Abstract
A mixture of pure Ta2O5 and Nb2O5 was dissolved using two different fluxes, namely NH4F·HF and Na2HPO4/NaH2PO4·H2O. Selective precipitation and ion exchange were used as separation techniques. Selective precipitation using p-phenylediamine in a fluoride matrix resulted in the isolation of 73(3)% tantalum accompanied by 23(5)% niobium. A separation factor of 11(4) was obtained. A single solvent extraction step using methyl-isobutyl ketone at a 4 M H2SO4 yielded excellent Ta and Nb separation in the fluoride solution with 80% of the Ta and only 2% Nb recovered in the organic layer. A two-step extraction recovered 100% Ta at 0.5–4 M H2SO4 with a separation factor of ~2000. A study of the extraction mechanism indicated that the stability of the protonated compounds such as H2TaF7/H2NbOF5 is in the extraction and separation determining steps in this process. A K′ (double de-protonated constant) of approximately 0.2 was calculated for H2TaF7. Only 91.7% Nb and 73.4% Ta were recovered from anion separation using strong Amberlite resin and 96.1% Nb and 52.3% using the weak Dowex Marathon resin from fluoride dissolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tantalum and niobium belong with vanadium to group Vb on the periodic table. The two elements, (Nb and Ta) are a congeneric pair with very similar chemical properties. The major source of niobium and tantalum is columbite-tantalite (coltan), with a general chemical formula of (Fe,Mn)(Nb,Ta)2O6, while pyrochlore (Na,Ca)2Nb2O6(OH,F) is a major source of niobium. The two elements also exist in more than 150 other minerals such as the metal oxides (Ta/Nb)2O5) and hydroxides, with the exception of behierite (Ta,Nb)(BO4) as borate mineral. Niobium is extensively used in the nuclear and super alloy industries,1,2 while tantalum plays an important role in the production of cell phones and laptop computers, while in the medical field it is used for joint arthroplasty.3,4
Dissolution and separation of the two main elements are challenging due to the metal oxides’ (and therefore the minerals’) high resistance to chemical attack5 and similar chemical properties. The major industrial processes for pyrochlore and tantalite improvement involve the use of harsh experimental conditions,6–14 which introduce halogens into the reaction mixture. Fluorination7,15–17 and chlorination16,18,19 of the primary material are the most successful methods for dissolution and subsequent separation techniques, which have led to the development of industrial processes. The conversion of the metal oxides to similar or different kinds of halide complexes under identical experimental conditions introduce sufficient differences in the chemistry of the two metals to allow for their separation. Chlorination is less favoured as an improvement process due to the sophisticated equipment required to avoid loss of volatile compounds of interest, complications in the purification process through boiling point interferences by complicated mineral matrixes and the generation of numerous volatile inorganic compounds. The hydrometallurgical process involving fluoride dissolution is much simpler and more economical.19
Current industrial processes make extensive use of HF, unaided at elevated temperatures or in combination with concentrated H2SO4 or HCl, to produce the much needed metal fluoride complexes. The Ames process used anhydrous HF to induce mineral dissolution, followed by solvent extraction (MIBK) to separate the two metals in the presence of H2SO4. This method replaced the Marignac process which utilized the solubility difference between the tantalum and niobium fluoride complexes.17 Agulyansky et al.20 reported the selective extraction of Ta from a HF-H2SO4 system with H2SO4 concentrations between 2 M and 4 M. Results obtained by Kabangu and Crouse21 indicated the simultaneous extraction of the two metals with 90% Ta and about 50% Nb (in tapiolite) from a NH4F·HF-H2SO4 system using 1-octanol and 2-octanol as extractants at 2.0 M H2SO4. Results of another study indicated that a TaF5/NbF5 mixture22 can be successfully separated using p-phenylediamine (PPDA) as precipitating reagent.
These hydrometallurgical processes are successful in the production of pure niobium and tantalum, but have serious disadvantages.23 These include (1) type and quantity of impurities in solution, (2) expensive regeneration of fluorine and the substantial loss of fluorine quantities in these processes, (3), generation of large amounts of residue, (4) the permanent loss of reagents, and (5) the environmental and hazardous nature of HF as a reagent. Recently, different alternative dissolution processes involving fluoride containing salts have been investigated. Nete et al.24 selectively extracted Ta from a NH4F·HF-H2SO4 system using different organic extractants while other fluoride salts such as KF·HF and NaF25,26 showed various degrees of successful tantalite and niobite dissolution.
All of the previous investigations lack a clear understanding of the mechanism and basic chemistry involved in the extraction process as well as the properties which allow for the separation of the two metals. This study investigated the separation of these two elements using a mixture of pure Ta2O5 and Nb2O5 as model to (1) identify the underlying chemical properties that permit separation, (2) to evaluate product mixture separation after dissolution using different fluxes (NH4·HF and Na2HPO4/NaH2PO4·H2O), and (3) to compare these results with those observed in the mineral processing. Selective crystallization using PPDA, solvent extraction using MIBK and ion exchange using both cationic and anionic exchangers were investigated for separation of the Ta and Nb dissolution products.
