Abstract
Acid-leaching behaviors of the titanium slag obtained by selective reduction of vanadium-bearing titanomagnetite concentrates were investigated. It was found that the optimal leaching of titanium and silicon were 0.7% and 1.5%, respectively. The titanium and silicon in the titanium slag were firstly dissolved in the acidic solution to form TiO2+ and silica sol, and then rapidly reprecipitated, forming hydrochloric acid (HCl) leach residue. Most of the silicon presented in the HCl leach residue as floccules-like silica gel, while most of the titanium was distributed in the nano-sized rod-like clusters with crystallite refinement and intracrystalline defects, and, as such, 94.3% of the silicon was leached from the HCl leach residue by alkaline desilication, and 96.5% of the titanium in the titanium-rich material with some rutile structure was then digested by the concentrated sulfuric acid. This provides an alternative route for the comprehensive utilization of titanium and silicon in titanium slag.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Panzhihua–Xichang region is widely recognized for its abundant vanadium-bearing titanomagnetite resources, and it accounts for 35.2% and 11.6% of world total titanium and vanadium resources, respectively.1,2 Generally, the titanomagnetite concentrates are smelted in a blast furnace to produce blast furnace slag (TiO2 22–25%).3,4 However, it is difficult to recover the titanium from the blast furnace slag because of the dispersive distribution with very fine-grained mineral phases.5,6
Recently, some alternative processes based on direct reduction and electric furnace smelting7,8,9,–10 or magnetic separation3,11,12 have been proposed. In the electric furnace smelting process, the obtained molten iron is smelted in a basic oxygen furnace to produce vanadium slag. This is then roasted with sodium salts at 750–850°C, and multiple-stage roasting is usually employed because of the very stable spinel structures, giving rise to an over-consumption of energy and resources.13,14 Moreover, the discharge of hazardous V5+ and Cr6+ in the roast-leach process also poses a great threat to the environment.15,16
To reduce the discharge of hazardous V5+ and Cr6+, the titanomagnetite concentrates are first selectively reduced, with subsequent magnetic separation to produce metallic iron powder and low-grade titanium slag, in which most of the vanadium and chromium is concentrated.11 Hydrochloric acid (HCl) leaching is the most commonly used technique to upgrade titanium slag and produce synthetic rutile,17,18,19,20,21,22,–23 and, as such, the titanium slag is leached with HCl at high temperatures to produce HCl leach residue and yield high leaching of vanadium and chromium. However, pulverization of synthetic rutile always occurs during HCl leaching, and higher temperatures induce the formation of fine-grained synthetic rutile.24 Thus, it is difficult to make the particle size of the HCl leach residue meet the requirements of the chloride process (above 85% of the particle size larger than 100 μm). Moreover, the efficient redox pretreatment25 seems to be unsuitable for the low-grade titanium slag because of the complicated processes. In order to figure out an alternative route for comprehensive utilization of the low-grade titanium slag, it is necessary to investigate in depth the leaching mechanism of titanium and the impurity of silicon during HCl leaching.
Materials and Methods
Materials
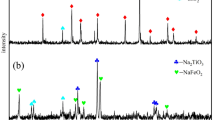
Titanium slag was prepared by selective reduction of the Hongge titanomagnetite concentrates with subsequent magnetic separation.11 The chemical composition is listed in Table I. The slag consists mostly of irregular granular particles with a very compact structure (Fig. 1a), and is mainly composed of titanomagnetite ((Fe2.5Ti0.5)1.04O4), pseudobrookite ((Mg,Fe)Ti2O5), ilmenite (FeTiO3), and amorphous silicates (Fig. 2a). All the other reagents used were of analytical grade.
Experimental Procedures
Pressure acid-leaching experiments were conducted in a 0.25-L Teflon autoclave with a stainless steel shell (see Fig. S1). Titanium slag was first mixed with a HCl solution in an autoclave. The autoclave was heated at a rate of 5°C/min after which it was affixed and sealed completely, and then held at the preset temperature for a certain time. After leaching, the autoclave was cooled quickly, and the slurry was filtered and washed with distilled water, forming HCl leach residue.
The HCl leach residue was mixed with sodium hydroxide (NaOH) solution in a 0.25-L cylindrical stainless steel reactor, and then the slurry was agitated at 350 rpm. After the reaction, the slurry was filtrated and washed with distilled water, forming titanium-rich material.
