Abstract
The hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag was investigated under different experimental conditions. The results indicate that particle size, hydrochloric acid concentration and reaction temperature were of significance to the leaching kinetics. Specifically, reaction temperature was the most important factor followed by hydrochloric acid concentration and particle size. The shrinking core model was used to describe the leaching process which was controlled by surface chemical reaction. The kinetic equation was obtained and the activation energy was found to be 43.16 kJ/mol. Iron and calcium species were almost completely dissolved in the acid when the extraction degree of titanium reached 99.84%. MgO (19.34 wt.%) and Al2O3 (32.45 wt.%) in the spinel were still in the leaching residue and SiO2 (43.53 wt.%) in the form of quartz remained in the leaching residue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vanadium titanomagnetite ore is a valuable raw material containing iron, vanadium, titanium and other metal elements. The Panzhihua-Xichang region of China is rich in vanadium titanomagnetite resources with reserves of about 9.66 billion tons. The reserves of iron, vanadium, titanium in Panzhihua-Xichang area account for 19.6%, 62.2% and 90.5% of that in China, respectively. In addition, the vanadium and titanium reserves account for 11.6% and 35.17% of that worldwide. Vanadium titanomagnetite resource has become increasingly important in China for its high values of utilization in high-tech industries.
Vanadium titanomagnetite concentrate and ilmenite concentrate are nowadays obtained by beneficiation methods in the exploitation and utilization of the vanadium titanomagnetite ore resource in the Panzhihua-Xichang region. The vanadium titanomagnetite concentrate contains about 52% titanium and 89% vanadium of the vanadium titanomagnetite ore. However, only iron and vanadium can currently be extracted from vanadium titanomagnetite concentrate by the conventional blast furnace process. Almost all the titanium remains in the slag which forms titanium-bearing blast furnace slag with 22–25 wt.% TiO2.1 Such slag is largely stockpiled without effective utilization. Obviously, it wastes titanium resources and simultaneously causes environmental pollution. Therefore, it is of great significance to develop effective methods to comprehensively recover iron, vanadium and titanium from vanadium titanomagnetite concentrate. The methods2–6 developed or under development are direct reduction-electric furnace smelting, sodium salt roasting-direct reduction-electric furnace smelting and direct reduction-magnetic separation and so on. The direct reduction-electric furnace smelting has been commercialized in South Africa and New Zealand,3 while the other methods are still under investigation due to technological problems.
Coke serves as fuel and reductant in blast furnace smelting. The excessive reduction potential in hearth causes difficult control of vanadium and titanium in the smelting process, which accounts for the inability of the blast furnace process to treat vanadium titanomagnetite concentrate without mixing other iron ores. When mixed with other iron ores, the lower titanium grade of blast furnace slag inhibits further treatment. Compared with the blast furnace process, direct reduction–electric furnace smelting does not rely on coke. It is short and environmental friendly because the pre-reduced concentrate (in the form of pellets) are heated by electrode. This process is beneficial to control the reduction tendencies of vanadium and titanium which favors smelting of the concentrate. The titanium-bearing electric furnace slag (TEFS) produced in the process has a high TiO2 grade of 48–52%, which is easier to recover and utilize compared to titanium-bearing blast furnace slag. Therefore, the direct reduction–electric furnace smelting process has received much attention in China.7,8 However, this process can only extract iron and vanadium. The titanium in the slag thus obtained cannot be recovered effectively though the TiO2 grade is higher than that of the titanium-bearing blast furnace slag.3 Thus, how to effectively extract titanium from TEFS has become a significant problem, which is crucial for the development of direct reduction–electric furnace smelting.
There are a few of studies on recovering and utilizing the titanium resource from TEFS.9 In spite of its high TiO2 grade, it contains many impurity elements such as calcium and magnesium. Consequently, it is not suitable to be utilized by the traditional methods aimed at producing high-quality synthetic rutile. Most of the reported studies focused on sulfuric acid leaching of TEFS for titanium dioxide preparation. With the development of anticorrosion materials, there has been increasing attention on the hydrochloric acid leaching method which has been used for the treatment of ilmenite and titanium-bearing slag.10–13 The cooled hydrochloric acid leaching solution can be the solvent to remove impurities and upgrade the titanium-bearing solution. The solution with high titanium grade can be hydrolyzed, filtered, dried and calcined to produce TiO2 pigment powders. Hydrochloric acid can also be regenerated by pyrohydrolysis.10 Other useful elements such as Al and Mg in the filtrate and leaching residue can be recycled through further treatments. Apparently, hydrochloric acid leaching may effectively extract the titanium resource and other useful elements from TEFS and thereby promote the process of direct reduction-electric furnace smelting by utilizing the titanium resource in the vanadium titanomagnetite concentrate.