Experimental
Apparatus
Flux fusions were performed in a Thermo Scientific Thermolyne Compact Benchtop Muffle Furnace. The Boeco SP series adjustable volume pipettes and polytetrafluoroethylene (PTFE) volumetric flasks (Merck) were used for sample solution preparations. Merck glass columns (height = 20 cm) with an internal diameter of 1.2 cm were used for ion exchange separations. Centrifugation was performed in a centrifuge supplied by MSE. Analytical determinations were performed under constant experimental conditions at 309.418 nm for Nb and at 240.068 nm for Ta using a Shimadzu ICPS-7510 ICP-OES sequential plasma spectrometer. The infrared (IR) spectra were obtained with a Scimitar Series Digilab spectrometer. The pH measurements were carried out using a Eutech CyberScan pH 1500 bench meter. The average values are based on at least three replicate analyses and are reported together with standard deviations to indicate the uncertainty in the last digit of the value throughout the paper.
Reagents
High-purity Ta2O5 (99.99%), Nb2O5 (99.9%), Na2HPO4 and NaH2PO4·H2O were bought from Sigma Aldrich. Ammonium bifluoride (NH4F·HF) and p-phenylenediamine were sourced from Merck. ICP standard solutions containing 1000 mg/L Ta and Nb were also obtained from Merck. Methyl isobutyl ketone (MIBK) was sourced from Saarchem. Analytical grade H2SO4 (97%), H3PO4 (85%) and HCl (32%) were bought from Associated Chemical Enterprises. Double-distilled water was used in all cases. A strong basic anion exchanger, Amberlite IRA-900 (16–50 mesh), and a weak basic anion exchanger, Dowex marathon (350–450 μm), as well as a strong cation exchanger, Amberlite IR-130C, resins were also purchased from Sigma Aldrich. Clinobrite 814 zeolite (cationic filter medium) was supplied by Pratley.
Sample Preparations and Measurements
Preparation of Standard Solutions
An amount of 2.0 g of the flux (NH4F·HF or 1:1 Na2HPO4/NaH2PO4·H2O) was weighed in a platinum crucible. The flux was heated to temperature above its melting point (NH4F·HF = 200°C and 1:1 Na2HPO4/NaH2PO4·H2O = 800°C) in a closed oven. The molten salt was cooled to room temperature and dissolved in 100 mL water. This flux solution was used for matrix matching of the standards and blank solutions. Standard solutions with concentrations ranging between 1.0 mg/L and 20.0 mg/L Ta and Nb were used for calibration of ICP-OES. The blank solutions were prepared by diluting a 5.0-mL solution of a flux melt in 10.0 mL H2SO4 (or 1:1 Na2HPO4/NaH2PO4·H2O fusion melt in 5.0 mL 85% H3PO4 for phosphate fusion analysis) to the 100.0 mL mark of the volumetric flask with double-distilled water and were used for background correction.
Dissolution of (Ta/Nb)2O5 Using Ammonium Bifluoride Flux
A mixture of Nb2O5 and Ta2O5 (0.1 g each) was dissolved using a NH4F·HF flux fusion method; details described in previous investigations.24,26 The IR spectra of Nb2O5/NH4F·HF, Ta2O5/NH4F·HF and a mixture (Ta/Nb)2O5/NH4F·HF fusion products were recorded in the range of 4000–400) cm−1 and the following characteristic stretching frequencies were obtained:
-
Nb2O5/NH4F·HF: ν(M-F) 476 cm−1 and 553 cm−1, ν(M-O) 892 cm−1, δ(M-O-M bridges and M-O bonds in oxyfluorides) 697 cm−1 and 1080 cm−1.
-
Ta2O5/NH4F·HF: ν(M-F) 478 cm−1 and 547 cm−1 δ(M-O-M bridges and M-O bonds in oxyfluorides) 701 cm−1 and 1075 cm−1.
-
(Ta/Nb)2O5/NH4F·HF: ν(M-F) 477 cm−1 and 558 cm−1, ν(M-O) 918 cm−1, δ(M-O-M bridges and M-O bonds in oxyfluorides) 698 cm−1 and 979 cm−1 and 1084 cm−1.
Dissolution of (Ta/Nb)2O5 Using Phosphate Flux
Approximately 0.1 g (accurately weighed to 0.1 mg) each of Nb2O5 and Ta2O5 (1.7:1 Nb:Ta mole ratio) was thoroughly mixed with 4.0 g each of Na2HPO4 and NaH2PO4·H2O (1:20 sample:flux mass ratio) in a platinum crucible.12 The mixture was heated at 800°C for 30 min and cooled to room temperature. The melt was dissolved with 30 mL water, quantitatively transferred to a 100-mL volumetric flask and diluted to the mark with double-distilled water.