Characterization
Chemical compositions were analyzed by an Optima 5300DV ICP-OES. At least triplicate analyses were carried out for each sample, and the relative standard deviation (n = 3) was less than 3%. Morphological changes were observed using a JSM-7001F scanning electron microscope (SEM). Phase compositions were investigated using an X’Pert PRO MPD x-ray Diffraction instrument (XRD). Infrared spectra were measured using a Fourier-transform infrared spectrometer (FT-IR; Spectrum GX) with a resolution of 4 cm−1. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo XPS ESCALAB 250Xi instrument. Solid-state 29Si CP/MAS nuclear magnetic resonance (NMR) measurements were performed using a Bruker Advance 400 spectrometer.
Results and Discussion
Optimization of Hydrochloric Acid Leaching
According to Fig. 2b, pseudobrookite still exists in the HCl leach residue after atmospheric acid leaching, indicating incomplete decomposition of the pseudobrookite, in which 31.7% of the vanadium and 21.7% of the chromium are distributed (see Table SI). To efficiently extract the vanadium and chromium, pressure acid leaching was employed (see Fig. S2), and the leaching of the vanadium and chromium were 90.9% and 92.3%, respectively, while the leaching of titanium and silicon was relatively stable (< 2%) under the conditions of initial acid concentration of 281 g/L, acid-to-slag mass ratio 4.5:1, leaching temperature 140°C, and leaching time 4 h. Most of the vanadium, chromium and impurities of Fe, Al, Ca, Mg were leached out, while almost all the titanium and silicon still remained in the HCl leach residue with the Ti/Si mass ratio preserved (Table I).
Acid Leaching Mechanism of Titanium and Silicon
To clearly elucidate the ‘abnormal phenomenon’ of titanium and silicon during HCl leaching, the XPS technique was used. Generally, bridging oxygen (Ob), non-bridging oxygen (Onb) and metal-bridging oxygen (Omb) refer to the oxygen that bonds two Si atoms together (Si-O-Si), bonds a Si atom to a metal cation (Si-O-Al and Si-O-Ti), and bonds two metal atoms together (Ti-O-Ti), respectively.26 The binding energy (BE) of O1 s signals usually decreased upon substitution of the Si atoms by less electronegative, more polarizable atoms (Ti and Al).27 Thus, Ob, Onb, and Omb signals for the titanium slag appeared at 531.8, 530.9, and 529.8 eV, respectively (Fig. 3a). After acid treatment, the Onb signal disappeared, and the Ob and Omb signals were observed (Fig. 3b). The surface composition of the titanium slag was about Ob/Omb = 1.23, which was much less than that of the HCl leach residue (Ob/Omb = 5.55). This was more likely to be caused by the adsorption of Si-rich species on the surface of Ti-rich species (Fig. 1b). Moreover, a relative symmetry could be observed in both Si2p and Ti2p3/2 peaks, indicating the presence of only one environment for Ti and Si atoms (both in tetravalent). A shift towards higher BE of Si2p and Ti2p3/2 was observed after acid treatment, probably indicating the Ti-rich and Si-rich species with more stable structures were formed. Thus, it could be speculated that titanium and silicon in the titanium slag might be first dissolved in the acidic solution and then precipitated with some Si-rich species adsorbed on the Ti-rich species.
To give more direct evidence for the dissolution of titanium and silicon, the effect of the initial heating time on the leaching of titanium and silicon was studied. As shown in Fig. 4, about 20% of the titanium and silicon were dissolved in the initial leaching period. However, almost all of the titanium and silicon were reprecipitated as the holding time prolonged to 1 h (see Fig. S2d).
FT-IR and solid state 29Si CP/MAS NMR techniques were used to specify the leaching behavior of silicon. According to Fig. 5a, Si-O stretching vibrations of the tetrahedral structure of the silicates (1002 cm−1) could be observed in the titanium slag.28,29 A shift of about 100 cm−1 towards higher wavenumbers of the Si-O stretching vibrations was observed as the acid attack occurred, and a new band at about 952 cm−1 appeared (Fig. 5b), which could be interpreted in terms of Si-OH groups.30 The NMR spectrum indicated that the silicon existed in the HCl leach residue as isolated silanol groups (SiO)3SiOH (Q3, − 101 ppm) and siloxane without hydroxyl groups (SiO)4Si (Q4, − 111 ppm),31,32,–33 with relative peak areas of 27.9% and 72.1%, respectively (see Fig. S3). This could be attributed to the more rigid structure of amorphous silica gel, in which the deformation of the Si-O-Si angles was more difficult than in the silicates.29
Therefore, titanium and silicon in the titanium slag were first dissolved in the acidic solution, forming TiO2+ and silica sol. TiO2+ and silica sol were then rapidly reprecipitated to form HCl leach residue, since higher temperatures and ionic strength induced the hydrolysis of TiO2+ and the gelation of silica sol. In conclusion, the acid leaching behaviors of titanium and silicon could be interpreted by a dissolution–reprecipitation mechanism,34,35 and, as such, the silicon in the HCl leach residue is expected to be dissolved in an alkaline solution, while the titanium is expected to be digested by concentrated sulfuric acid (H2SO4) because of the very strong interfacial/surface free energy caused by crystallite refinement and intracrystalline defects (Figs. 1b and 2c).