As expected, hydrochloric acid leaching is affected by a variety of parameters. In the present study, the kinetics of hydrochloric acid leaching of TEFS was investigated, with an emphasis on the effects of particle sizes, hydrochloric acid concentration, and reaction temperatures on the leaching process. The findings will provide a technical basis for titanium dioxide preparation by hydrochloric acid leaching process of TEFS.
Materials and Methods
Materials
The TEFS used in this study was provided by the Chongqing Iron & Steel Company (Sichuan, P.R. China). The particles with size < 1 mm were used in the experiments. The chemical composition of the sample is listed in Table I. It can be seen that the TiO2 content of the TEFS was 51.8 wt.%, and the major impurities were aluminum, silicon, magnesium, and calcium-bearing diopside and spinel. The XRD pattern of the TEFS is shown in Fig. 1a. The major phases in the TEFS were M x Ti3−x O5(0 ≤ x ≤ 2), diopside and spinel. The microstructure of the TEFS is shown in Fig. 1b. All reagents used in the experiments were of analytical grade.
Experimental Apparatus and Procedure
Figure 2 shows the structure of the leaching experimental apparatus used in this study. Leaching experiments were carried out in a 60-mL cylindrical stainless steel reactor with a PTFE lining. The reactors were heated in a thermostatically controlled glycerol bath. The TEFS samples were crushed, ground, dried and screened into different parts with different average particle sizes of 30.5 µm, 57.5 µm, and 92.0 µm, respectively. A constant solid/liquid ratio of 1:16 g/mL was used in all experiments. The experimental procedure was initiated by pouring 3.125 g of TEFS and 50 mL of the required concentration hydrochloric acid into the reactor. The reactor with the acid/slag mixture was well sealed and rapidly heated to the target reaction temperature in a glycerol bath in 1 min and then the reaction time was, measured. The rotation speed of the cylindrical stainless steel reactor was kept at 30 rpm to obtain a homogenous suspension in all experiments. After the reaction, the reactor was cooled to 25°C by water in 2 min. The slurry was filtered and washed with distilled water and the washed residue was dried for 4 the h at 110°C for the chemical composition and XRD analyses, etc.
Experimental Conditions
The effects of reaction temperature, particle size and hydrochloric acid concentrate on the leaching kinetics were investigated in this work. Five temperature points in the temperature range from 80°C to 160°C with the interval of 20°C were examined. The average particle sizes tested were 30.5 µm, 57.5 µm, and 92.0 µm, respectively. The hydrochloric acid concentration was varied from 30% to 36% to show its effect on the titanium extraction.
Definition of Parameters
The extraction degree of titanium is defined as follows:
where X is the titanium extraction degree, m is the mass of the dry leaching residue (g), w Ti is the weight fraction of Ti in the leaching residue (wt.%), m 0 is the initial mass of the dry TEFS sample (g), and w 0 is the weight fraction of Ti in the TEFS sample (wt.%).
Analysis and Characterization
The chemical compositions of the TEFS, residue and leach solution were analyzed by EDTA titration and ICP analysis. The particle size was measured by a laser particle size analyzer (Mastersizer 3000).
The TEFS samples were mounted into epoxy resin, mechanically ground and polished. Then, the polished surface was characterized by an electron microscope (LEICA DMI5000 M).
The compositions of the TEFS and residue were characterized by x-ray diffraction (Cu Ka radiation, λ = 0.154056 nm, 40 kV, 250 mA, SCAN: 10.0/80.0/0.02/.15 s, D/max2550PC; Rigaku, Japan).
Results and Discussion
Dissolution Reactions
The main mineral of TEFS is M x Ti3−x O5, which could react with hydrochloric acid as follows:
Because TiCl4 is easily hydrolyzed, the titaniferous product is often TiOC12, even H2TiO3 or H4TiO4.14 Actually, a large number of unreacted acids existed in the leaching solution, and a titanium ion was surrounded by many acid molecules to keep it from hydrolyzing. It is implied that the phase of titanium in the hydrochloric should be TiOC12·nHCl.15
The main gangues in the TEFS are spinel and diopside. The main reactions occurred in the solution are as follows.
Most of the TEFS could react with hydrochloric acid except spinel which was partially dissolved or undissolved at the leaching temperature range (80–160°C) in this study. A high liquid–solid ratio of 1:16 g/mL with a large excess of hydrochloric acid was used in this work as mentioned above; that is, the hydrochloric acid concentration was kept constant in the whole leaching process as the acid consumption of the reactions with M x Ti3−x O5 and gangues could be neglected.