Selective Precipitation of Nb and Ta in Different Matrices
A 5.0-mL aliquot of the fluoride solution (“Dissolution of (Ta/Nb)2O5 Using Ammonium Bifluoride Flux” section) was transferred to a clean 50-mL beaker. Approximately 0.06 g of PPDA was accurately weighed and dissolved in 10.0 mL double-distilled water. The PPDA solution was added to the Ta/Nb-containing solution with an immediate colour change from colourless to blue and then precipitation formation. The pH of the solution was determined to be 5.42. The white precipitate was separated from the solution by centrifugation and decanted. The filtrate solution was acidified using 97% H2SO4 to match the standard and blank solutions. The precipitate was dissolved in 10 mL of 97% H2SO4 and the solution was diluted in a 100-mL volumetric flask with double-distilled water. Metal concentrations were determined in both the filtrate and precipitate solutions using ICP-OES. The precipitate yielded recoveries of 73(3)% Ta and 23(5)% Nb while the overall mass balances (total elemental recoveries of five replicate analyses in both the precipitate and filtrate) of 97(3)% Ta and 101(3)% Nb were obtained. The following IR stretching frequencies for the precipitate product and PPDA ligand were obtained:
-
Ta2O5/NH4F·HF/PPDA: 1419 cm−1, 951 cm−1, 832 cm−1 and 617 cm−1.
-
PPDA: 1513 cm−1, 1260 cm−1, 823 cm−1 and 574 cm−1.
The precipitation procedure was repeated with the phosphate solution (“Dissolution of (Ta/Nb)2O5 Using Phosphate Flux” section). No precipitation or colour change was observed even after 12 h. The pH of the final solution was found to be 6.89. The experiment was repeated and the pH of the final solution was dropped to 5.10 and below using 0.1 M H3PO4 solution. Even under these new set of experimental conditions, no precipitate formation or colour change was observed at the end of the 12-h period.
Solvent Extraction and Subsequent Striping of Ta and Nb in Different Matrices
Aliquots (5.0 mL) of the solution (“Dissolution of (Ta/Nb)2O5 Using Ammonium Bifluoride Flux” section) were pipetted into glass separation funnels followed by the addition of 5-mL aliquots of a freshly prepared H2SO4 solution, which varied between 1 M and 8 M. Then, 10.0-mL aliquots of MIBK were added to the different metal/acid mixtures and were shaken for 5 min. Back extraction was accomplished using double-distilled water (10 mL). The concentration of metals in the two liquid phases (back extraction and original acidic solution) was measured by ICP-OES and the average results of three replicate analyses of this single extraction are reported in Table I. The single extraction did not provide quantitatively satisfactory Ta recoveries. Therefore, the procedure was repeated for two extractions (also in triplicate) and the results are reported in Table II.
The solvent extraction procedure was repeated with the phosphate solution (2.3.3). Analytical results obtained in this study indicated that the separation of Ta and Nb in phosphate medium by solvent extraction using MIBK was not visible. Maximum extractions of 0.75% Ta and 0.69% Nb were obtained at 3.0–4.0 M acidity.
Ion Exchange Separation of Ta and Nb in Fluoride and Phosphate Matrices
Two anionic exchange resins namely, Dowex Marathon wba and Amberlite IRA-900, were separately added to double-distilled water and stirred in a 250-mL beaker to form a free-flowing slurry. The slurry was transferred to the column of 1.2 cm diameter packed to a height of 20 cm, and washed with 20 mL 2.0 M HCl. A 5.0-mL volume of the sample solution (“Dissolution of (Ta/Nb)2O5 Using Ammonium Bifluoride Flux” section) was transferred to the column and sequentially eluted with 30-mL aliquots of different concentrations of HCl ranging from 0.1 M to 6.0 M at a rate of 0.7 mL/min. The typical elution curves are shown in Figs. 1 and 2 for the strong Amberlite and weak Dowex Marathon anionic resins, respectively. It is important to note that Figs. 1 and 2 only illustrate the general trend observed during the elution of tantalum and niobium and which were obtained from this separation technique.
The ion exchange separation procedure was repeated with Ta/Nb phosphate solution on a weakly acidic clinobrite zeolite and strong Amberlite IR-130C cation resins as well as on weakly basic Dowex Marathon resin. Elution was performed with dilute H3PO4 solutions (up to 4.0 M). The analytical results of the cation exchange separation are presented in Table III.
Results and Discussion
Sample Dissolution
Successful sample dissolution was accomplished with fusion using both fluoride (NH4F·HF) and phosphate (Na2HPO4/NaH2PO4·H2O) as fluxes. Recoveries of 100.4(9)% and 100(1)% for Nb and Ta were obtained for NH4F·HF while Na2HPO4/NaH2PO4·H2O yielded 99.9(4)% and 100.6(6)% recovery, with a precision of 0.4% and 0.6% RSD for Nb and Ta, respectively. The elemental recovery using NH4F·HF is also very similar to that obtained for the tantalite mineral,25,26 which recovered 100.3(5)% Nb and 100.0(9)% Ta. This result clearly suggests that the two metal compounds (oxides) are identical in both the two samples. The literature28 predicts that fluoride compounds with formulae (NH4)3NbOF6, (NH4)2NbOF5, (NH4)2TaF7 and NH4TaF6 are formed during the NH4F·HF dissolution process. The formation of soluble linear poly meta-phosphates is suggested29 during the phosphate dissolution, arguably due the strong complexing properties of the phosphates. Phosphate, however, is much better known to form insoluble products or precipitates,30,31 with few references of phosphates’ complexing ability even on the spectrochemical series,32 and it is anticipated that hydrated metal species are formed during the dissolution of this melt.