Alkaline Desilication and H2SO4 Decomposition
The HCl leach residue after pressure acid leaching was leached with 175 g/L NaOH solution and liquid-to-slag mass ratio 3:1 at 40°C for 35 min, resulting 94.3% of the silicon being leached out, forming a titanium-rich material with TiO2 purity of 91.38%. The floccules-like Si-rich species disappeared after alkaline treatment while titanium was still presented in nano-sized rod-like clusters (Fig. 1c), indicating the efficient removal of amorphous silica gel. The obtained alkaline Na2SiO3 solution might be used for water glass production.
The obtained titanium-rich material with above 88% of the particle size less than 13 μm (see Fig. S4) could not meet the requirements of the chloride process, but the main phase was identified as a rutile structure (Fig. 2c), which is widely considered to be resistant to H2SO4. However, most of the titanium presented in the titanium-rich material as nano-sized rod-like clusters with very strong interfacial/surface free energy. Therefore, the titanium-rich material was digested by 98% concentrated H2SO4 with a H2SO4/TiO2 molar ratio of 1.7:1 at 190°C for 3 h in which 96.5% of the titanium was leached out in the following dilute acid leaching, together with a titanyl sulfate solution with TiO2 concentration of 144.8 g/L and H2SO4/TiO2 mass ratio of 2.07, indicating complete digestion of the titanium-rich material by concentrated H2SO4.
Conclusions
-
(1)
The optimal leachings of titanium and silicon were 0.7% and 1.5%, respectively, while those of vanadium and chromium were 90.9% and 92.3%, respectively, when using pressure acid leaching.
-
(2)
Hydrochloric acid leaching behaviors of the titanium slag could be clearly interpreted by a dissolution–reprecipitation mechanism, in which the titanium and silicon were first dissolved in the acidic solution to form TiO2+ and silica sol, and then rapidly reprecipitated to form HCl leach residue.
-
(3)
Most of the silicon presented in the HCl leach residue as floccules-like silica gel, while most of the titanium presented as nano-sized rod-like clusters with crystallite refinement and intracrystalline defects, and, as such, 94.3% of the silicon could be leached out by the NaOH solution, and 96.5% of the titanium could then be digested by concentrated H2SO4 despite its rutile structure.
References
P.R. Taylor, S.A. Shuey, E.E. Vidal, and J.C. Gomez, Miner. Metall. Process. 23, 80 (2006).
H.G. Du, Theory of Smelting Vanadium-Bearing Titanomagnetite by Blast Furnace (Beijing: Science Press, 1996).
D.S. Chen, L.S. Zhao, Y.H. Liu, T. Qi, J.C. Wang, and L.N. Wang, J. Hazard. Mater. 244–245, 588 (2013).
D.S. Chen, B. Song, L.N. Wang, T. Qi, Y. Wang, and W.J. Wang, Miner. Eng. 24, 864 (2011).
W.G. Fu, Y.C. Wen, and H.E. Xie, J. Iron. Steel Res. Int. 18, 7 (2011).
L. Zhang, L.N. Zhang, M.Y. Wang, G.Q. Li, and Z.T. Sui, Miner. Eng. 20, 684 (2007).
V.E. Roshchin, A.V. Asanov, and A.V. Roshchin, Russ. Metall. 11, 1001 (2010).
V.E. Roshchin, A.V. Asanov, and A.V. Roshchin, Russ. Metall. 6, 499 (2011).
G.Z. He, X.H. Du, K. Zhang, Z.S. Tang, T.P. Lou, and G.F. Tu, J. Mater. Metall. 13, 15 (2014).