Parameters Effect on Titanium Extraction Degree
This study discusses the effects of experimental parameters (reaction temperature, TEFS particle size and concentration of hydrochloric acid) on the titanium extraction degree from TEFS.
Reaction Temperature
Figure 3a shows a series of experiments conducted in a temperature range from 80°C to 160°C under the conditions of average particle size of 30.5 µm and initial acid concentration at 36%. It was found that the titanium extraction degree increased with increasing reaction temperature. For leaching at 80°C for 30 min, the titanium extraction degree was only 6.0%. When the reaction temperature was increased to 160°C, the titanium extraction degree increased to 80.52%. This shows that the reaction temperature had a significant effect on the titanium extraction degree.
Effects of different parameters on titanium extraction degree: (a) effect of reaction temperature (average particle size 30.5 µm, initial acid concentration 36%); (b) effect of particle size (leaching temperature 120°C, initial acid concentration 36%); (c) effect of initial hydrochloric acid concentration (leaching temperature 120°C, average particle size 30.5 µm)
Particle Size
The average particle sizes of these samples were 30.5 µm, 57.5 µm and 92.0 µm, respectively. The titanium extraction degree increased with decreasing particle size, as presented in Fig. 3b, which shows the effect of particle size on the titanium extraction degree at leaching temperature of 120°C and initial acid concentration at 36%. The maximum titanium extraction degree reached 96.65% with the average particle size of 30.5 µm leaching for 180 min, whereas only 65.46% Ti was extracted with the average particle size of 92.0 µm under the same conditions. The larger specific surface area of finer particles increased the interface between the hydrochloric acid and the TEFS particle, which effectively promoted the leaching reactions.
Hydrochloric Acid Concentration
TiOC12·nHCl may hydrolyze when the hydrochloric acid concentration is less than 9.5 mol/L (about 30 wt.%).15 Therefore, the minimum hydrochloric acid concentration was selected to be 30% in this study. Figure 3c shows the effect of initial hydrochloric acid concentration on the titanium extraction degree at leaching temperature of 120°C and average particle size of 30.5 µm. The results show that the titanium extraction degree increased with increasing hydrochloric acid concentration. When the hydrochloric acid concentration was 30%, the titanium extraction degree reached 70.28% after leaching for 120 min. The titanium extraction degree increased to 85.98% when the hydrochloric acid concentration was raised to 36%. The increase of the titanium extraction degree became slower after 120 min.
Selection of Leaching Kinetics Model
The typical solid–fluid reaction models are shrinking core, homogeneous, grain and pore models. Because TEFS particles consist of essentially dense grains which could be regarded as non-porous particles, and the slag grains gradually shrink during leaching, the most appropriate reaction model should be the shrinking core model in this study. Other models are usually applied to the porous solid–liquid systems.16 Thus, the shrinking core model was chosen as the leaching kinetics model.
The experimental data obtained in this study were analyzed by the shrinking core model, and it was determined that the data fit the shrinking core model quite well. The steps of shrinking core model can be described as: diffusion through the liquid film, diffusion through the ash/product layer and chemical reaction at the surface of the solid particles. The step with the highest kinetic resistance is the rate-controlling step.17,18 Integrated rate equations for shrinking core model are shown in Table II.
In the concentrated hydrochloric acid system, the silicic acid formed by dissolving silicate minerals will transform into gel or condensate producta. These products may be attached to the surface of unreacted TEFS particles, resulting in the leaching process being controlled by the diffusion through the ash or production layer. Therefore, it is necessary to determine the rate-controlling step. The kinetic data were treated by equations in Table II.
All the experimental data were analyzed by linear regression using the equations in Table II. The results show the plots of 1 − (1 − X)1/3 obtained a very good linear relationship with time. Figure 4 shows the comparison between the plots of 1 − (1 − X)1/3 and 1 − 2/3X − (1 − X)1/3 versus time at a leaching temperature of 140°C with average particle size of 30.5 µm and initial acid concentration of 36%. This indicates that the leaching rate was controlled by surface chemical reaction. The plots of 1 − (1 − X)1/3 versus reaction time under different conditions are given in Fig. 5.
Plot of 1 − (1 − X)1/3 versus time for different parameters: (a) reaction temperature (average particle size at 30.5 µm, initial acid concentration 36%); (b) particle size (leaching temperature 120°C, initial acid concentration 36%); (c) initial hydrochloric acid concentration (leaching temperature 120°C, average particle size 30.5 µm)
Determination of Rate Constant
The apparent rate constant k r is affected by particle size, hydrochloric acid concentration and reaction temperature as indicated in Eq. 12 from Eq. 9 in Table 2.