Product Characterisation Using Infrared Spectroscopy
Some of the stretching frequencies of the metal oxides (especially in the 500–700 cm−1 region) as well as those for the pentafluorides were comparable to those obtained in the fusion products suggesting possible oxide-fluoride type of compounds. The comparison between the IR spectra of the starting materials (Ta2O5, Nb2O5, TaF5 and NbF5) with those of the oxides/NH4F·HF fusion products also showed that the compounds formed during this NH4F·HF fusion digestion step were chemically different from both the pentoxides and pentafluorides.
The stretching frequencies for the isolated Ta and Nb products (in this study) were compared with that literature17 stretching frequencies for the compounds (NH4)3NbOF6 and (NH4)3TaOF6, which were obtained by a similar digestion procedure. The stretching frequencies in the 476–477 cm−1 region compared well with the recorded stretching frequencies of 470 cm−1 for the Nb-F in (NH4)3NbOF6 and 465 cm−1 for Ta-F in (NH4)3TaOF6 compounds. The stretching frequency at 892 cm−1 obtained in this study was similar to the 910 cm−1 for the Nb-O vibrations in (NH4)3NbOF6, while the stretching frequencies at 553–558 cm−1 and 918 cm−1 regions were similar to the frequencies of 550 cm−1 for Nb-F and 920 cm−1 for Nb-O in the (NH4)2NbOF5.17
The IR spectrum for the Ta2O5/NH4F·HF fusion product displayed a stretching frequency at 547 cm−1, which is similar to the literature33 value of 550 cm−1 for the Ta-F vibration in (NH4)3TaOF6. The IR stretching frequencies in the 1396–1406 cm−1 region compared well with the vibrations of NH4 + ion as reported in the literature.33 The strong stretching frequencies at 693–706 cm−1 and weak stretching frequencies at 975–1087 cm−1 observed in all the spectra were similar to the literature values for the vibrations of the M-O-M bridges and the M-O bond of the metal oxyfluoride17,34 indicating a possible mixture of fluorotantalates and oxyfluorotantalates in the case of the Ta2O5/NH4F·HF reaction.
Selective Precipitation of Ta and Nb
Selective Precipitation of Ta and Nb in Fluoride Solution
The addition of PPDA after the (Ta/Nb)2O5/NH4F·HF fusion yielded Nb recoveries of 19.8–28.7% and 70.2–76.2% for Ta in the white precipitate (“Selective Precipitation of Nb and Ta in Different Matrices” section) with the remaining portion of the elements (mass balance) present in the solution. The distribution coefficients (D) were calculated as D (Ta) = 3.377 and D (Nb) = 0.307 while the separation factor (α) was calculated as 11(4). The formation of the blue-coloured complex immediately after the addition of the DPPA is indicative of the formation of metal/PPDA complexes.35 Gross and Kaim35 described the formation of the blue complex as a result of the possible oxidative deprotonation of the amino functional in PPDA. These intensely blue- and red-coloured complexes, also known as Wurster’s salts, are normally produced by one e− oxidation reaction and have been known for more than a century.35,36 The formation of the blue complex in the current study is most probably due to the oxidation of the amine group through a loss of a proton, resulting in the formation of PPDA anion. This anion can then react with the MF5 complex to produce an intensely coloured intermediate complex.
The results of this study clearly indicate that PPDA reacted preferentially with Ta, even in the presence of Nb, and thus has some potential as a separating reagent when the oxides have been digested and fluorinated with NH4F·HF. This method may not be ideal for the separation of the two main elements, but certainly has the potential for Ta enrichment in mineral ores containing larger concentrations of Ta.
In a previous study,22 more than 80% Nb were selectively precipitated (and only 4% Ta) from a 50% mixture of NbF5 and TaF5 using the same complexing/precipitating agent. It is apparent from these experimental results that the NH4F·HF dissolution process results in the formation of Ta/Nb fluoride complexes, which do not only behave differently from the pentafluorides (different separation factors) but also produce different types of complexes or the same complexes with substantially different chemical properties (Nb precipitation versus Ta precipitation).
The IR spectra of the precipitate product of (Ta2O5/NH4F·HF with PPDA) and unreacted PPDA were recorded and compared to determine if there has been any chemical reaction between Ta2O5/NH4F·HF and PPDA. The stretching frequencies of 1419 cm−1 and 832 cm−1 in the product (precipitate) correspond with the peaks at 1513 cm−1 and 823 cm−1 that were obtained for the PPDA ligand. The appearance of two strong peaks at 617 cm−1 and 950 cm−1 in the product spectrum indicated that a new product was formed during the reaction between Ta2O5/NH4F·HF and PPDA.
Selective Precipitation of Ta and Nb in Phosphate Solution
The absence of any precipitation or a solution colour change (“Selective Precipitation of Nb and Ta in Different Matrices” section) with the addition of PPDA to a Ta/Nb-containing solution after phosphate flux dissolution, emphasizes the importance of the type of dissolution method in the subsequent separation process. The use of the phosphate flux produces soluble metal complexes which react completely differently (Fig. 3) from the products obtained after dissolution with fluoride-containing reagents.
