Abstract
The similarity between Ta and Nb chemistry makes it difficult to find the appropriate reagents and chemical reactions for the separation of the two elements. This study investigated the precipitation behavior of TaF5 and NbF5 with p-phenylenediamine (PPDA). PPDA preferentially precipitated Nb from a 1:1 ratio of NbF5 and TaF5. Niobium recoveries of >80%, and only 4% Ta, were found in the precipitate of the reaction between (Nb/Ta)F5 and PPDA in ethanol. A separation factor of 100(9) indicated the potential for successful separation of Nb and Ta in a fluoride environment. A spectrophotometric study of the formation ratio of the newly formed Nb compound indicated a 1:1 metal:ligand ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The successful separation and isolation of Nb and Ta from their primary sources have always depended on the utilization of the halogen chemistry of the two elements.1,2 The most important and commercial processing routes entail the halogenation of the two elements in mineral ore or scrap during dissolution, using fluoride sources such as concentrated HF or acid combinations such as HF/H2SO4, HF/HCl or HF/NO3. Lately, molten salts such as NH4F.HF, KF and KF.HF have proved to be effective fluoride sources, which produce MF7 2−/MOF5 2− (M = Ta and/or Nb) complexes for separation.3
The de Marignac fractional crystallization process that separated Ta and Nb as potassium heptafluorotantalate and potassium oxypentafluoroniobate had low recoveries or product contamination through the co-precipitation of impurities in the solution.4 The subsequent liquid–liquid extraction utilized solubility differences of TaF7 2−/NbOF5 2− in different organic solutions.5 The importance of the two metals in modern technology products has prompted the development of a number of alternative processing procedures.6–13
Recently, the separation of TaF5/NbF5 using different organic solvents in a solvent extraction system in different acidic media was investigated.14 The results indicated differences in extraction of Ta and Nb in some solvent systems, but no clean or satisfactory separation was obtained.
This study investigated the precipitation of TaF5 and NbF5 with p-phenylenediamine ligand. Both Ta and Nb have the potential to increase their coordination number to 8.15 This ability to expand the coordination number around the metal center offers the potential for reactions between the metal complex and mono- and bidentate ligands to produce new Ta and Nb complexes, which may differ sufficiently to allow separation.
Experimental
Reagents and Equipment
Analytical grade reagents were used and precipitation reactions were carried in ethanol. Precipitates were separated from the solution by centrifugation using a rotor supplied by MSE. Metal concentrations were determined using an inductively coupled plasma spectrometer (Shimadzu ICPS-7510 ICP-OES) at 309.418 nm for Nb and at 240.068 nm for Ta. A Bruker Avance II 600 MHz NMR was used for the fluoride quantification in the precipitate. A Bruker Avance III 400 MHz NMR spectrometer, equipped with a 4-mm CP-MAS SB probe and a 4.0-mm rotor with KEL-F cap and a spinning rate of 14 kHz, was used for the 13C determination. A LECO TruSpec Micro CHNS was used for determination of elemental carbon, hydrogen and nitrogen. Absorbance measurements were performed on a Cary 50 UV–Visible spectrophotometer. The infrared spectra were obtained with a Scimitar Series Digilab spectrometer. pH data were collected using a Eutech CyberScan pH 1500 bench Meter.
Precipitation Procedure
Double-distilled water was used in the preparation of solutions for ICP-OES analyses. A 50% mixture of NbF5 and TaF5 was dissolved in 10 mL ethanol. The metal solution was mixed with the PPDA ethanol solution and left for 12 h to allow for precipitate formation. The precipitate was separated from the supernatant solution and re-dissolved using 97% H2SO4. The precipitate and filtrate solutions were analyzed for Nb and Ta concentrations using ICP-OES. Precipitates were also characterized using infrared spectroscopy (IR), UV–Vis spectrophotometry, elemental CHN microanalysis and NMR spectroscopy.
Analytical Determinations
The blank and metal’s calibration solutions, ranging between 1.0 mg/L and 20.0 mg/L Nb/Ta, were prepared in 10 mL 97% H2SO4. Standard deviations were calculated based on at least three replicate measurements. Average values were reported with standard deviations to indicate uncertainty in the results.
Fluoride concentration in the precipitate was determined using 19F-NMR, as reported by He et al.16 and Deng et al.17 A stock solution of 1500 mg/L fluoride was prepared using NaF in double-distilled water. The standard and sample matrices were matched as closely as possible. Fluoride solutions of 150–1000 mg/L were prepared in 10.0-mL volumetric flasks and contained 1 mL 97% H2SO4, 3 mL D2O and double-distilled water.
Results and Discussion
Reactions of TaF5 and NbF5 with p-Phenylenediamine Reagent
The reactions between the metal fluorides and p-phenylenediamine resulted in precipitate formation as well as an intense blue color, which had an absorption maximum at 660 nm. The metal quantification results indicated Nb enrichment in the precipitate with concentration ratios of 4:1 Nb:Ta. Niobium recovery of 57(2)% versus 14.3(9)% of Ta in the precipitate indicated that Nb formed a compound with a lower solubility with PPDA compared to Ta, which remained in the ethanol filtrate.