J. Deng, X. Xue, and G.G. Liu, J. Mater. Metall. 6, 83 (2007).
L.S. Zhao, L.N. Wang, T. Qi, D.S. Chen, H.X. Zhao, and Y.H. Liu, Hydrometallurgy 149, 106 (2014).
L.S. Zhao, L.N. Wang, D.S. Chen, H.X. Zhao, Y.H. Liu, and T. Qi, Trans. Nonferrous Metal. Soc. 25, 1325 (2015).
B. Liu, H. Du, S.N. Wang, Y. Zhang, S.L. Zheng, L.J. Li, and D.H. Chen, AIChE J. 59, 541 (2013).
H.X. Fang, H.Y. Li, and B. Xie, ISIJ Int. 52, 1958 (2012).
W.M. Mayes, P.L. Younger, and J. Aumonier, Water Air Soil Pollut. 195, 35 (2008).
L.H. Xu, W.C. Li, S. Volodymyr, M. Liu, H. Wang, S.M. Bi, and Y.B. Bi, Mater. Manuf. Process. 23, 743 (2008).
M.H.H. Mahmoud, A.A.I. Afifi, and I.A. Ibrahim, Hydrometallurgy 73, 99 (2004).
N. El-Hazek, T.A. Lasheen, R. El-Sheikh, and S.A. Zaki, Hydrometallurgy 87, 45 (2007).
C. Li, B. Liang, and H.Y. Wang, Hydrometallurgy 91, 121 (2008).
L. Zhang, H.P. Hu, Z. Liao, Q.Y. Chen, and J. Tan, Hydrometallurgy 107, 40 (2011).
L. Zhang, H.P. Hu, L.P. Wei, Q.Y. Chen, and J. Tan, Sep. Purif. Technol. 73, 173 (2010).
L.S. Zhao, Y.H. Liu, L.N. Wang, H.X. Zhao, D.S. Chen, B.N. Zhong, J.C. Wang, and T. Qi, Ind. Eng. Chem. Res. 53, 70 (2014).
F.Q. Zheng, F. Chen, Y.F. Guo, T. Jiang, A.Y. Travyanov, and G.Z. Qiu, JOM 68, 1476 (2016).
H.B. Cheng, D.J. Wang, B.W. Huang, and G. Sun, Nonferr. Metal. 56, 82 (2004).
J.B. Zhang, Q.S. Zhu, Z.H. Xie, and H.Z. Li, Hydrometallurgy 157, 226 (2015).
K.N. Dalby, H.W. Nesbitt, V.P. Zakaznova-Herzog, and P.L. King, Geochim. Cosmochim. Acta 71, 4297 (2007).
B.J. Aronson, C.F. Blanford, and A. Stein, Chem. Mater. 9, 2842 (1997).
J. Madejova, J. Bujdak, M. Janek, and P. Komadel, Spectrochim. Acta A 54, 1394 (1998).
M.A. Vicente-Rodriguez, M. Suarez, M.A. Banares-Munoz, and J.D. Lopez-Gonzalez, Spectrochim. Acta A 52, 1685 (1996).
B.L. Newalkar, J. Olanrewaju, and S. Komarneni, Chem. Mater. 13, 552 (2001).
W.H. Zhang, J.Q. Lu, B. Han, M.J. Li, J.H. Xiu, P.L. Ying, and C. Li, Chem. Mater. 14, 3413 (2002).
G. Li and X.S. Zhao, Ind. Eng. Chem. Res. 45, 3569 (2006).
Z.Y. Wu, Y.F. Tao, Z. Lin, L. Liu, X.X. Fan, and Y. Wang, J. Phys. Chem. C 113, 20335 (2009).
A. Putnis and C.V. Putnis, J. Solid State Chem. 180, 1783 (2007).
A. Putnis, Mineral. Mag. 66, 689 (2002).
Acknowledgements
This research was sponsored by Key Research Program of Frontier Sciences of Chinese Academy of Sciences (QYZDJ-SSW-JSC021), Science and Technology Service Network Initiative (KFJ-SW-STS-148, KFJ-STS-ZDTP-040), National Natural Science Foundation of China (51374191, 21506233, 51402303, 21606241), and Open Cooperation Program of Science and Technology of Henan Province (172106000012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, L., Wang, L., Qi, T. et al. Leaching of Titanium and Silicon from Low-Grade Titanium Slag Using Hydrochloric Acid Leaching. JOM 70, 1985–1990 (2018). https://doi.org/10.1007/s11837-018-2929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2929-6