The results illustrate the importance of the digestion method on the identity of the chemical products obtained and their applicability in the subsequent separation methodology.
Solvent Extraction of Ta and Nb
Solvent Extraction and Solvent Stripping of Ta and Nb in the Fluoride Matrix
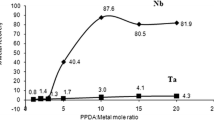
Elemental recoveries obtained for the Ta/Nb extraction with MIBK in the presence of different H2SO4 concentrations (Fig. 4) indicated excellent element separation with one extraction step. The quantitative results showed that Ta was the major metal compound present in the organic solvent (from 0.5 M to 4 M H2SO4) leaving the majority of Nb in the aqueous solution. More than 80% Ta is extracted into the organic solvent at 4 M H2SO4 while Nb remained in the original aqueous solution (0.10–2.04% in the organic portion). The distribution ratios (D) for the extraction of both metal compounds were calculated using different equations (\( q = \left( {\frac{1}{{DV_{R} + 1}}} \right)^{n} \) and D = [M]org/[M]aq) at different acid concentrations [using concentration (mg/L) data for Table I] and excellent agreement between the values using the two different equations were found. The distribution ratios for Nb increased from 0.006 to 0.016 in the 0–4.0 M H2SO4 range while the Ta extraction ratios increased from 1.45 to 3.4 in the same [H2SO4] range confirming the Ta enrichment in organic extractant (Fig. 4).
Experimental and calculated (Eq. 6 for Ta) % extraction (for n = 2) of Nb2O5 and Ta2O5 in MIBK as a function of [H2SO4]
Calculations also indicated (Table I) that the number of extractions required to reduce the % Ta in the aqueous layer to 0.1% (or 99.9% extracted), decrease from 2.5 ([H2SO4] = 0.5 M) to 1.5 ([H2SO4] = 4.0 M). This observation indicates the acid dependence of the Ta complex extraction process into the organic phase. These calculations as well the results in Fig 5 predict that two or more extractions (n = 2) are needed to extract Ta quantitatively under current experimental conditions (0.0–4.0 M H2SO4) from the sample mixture. Linear extrapolation of the graph in Fig. 5 predicts that ca. 6.0 M H2SO4 will be needed to extract 99.9% Ta with a single extraction step. A quantitative extraction of Nb on the other hand would require more than 100 consecutive extractions from the mixture using similar experimental conditions as described in “Solvent Extraction and Subsequent Striping of Ta and Nb in Different Matrices” section. Similar results were reported during the separation of Nb and Ta in a tantalite mineral also using solvent extraction.20 Tantalum was selectively extracted into the organic phase for all five different solvents, which were investigated leaving the Nb in the aqueous phase.20 The calculated distribution ratios of 3.43 for Ta in pentoxide mixture (this study) and 3.66 for Ta in tantalite using MIBK are in excellent agreement with one another. The distribution ratios obtained for niobium were extremely small [0.0122 in tantalite compared to 0.016 in (Ta/Nb)2O5 for MIBK] and indicates the inability of this element to dissolve in the organic solvents at [H2SO4] between 0 M and 4.0 M. The results indicate the similarity of the chemical behaviour between Nb and Ta compounds in the mineral and that of pure Nb and Ta pentoxides. This observation allows for the development and validation of possible dissolution and separation methods on the pure metal oxides, which will be extremely relevant for separation of Ta and Nb elements in minerals.
Results of the two-step extraction process (Table II) showed that Ta was successfully separated (~100% Ta in MIBK) from Nb by this solvent extraction method. No extraction of the niobium complex was observed in this H2SO4 concentration range. The distribution ratios for Ta (D (Ta)) were found to increase with an increase in H2SO4 concentration and a plot of D (Ta) against [H2SO4] produced a linear curve (Fig. 6), which demonstrates the dependence of the tantalum extraction efficiency on H2SO4 concentration.
This observation can be explained using Eq. 1 where D is expressed as a function of the distribution constant (K D), the acid dissociation constant (K a) of the Ta complex and the actual acid concentration predicts that D ≈ K D 27 at high acid concentrations ([H+] ≫ K a). The acid dependence of D on [H+] at these high H2SO4 concentrations (4 M) suggest that the real/final distribution constant (K d) is still larger than the highest D value calculated in this study. The niobium distribution coefficient on the other hand remains almost constant in this H2SO4 concentration range (0.5–4.0 M).
Extraction Mechanism
A literature study17 indicated that NbOF5 2− and TaF7 2− are the most stable and probable metal complexes that are formed after fluoride source dissolution (such as HF/H2SO4 or NH4F·HF). These species will be regarded as the main species in solution for the rest of the discussion. Counterbalancing of the negative charges on these metal complexes by protonation is the key step that will allow them to be soluble in an organic medium (neutral species) as indicated in Eqs. 2 and 3.
The difference in either the solubility or the extent of formation of these compounds (H2NbOF and H2TaF7) holds the key to their extraction and possible separation. The different steps in the extraction process are illustrated in Fig. 7. The extraction process involves the dissolution of H2SO4 (source of H+), the neutralization of the metal compounds, TaF7 2− in this case, with the formation of the neutral H2TaF7 complex in the aqueous phase and its subsequent movement to the organic layer. A close inspection of the different individual steps (equilibrium constants) associated with the extraction process (K ext or D), can shed light on the importance of [H+] in the successful extraction of the Ta product into the organic layer.