The empirical formulas of k r versus particle size, hydrochloric acid concentration and reaction temperature can be established. Therefore, the rate constant can be determined by quantitatively calculating the relationships between each factor and k r.
Reaction Temperature
The relationship between reaction temperature and k r obeys the Arrhenius equation.
The Arrhenius plot which describes the relationship of apparent rate constant k r and temperature is shown in Fig. 6a. The correlation coefficient (R 2) of the straight line in Fig. 6a is 0.9946. The activation energy of the reaction calculated from the slope of the line is 43.16 kJ/mol. This value confirms that the dissolution rate was controlled by chemical reaction. It has been reported that the activation energy of the reaction controlled by surface chemical reaction is in excess of 40.0 kJ/mol.18,19
Relationship between lnk r and 1/T, lnr 0, lnC 0 : (a) the Arrhenius plot of the dissolution process. (average particle size 30.5 µm, initial acid concentration 36%); (b) relationship between lnk r and lnr 0 (leaching temperature 120°C, initial acid concentration 36%); (c) relationship between lnk r and lnC 0 (leaching temperature 120°C, average particle size 30.5 µm)
The const was calculated to be 7.68. The relationship between k r and T can be expressed as:
Particle Size
Equation 12 shows that the apparent rate constant k r is inversely proportional to the particle size. While \( kC_{0}^{n} M/\rho \) is regarded as constant A 1, Eq. 12 can be transformed into:
The plots of lnk r versus lnr 0 is presented in Fig. 6b. The slope of the fitted straight line was calculated to be −0.75 according to Fig. 6b. Then the relationship between k r and r 0 can be written as:
Hydrochloric Acid Concentration
While \( kM/\rho {\text{r}}_{0} \) is regarded as constant A 2, Eq. 12 can be transformed into:
The plot of lnk r versus lnC 0 is shown in Fig. 6c. The slope of the fitted straight line was calculated to be 1.21 according to Fig. 6c. The relationship between k r and C 0 is:
Determination of Leaching Kinetics
The relationships between the tree factors and k r are established in Eqs. 14, 16 and 18. The semi-empirical kinetic equation can be obtained by synthesizing the three relationships.
where A 3 is frequency factor, \( A^{\prime} = \frac{{MA_{3} }}{\rho } \).
The Arrhenius equation obtained from Fig. 6a is \( k_{\text{r}} = 2154.04e^{ - 43160/RT} \), and then, \( A^{\prime}\frac{{C_{0}^{1.21} }}{{r_{0}^{0.75} }} = 2154.04 \).
Substituting C 0 = 11.6 mol/L and r 0 = 15.25 × 10−6 m, gives A′ = 2.85 × 10−2.
Therefore, the semi-empirical kinetic equation is
where X is the fraction of Ti extracted, C 0 is the initial concentration of hydrochloric acid (mol/L), r 0 is the average particle radius (µm), R is the molar gas constant (8.314 J mol−1 K−1), T is the reaction temperature (K), and t is the reaction time (min).
The semi-empirical kinetic equation shows that the effects of the parameters on the titanium extraction degree in the order of significance were: reaction temperature, hydrochloric acid concentration, and particle size. The final semi-empirical kinetic equation for titanium extraction from TEFS by hydrochloric acid leaching obtained in this work is Eq. 20.
Comparison of Experimental and Calculated Results
A comparison between the experimental values of the titanium extraction degree and those calculated by the semi-empirical kinetic equation developed above using Eq. 20 is shown in Fig. 7. This indicates that the calculated results agreed well with the experimental ones.
Comparison between the calculated titanium extraction degree using Eq. 20 and the experimental results for all the runs made in this work
Leaching Residues
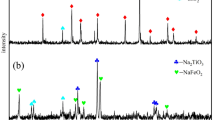
The impurities were also dissolved in the hydrochloric acid in the process. Figure 8 shows the XRD patterns of different leaching residues and original TEFS. The results indicate that the peak strength of M x Ti3−x O5 remained weak with increasing the titanium extraction degree. When the titanium extraction degree was 85.98%, the phases of diopside disappeared. The silicic acid, which was formed from silicate dissolved in the hydrochloric acid, eventually converted into SiO2 in the leaching residue. When the titanium extraction degree was 99.84%, the main phases of leaching residues were spinel and quartz.
