) The IUCN Red List of Threatened Species. Version 2019–1. http://www.iucnredlist.org. Accessed 20 Oct 2019
Joshi HG, Ghose M (2014) Community structure, species diversity, and aboveground biomass of the Sundarbans mangrove swamps. Trop Ecol 55(3):283–303
Kasawani I, Kamaruzaman J, Nurun-Nadhirah MI (2007) Biological diversity assessment of Tok Bali mangrove forest, Kelantan, Malaysia. WSEAS Trans Environ Dev 3(2):30–385
Kathiresan K, Rajendran N, Thangadurai G (1996) Growth of mangrove seedlings in the intertidal area of Vellar estuary, southeast coast of India. Indian J Mar Sci 25:240–243
Kathiresan K, Gomathi V, Anburaj R, Saravanakumar K, Asmathunisha N, Sahu SK, Shanmugaarasu V, Anandhan S (2013) Carbon sequestration potential of Rhizophora mucronata and A. marina as influenced by age, season, growth and sediment characteristics in southeast coast of India. J Coast Conserv 17(3):397
Komiyama A, Moriya H, Prawiroatmodjo S, Toma T, Ogino K (1988) Forest primary productivity. In: Ogino K, Chihara M (eds) Biological system of mangrove. Ehime University, Ehime, pp 97–117
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21(4):471–477
Lakyda P, Shvidenko A, Bilous A, Myroniuk V, Matsala M, Zibtsev S, Schepaschenko D, Holiaka D, Vasylyshyn R, Lakyda I, Diachuk P, Kraxner F (2019) Impact of disturbances on the carbon cycle of forest ecosystems in Ukrainian Polissya. Forests 10:337–360
Lal JB (2007) Forest ecosystems and carbon sequestration in India, ecosystem diversity and carbon sequestration, challenges and a way out for ushering in a sustainable future. Ecosyst Chapter 5:9–14
Le Quere CL, Andrew RM, Friedlingstein P, Sitch S, Zheng B (2018) Global carbon budget 2018. Earth Syst Sci Data 10:2141–2194
Lee SY, Primavera JH, Dahdouh-Guebas F, McKee K, Bosire JO, Cannicci S, Diele K, Fromard F, Koedam N, Marchand C, Mendelssohn I, Mukherjee N, Record S (2014) Ecological role and services of tropical mangrove ecosystems: a reassessment. Glob Ecol Biogeogr 23(7):726–743
Lu D (2006) The potential and challenge of remote sensing–based biomass estimation. Int J Remote Sens 27(7):1297–1328
Lugo AE, Snedaker C (1974) The ecology of mangroves. Annu Rev Ecol Evol Syst 5:39–64
Mall LP, Singh VP, Garge A (1991) Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric mangrove and mixed mangrove forests of Andaman Islands. Trop Ecol 32:144–152
Mitra A (2013) Mangroves: a unique gift of nature. In: Mitra A (ed) Sensitivity of mangrove ecosystem to changing climate. Springer, New Delhi, pp 33–54
Mitra A, Sundaresan J (2016) How to study stored carbon in mangroves, published by CSIR-National Institute of Science Communication and Information Resources (NISCAIR), ISBN: 978-81-7236-349-9
Mitra A, Banerjee K, Sengupta K, Gangopadhyay A (2009) Pulse of climate change in Indian Sundarbans: a myth or reality? Natl Acad Sci Lett 32:1–2
Mitra A, Chowdhury R, Sengupta K, Banerjee K (2010) Impact of salinity on mangroves of Indian Sundarbans. J Coast Environ 1(1):71–80
Mitra A, Sengupta K, Banerjee K (2011) Standing biomass and carbon storage of above-ground structures in dominant mangrove trees in the Sundarbans. For Ecol Manag 261(7):1325–1335
Murdiyarso D, Purbopuspit J, Kauffman JB, Warren MW, Sasmito SD, Donato DC, Manuri S, Krisnawati H, Taberima S, Kurnianto S (2015) The potential of Indonesian mangrove forests for global climate change mitigation. Nat Clim Change 5:1089–1092
Nguyen H, Cao D, Schmitt K (2013) Soil particle–size composition and coastal erosion and accretion study in Soc Trang mangrove forests. J Coast Conserv 17(1):93–104
NOAA (2019) Global climate report, 2019
Omar R, Masera Garza-Caligaris JF, Kanninen M, Karjalainen T, Liski J, Nabuurs GJ, Pussinen A, Jong BHJ, Mohren GMJ (2003) Modeling carbon sequestration in afforestation, agroforestry and forest management projects: the CO2 FIX vol 2 approach. Ecol Mode 164(2–3):177–199
Panda S, Panda S (2015) Carbon sequestration—a new vista towards sustainable development. J Emerg Technol Adv Eng 5(5):111–118
Putz F, Chan HT (1986) Tree growth, dynamics, and productivity in a mature mangrove forest in Malaysia. For Ecol Manag 17:211–230
Ren H, Chen H, Li ZA, Han W (2010) Biomass accumulation and carbon storage of four different aged Sonneratia apetala plantations in Southern China. Plant Soil 327:279–291
Roy Choudhuri PK (1991) Biomass production of mangrove plantation in Sundarbans, West Bengal (India)—a case study. Indian For 177:3–12
Singh VP, Odaki K (2004) Mangrove ecosystem structure and function. Scientific Publishers, Jodhpur, pp 1–279
Spalding M, Kainuma M, Collins L (2010) World atlas of mangroves. ISME publication, London, p 320
Steininger MK (2000) Satellite estimation of tropical secondary forest aboveground biomass data from Brazil and Bolivia. Int J Remote Sens 21:1139–1157
Suzuki E, Tagawa H (1983) Biomass of a mangrove forest and a sedge marsh on Shigaki Island, South Japan. Jpn J Ecol 33:231–234
Thenkabail PS, Stucky N, Griscom BW, Ashton MS, Diels J, van der Meer B, Enclona E (2004) Biomass estimations and carbon stock calculations in the oil palm plantations of African derived savannas using IKONOS data. Int J Remote Sens 25:5447–5472
Twilley RR, Chen RH, Hargis T (1992) Carbon sinks in mangrove forests and their implications to the carbon budget of tropical coastal ecosystems. Water Air Soil Pollut 64:265–288
UNEP-WCMC (2006) Shoreline protection and other ecosystem services from mangroves and coral reefs, UNEP-WCMC, Cambridge, UK
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–37
Wijaya A, Liesenberg V, Gloaguen R (2010) Retrieval of forest attributes in complex successional forests of Central Indonesia: modeling and estimation of bitemporal data. For Ecol Manag 259(12):2315–2326
Woodroffe CD (1985) Studies of a mangrove basin, Tuff Crater, New Zealand. I: mangrove biomass and production of detritus. Estuar Coast Shelf Sci 20:265–280
Acknowledgements
The authors are grateful to Ministry of Earth Sciences, Govt. of India project (Sanction No. MoES/36/OOIS/Extra/44/2015 dated 29th November, 2016) for providing financial support. We would like to thank Institute of Forest Biodiversity, Hyderabad for helping us in analysing the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, K., Sahoo, C.K., Bal, G. et al. High blue carbon stock in mangrove forests of Eastern India. Trop Ecol 61, 150–167 (2020). https://doi.org/10.1007/s42965-020-00072-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-020-00072-y