The distribution ratio, D, can be expressed as a combination of the different equilibria illustrated in Fig. 5 resulting in Eq. 4
where P L = [H2SO4]org/[H2SO4]aq) or solubility of H2SO4 in the organic layer, K L = [H+][HSO4 −]/[H2SO4] or dissolution of H2SO4, K D = [H2TaF7]org/[H2TaF7]aq or solubility of neutral tantalum compound in the organic layer and finally K M = [TaF7 2−][H+]2/[H2TaF7] or the stability/dissociation of the neutral tantalum compound in the aqueous layer.
It is anticipated that the solubility of the extractant (H2SO4 in this case), represented by PL (≪1) in Fig. 7, is extremely low in the organic layer. This can easily be explained since H2SO4 is a strong acid and therefore dissociated completely to form H+ and HSO4 − [which is also favourable for extraction as KL ≫ 1 (K a for H2SO4)] and these polar or ionic species will be highly insoluble in the organic layer.
The results in Fig. 4 (zero extraction of Ta with no addition of acid) also demonstrate that no or very little of the H2TaF7 compound is present in the aqueous phase and only with the addition of H+ (H2SO4) is formed in substantial amounts, which can then migrate to the organic layer, therefore suggesting a K M value of substantial magnitude. Alternatively, it indicates that H2TaF7 is a moderately strong acid, which dissociates easily (poor stability) in the aqueous layer. The extraction results presented earlier in Table II indicate that the neutral Ta species are easily extracted into the organic layer, once they are formed, and hence a favourable K D value (K D > 1 as calculated in Table I). The easy stripping of this compound, in the absence of additional H+, proves the tendency of H2TaF7 to dissociate very easily. It is essentially the size of the K M value in Eq. 4, which controls the extraction and hence is the extraction/separation determining step in this process. It is therefore argued that the addition of H+ to the reaction mixture suppresses the dissociation of the H2TaF7 or enhances the formation of the compound and shifts the equilibrium to the right hand side of the chemical reaction as indicated in Fig. 7 and Eq. 1 to afford the extraction of the Ta. The extraction process is also be presented by Eq. 5, which involves the stepwise protonation of TaF7 2− anion and then the product’s extraction into the organic layer.
or
with the formation of the neutral metal species representing the extraction determing step. The total equilibrium constant K” (= K1K2KD), taking Eq. 6 as well as mass balance into account, can be expressed by Eq. 7
where %Ta = amount of Ta at different [H+] and %Ta0 the initial amount extracted into the organic layer of %Ta∞ = amount at t = ∞.
A plot (calculated extraction curve in Fig. 4) of the data in Table I (%Ta(org) versus [H2SO4]) yielded a K″ value equal to 16(7) and a fairly good fit37 to the experimental results (see broken line). The relatively large error in K″ is not unexpected due to the few data points in 0–0.5 M H2SO4 region. Using the K D value calculated at 4 M H2SO4 (K D = 3.4), a K′ equal to 4.7 is obtained for the product of the two protonation reactions (K 1 K 2). The inverse of this value (K M) is equal to 0.2, which indicates the combined acid dissociation constant for the two acids, (combined pK a = 0.69) confirming the moderate nature of H2TaF7 as predicted by the need for additional H+ for extraction ([Ta] ~ 0 at low [H2SO4]) and the easy stripping (dissociation) of the acid from the organic layer.
Niobium of the other hand is only extracted at much higher [H+] as seen in Fig. 4 and Table I (increasing amounts of Nb in the organic layer) and as reported in literature20–24 suggesting that K D is also favourable for Nb extraction (K D > 1 at very high [H+]) with P L and K L remaining the same in the extraction process. Poor Nb extraction at lower [H+] (0–4 M H2SO4) suggests that the K M value for Nb is much larger and that K2NbOF5 is extremely unstable and dissociates very easily compared to the Ta compound. A larger K M value (stronger acidic properties of Nb) suggests the formation of less H2NbOF5 with the addition of H+ (equilibrium lies far to the left in Eq. 1) and hence less Nb product to be extracted into the organic layer. The key to the separation of these two elements therefore seems to be the difference in the K M values (stability of acid dissociation constants) of the two compounds as illustrated by the metal recoveries in Table II.
Extraction of Nb and Ta by MIBK from Phosphate Fused Solution
Quantitative results obtained from the separation of Nb and Ta in a phosphate environment showed that solvent extraction using MIBK was ineffective for the extraction of these metals. Elemental recoveries in the organic fraction were less 1.0% for both Nb and Ta. This observation clearly shows the difference in chemical identity and behaviour of the Ta/Nb phosphate compounds towards MIBK extraction compared to the Ta/Nb fluoride and further proves the importance of the selection of an appropriate sample dissolution method to ensure that the chemical yield is comparative with the subsequent separation processes.