The titanium extraction degree of the slag was 99.84% when leached for 45 min at a leaching temperature of 160°C with average particle size of 30.5 µm and initial acid concentration of 36%. The chemical composition of the leaching residue under this condition is listed in Table 3, and the extraction degrees of the main elements are shown in Table 4. It was found that iron and calcium were almost completely dissolved in the acid when the titanium extraction degree was 99.84%, while part of magnesium and aluminum in the spinel still existed in the leaching residue and silicon in the form of SiO2 remaining in the residue.
Conclusion
The main minerals of TEFS are M x Ti3−x O5, diopside and spinel. The titanium extraction degree obviously increased with increasing reaction temperature, higher initial hydrochloric acid concentration and smaller particle size. The results indicate that these three parameters were of significance to the leaching kinetics, and that the reaction temperature was the most important factor followed by hydrochloric acid concentration and particle size. The shrinking core model was selected to describe the leaching kinetics of the TEFS in hydrochloric acid solution, which was controlled by the surface chemical reaction. The leaching equation was obtained with the activation energy of 43.16 kJ/mol.
The titanium extraction degree reached as high as 99.84% after leaching for 45 min at 160°C with average particle size of 30.5 µm and initial acid concentration of 36%. Iron and calcium mainly in the diopside were almost completely dissolved in hydrochloric acid. The MgO (19.34 wt.%) and Al2O3 (32.45 wt.%) in the spinel were still in the leaching residue and SiO2 (43.53 wt.%) in the form of quartz remained in the leaching residue.
References
G.H. Han, T. Jiang, Y.B. Zhang, Y.F. Huang, and G.H. Li, J. Iron. Steel Res. Int. 18, 14 (2011).
P.R. Taylor, S.A. Shuey, E.E. Vidal, and J.C. Gomez, Miner. Metall. Proc. 23, 80 (2006).
B.C. Jena, W. Dresler, and I.G. Reilly, Miner. Eng. 8, 159 (1995).
D.S. Chen, H.X. Zhao, G.P. Hu, T. Qi, H.D. Yu, G.Z. Zhang, L. Wang, and W.J. Wang, J. Hazard. Mater. 294, 35 (2015).
S. Samanta, S. Mukherjee, and R. Dey, JOM 67, 467 (2015).
Z. Peng and J.Y. Hwang, Int. Mater. Rev. 60, 30 (2015).
Y.F. Guo, M.J. Tang, T. Jiang, L.J. Qing and J.F. Zhou, in 4th International Symposium on High-Temperature Metallurgical Processing, TMS Annual Meeting, San Antonio, Texas, USA, (2013), pp: 87–94.
G.G. Liu, Appl. Mechan. Mater. 217–219, 441 (2012).
Y. Li, Y. Yue, Z.Q. Que, M. Zhang, and M. Guo, Int. J. Miner. Met. Mater. 20, 1012 (2013).
D. Verhulst, B. Sabacky, T. Spitler, and W. Duyvesteyn, CIM Bull. 95, 89 (2002).
M.H.H. Mahmoud, A.A.I. Afifi, and I.A. Ibrahim, Hydrometallurgy 73, 99 (2004).
N. El-Hazek, T.A. Lasheen, R. El-Sheikh, and S.A. Zaki, Hydrometallurgy 87, 45 (2007).
D.A. Hansen and D.E. Traut, JOM 41, 34 (1989).
T.H. Wang, A.M. Navarrete-López, S. Li, D.A. Dixon, and J.L. Gole, J. Phys. Chem. A 114, 7561 (2010).
S.X. Gu and X.H. Luo, Rare Met. 2, 96 (1987) (in Chinese).
R.J. Ma, Principle of Hydrometallurgy (Beijing: Metallurgical Industry Press, 2007), pp. 325–342. (in Chinese).
O. Levenspiel, Chemical Reaction Engineering, Third Edition ed. (New York: Wiley, 1999), pp. 566–570.
H.Y. Sohn and M.E. Wadsworth, Rate Processes of Extractive Metallurgy (New York: Plenum Press, 1979), pp. 135–143.
F. Habashi, Principles of Extractive Metallurgy, General Principles, 2nd ed. (New York: Gordon and Breach Science Publishers, Inc, 1969), pp. 111–169.
Acknowledgements
The authors are grateful to the Program for New Century Excellent Talents in University from Chinese Ministry of Education (NCET-10-0834) and the Visiting Scholar Program from China Scholarship Council ([2013]3018) for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, F., Chen, F., Guo, Y. et al. Kinetics of Hydrochloric Acid Leaching of Titanium from Titanium-Bearing Electric Furnace Slag. JOM 68, 1476–1484 (2016). https://doi.org/10.1007/s11837-015-1808-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1808-7