Ion Exchange Separation of Ta and Nb in Different Chemical Environments
Anion Exchange Separation Using the NH4F·HF Fused (Nb/Ta)2O5 Mixture
Two different anion exchange resins, namely, Dowex Marathon wba (a weakly basic anion exchanger, -N+H2CH3), and Amberlite IRA-900 (a strongly basic anion exchanger, -N+(CH3)3), were evaluated for possible separation of Nb and Ta from a pentoxide mixture. The results of the elution of Ta and Nb with HCl from the strong Amberlite anionic exchange resin (Fig. 1) indicated ineffective separation between the two elements. Both Nb and Ta were eluted simultaneously from the column with HCl as eluate at concentrations ranging between 0.5 M and 4.0 M in this column. The weak Dowex Marathon anion exchange column (Fig. 2) on the other hand retains these elements to a greater extent than the strong Amberlite anion column. Total recoveries of 91.69% Nb and 73.39% Ta were obtained from the strong Amberlite anion column and 96.05% Nb and 52.34% Ta were obtained from the weak Dowex Marathon anion exchange column. The incomplete Ta recovery from these columns is possibly due to the formation of stronger TaF7 2−-N+H2CH3-R interactions (Eqs. 2 and 3) or the presence of other Ta species in solution, which results in poor Ta elution. Literature studies17,38 have verified that the change of H+ with K+ produces the slightly soluble K-salt (K2TaF7), which was previously used to separate Ta from Nb.
Retention times and the column widths were calculated from the experimental results and the corresponding semi-quantitative parameters such as distribution coefficients (k), separation factor (α) and the number of theoretical plates (N) are reported in Table III using Eq. 8.27
The k value of 4.00 for both metals and the corresponding α = 1 confirmed the ineffective separation of Nb and Ta by these resins. Although the separation of the two metals was not achieved by these resins, the total recovery of the metals indicated that Ta and Nb had different adsorption properties towards these anionic columns. Ta was the more retained element of the two, confirming a slight difference in the chemical behaviour of the products of the two elements. Similar results were obtained for the separation of these elements in tantalite (k = 4.33 and W = 10 for Nb)24 and the inability of the ion exchange to separate Ta from Nb required Ta removal via solvent extraction prior to ion exchange.
Cation and Anion Exchange for Na2HPO4/NaH2PO4·H2O Fused (Nb/Ta)2O5 Mixture
It is important to note that at this stage the type of complexes formed between (Nb/Ta)2O5 and Na2HPO4/NaH2PO4·H2O during flux fusion have not been properly characterized. Only the formation of insoluble oxy-orthophosphate compounds (TaOPO4 and NbOPO4) have been reported as products after the reaction between phosphoric acid and the pentoxides.30,31
The results of this study using cationic exchange resins indicated that Nb and Ta were easily and quantitatively eluted with dilute H3PO4 solutions (Table IV) from both the cationic clinobrite zeolite and the strong Amberlite IR-130 cation resins. These results indicate that the products formed during the (Nb/Ta)2O5/phosphate fusion step are not positively charged and are simply eluted from the column without retention/adsorption. However, results obtained from the use of weak Dowex Marathon anion exchanger indicated strong adsorption of both Ta and Nb phosphate complexes and thereby suggesting the possible existing of negatively charged Ta and Nb species in the phosphate matrix. The elution was investigated with up to a 4.0 M H3PO4 solution without any sign of Ta or Nb been desorbed from the column.
Conclusion
The separation of tantalum and niobium after NH4F·HF flux dissolution was investigated using three different separation methods, namely selective precipitation, solvent extraction and ion exchange. Selective precipitation of Nb and Ta from a (Nb/Ta)2O5/NH4F·HF solution using PPDA as a chelating agent indicated elemental recoveries of 73(3)% Ta and 23(5)% Nb in the precipitate. A separation factor of 11(4) was obtained. Quantitative extraction of tantalum into the organic phase from a (Nb/Ta)2O5/NH4F·HF solution with two extractions with MIBK was achieved at 4.0 M H2SO4. A separation factor of ~2000 was obtained. A single extraction would require a 6.0 M H2SO4 solution for complete Ta extraction.
The use of the two anion resins, Amberlite IRA-900 and Dowex marathon wba, for Ta and Nb separation in a fluoride matrix showed some absorption differences. However, poor separation between the two metals rendered these resins ineffective for this purpose. Selective precipitation and solvent extraction also proved to be highly ineffective after phosphate flux fusion dissolution, confirming the presence of different metal complexes in the solution. The cationic resins, Amberlite IR-130C and Clinobrite 814 zeolite were also unsuccessful in the separation of the two elements. Both Ta and Nb phosphate complexes were strongly retained by a weakly basic Dowex Marathon anion which indicated the possible existence of negatively charged Ta and Nb phosphate compounds in solution.
It is clear that the solvent extraction results obtained in this study (NH4F·HF dissolution of the pure metal oxides) produce Ta and Nb compounds that behave similarly to those obtained for HF dissolution. The results further showed very similar chemistry between the pure oxides (in this study) and the tantalite mineral, signifying the relevance of using the pure oxides to develop or investigate new beneficiation procedures.
References
D. Bayot and M. Devillers, Coord. Chem. Rev. 250, 2610 (2006).
D.R. Sadoway and S.N. Flengas, Metall. Trans. B. 11B, 57 (1980).
Roskill Information, The Economics of Tantalum, 9th ed. (London: Roskill Information Services Ltd., 2005).
W.A. Serjak, Technical Promotion Officer Tantalum-Niobium (International Study Center, 40 Rue Washington, 1050 Brussels, Belgium).
K.M. Mackay, R.A. Mackay, and W. Henderson, Introduction to Modern Inorganic Chemistry, 5th ed. (Cheltenham: Stanley Thornes (Publishers) Ltd., 1996), p. 261.
M.H. Cockbill, Analyst 87, 611 (1962).
W. Kock and P. Paschen, JOM 41, 33 (1989).
G.W. Sears and L. Quill, J. Am. Chem. Soc. 47, 922 (1925).
J.E.S. Uria, C.G. Ortiz, A.M. Garcia, and A. Sanz-Medel, Mikrochim. Acta [Wien] 2, 195 (1987).
M.E. Pennington, J. Am. Chem. Soc. 18, 38 (1896).
X. Wang, S. Zheng, H. Xu, and Y. Zhang, Hydrometallurgy 98, 219 (2009).
P.L. Mahanta, V.V. Hanuman, R. Radhamani, and P.K. Srivastava, At. Spectrosc. 29, 172 (2008).
T.A. Theron, M. Nete, J.A. Venter, W. Purcell, and J.T. Nel, S. Afr. J. Chem. 64, 173 (2011).
M. Nete, W. Purcell, E. Snyders, and J.T. Nel, S. Afr. J. Chem. 63, 130 (2010).
O.N. Grebneva, I.V. Kubrakova, T.F. Kudinova, and N.M. Kuz’min, Spectrochem. Acta Part B. 52, 1151 (1997).
G.E.M. Hall and J.C. Pelchat, J. Anal. At. Spectrom. 5, 339 (1990).
A. Angulyansky, The Chemistry of Tantalum and Niobium Fluoride Compounds (Amsterdam: Elsevier, 2004), p. 263.
W.S. Arlesheim and F.K. Benningen, Process for separating niobium and tantalum from materials containing these metals, U.S. Patent US 2842424 (1958).
G. Choi, J. Lim, N.R. Munirathnam, and I. Kim, Met. Mater. Int. 15, 385 (2009).
A. Agulyansky, L. Agulyansky, F. Viktor, and V.F. Travkin, Chem. Eng. Process. 43, 1231 (2004).
M.J. Kabangu and P.L. Crouse, Hydrometallurgy 129–130, 151 (2012).
M. Nete, Separation and purification of niobium and tantalum from synthetic and natural compounds, PhD Thesis, University of the Free State, Bloemfontein, South Africa, 2013.
E.G. Polyakov and L.P. Polyakova, Metallurgist 47, 33 (2003).
M. Nete, W. Purcell, and J.T. Nel, Hydrometallurgy 149, 31 (2014).
M. Nete, W. Purcell, and J.T. Nel, Evaluation of ammonium bifluoride dissolution on different tantalum and niobium mineral samples, Precious Metals 2013 Conference, (Cape Town, South Africa, 2013), p. 21.
M. Nete, W. Purcell, and J.T. Nel, J. Fluorine Chem. 165, 20 (2014).
D.G. Christian, Analytical Chemistry, 5th ed. (New York: Wiley, 1994), p. 484.
F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 5th ed. (New York: Wiley, 1988), p. 703.
R. Radhamani, P.L. Mahanta, P. Murugesan, and G. Chakrapani, J. Radioanal. Nucl. Chem. 285, 287 (2010).
R.B. Hahn, J. Am. Chem. Soc. 73, 5091 (1951).
M.L.C.P. da Silva, G.L.J.P. da Silva, and D.N. VillelaFilho, Mater. Res. 5, 71 (2002).
C.K. Jörgensen, Absorption Spectra and Chemical Bonding in Complexes (New York: Pergamon Press LTD, 1962), p. 107.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, 2nd ed. (New York: Wiley, 1970), pp. 151–225.
Y.A. Buslaev, E.G. Ii’in, V.D. Kopanev, and O.G. Gavrish, Inorg. Anal. Chem. 6, 1055 (1971).
R. Gross and W. Kaim, Inorg. Chem. 26, 3596 (1987).
J.W. Sibert, Wurster’s crown ligands, U.S. Patent US 6441164 B2 (2002).
MicroMath, Scientist Handbook, MicroMath, Salt Lake, USA; 1986–2004.
V. Langer, L. Smrčok, and M. Boča, Acta Cryst. E62, i91 (2006).
Acknowledgements
The authors would like to thank the Research Fund of the University of the Free State, the National Research Foundation (NRF) of South Africa, the South Africa Nuclear Energy Corporation SOC Limited (Necsa) and the New Metals Development Network (NMDN) of the Advanced Metals Initiative (AMI) of the Department of Science and Technology of South Africa (DST) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nete, M., Purcell, W. & Nel, J.T. Hydrometallurgical Separation of Niobium and Tantalum: A Fundamental Approach. JOM 68, 556–566 (2016). https://doi.org/10.1007/s11837-015-1711-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1711-2