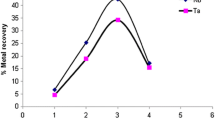
For a precipitation reaction, the ions involved in precipitate formation have to exist in solution in sufficient concentrations to produce a supersaturated solution and have to exceed the solubility product (K sp) value of the resulting compound. The optimum concentration of the ligand for the selective precipitation to occur was determined by varying the ligand concentration from metal:PPDA molar ratios of 1:5 to 1:20. At higher ligand concentrations, precipitation as well as the blue color developed immediately after mixing the reagents. No observable precipitate formed in the 12-h period for the ligand:metal ratio concentration equal or below 1:3 [Nb (0.02 g, 0.053 mmol):PPDA (0.0171 g, 0.16 mmol)], but the quick blue coloration was still observed even at these low ligand:metal ratios. Precipitation was immediately observed at metal:PPDA molar ratios from 1:5 to 1:20. Niobium recoveries up to 87.6% and a maximum Ta recovery of 4.32% were obtained at a metal:PPDA mole ratio of 1:20. The selective precipitation of Nb was possibly due to the difference in the formation constant (K f) with K f(Nb) ≫ K f(Ta) as well as the subsequent dissolution reaction (reverse) with K sp(Nb) ≪ K sp(Ta) (Eq. 1). Thus, PPDA has the potential to afford good TaF5/NbF5 separation in only one precipitation step. The improved Nb recoveries at higher PPDA concentrations could be due to surpassing of the Kf value and the higher PPDA concentrations resulted in larger amounts of NbF5 precipitating.

The increase in ligand concentration to 1:10 metal:PPDA mole ratio produced an optimum Nb recovery and a further increase to 1:15 or 1:20 mol ratio had little or no additional influence (Fig. 1). Repetitions of the experiment under the identified experimental conditions gave highly reproducible results for Nb precipitation with an average recovery of 80(1)% and RSD = 1.64%, but a poor Ta recovery of 3.7(7)% and RSD = 17.91%.
The Ta and Nb of the supernatant were quantified to ensure that there was no significant loss of precipitated product in the preparation. The graphs obtained from the ICP-OES analyses of both the precipitate and the supernatant (Figs. 1 and 2) are mirror images of each other with the total recovery of approximately 100% (within experimental error) indicating little or no loss of matter in the separation process.
Characterization of the NbF5/PPDA Products
The reaction between the metal pentafluorides [(Nb/Ta)F5] and PPDA indicated two different, very fast reactions, namely the rapid blue complex formation as well as the white precipitate formation at relatively high PPDA concentrations. Uncertainty exists whether the precipitate formation is the result of a successive or a parallel process. The two possible processes are illustrated in Fig. 3. In the parallel reactions (Fig. 3), the blue complex formation results from a reaction between NbF5 and PPDA while the precipitate formation results from another, but different, reaction between the two starting materials. Both reactions were, however, too fast to identify which reaction occurred first. In the successive process, the blue complex formation is followed by precipitate formation. The general reaction between the PPDA ligand and NbF5 is proposed in Eqs. 2 and 3 with coordination number retention in Eq. 2 and expansion depicted in Eq. 3.


Complex Composition Determination by Spectrophotometric Analyses
The UV–Vis spectrum of the reaction mixture was obtained after the mixing of PPDA and NbF5 (Fig. 4). The color rapidly changed from a brownish for PPDA solution to an intense blue, suggesting some charge transfer between the metal ion and PPDA. The blue coloration was also observed for Ta, but as expected at much higher [PPDA] with increasing spectrum absorption due the precipitation.
Mole Ratio Determination
The color change of the solutions was utilized to determine the ratio of the ligand to metal in the newly formed metal complex. The solution was analyzed using Job’s continuous variation, mole ratio and slope ratio procedures.18 The results (Table I) indicated that the colored complex formed by Nb (or Ta) and PPDA has a 1:1 metal:ligand mole ratio. The kinetic study of the product formation was unsuccessful due to the fast reaction and the immediate precipitate formation.
Determination of Nb-PPDA Complex Formation Constant and Molar Extinction Coefficient
The spectrophotometric analysis of the NbF5/PPDA solutions was performed to determine the formation constant and molar extinction coefficient of the product. A linear Benesi-Hildebrand plot of [Nb]/Abs versus 1/[PPDA] (Fig. 5) with the slope = 1/εK f and a y-intercept = 1/ε was obtained. The formation constant and molar extinction coefficient, which were calculated from the slope and the y-intercept were K f = 7.74 × 102 M−1 and ε = 1.48 × 105 M−1cm−1, respectively. The magnitude of the formation constant obtained for the blue complex indicated a thermodynamically favorable reaction for the formation of this complex as was predicted with the high recovery of Nb in the separation step. This in turn would produce the complex in large amounts for a rapid subsequent precipitation reaction (Fig. 3) if the complex is an intermediate in the formation of the final precipitation (successive reaction).
Infrared Analysis
The infrared spectra (Fig. 6a–c) of PPDA, NbF5 and product indicated the successful isolation of a new product. The vibrational frequencies of 1615 cm−1, 1514 cm−1, 1258 cm−1 and 818 cm−1 in the product (Fig. 6b) corresponded well with the peaks at 1626 cm−1, 1513 cm−1, 1259 cm−1 and 822 cm−1 that were obtained for the pure PPDA ligand (Fig. 6a), while the vibrational frequencies at 1740 cm−1 and 1366 cm−1 in the product compared well with the NbF5 vibrational frequencies at 1737 cm−1 and 1367 cm−1 (Spectrum c). The appearance of a large number of new vibrational frequencies in the 1000–900 cm−1 region indicates a more complicated product is formed during the reaction between NbF5 and PPDA.
The vibrational frequencies at 3065 cm−1 in the precipitate product and at 3196 cm−1 in the PPDA spectra could be due to the aromatic C–H vibration. The vibrational frequency at 1626 cm−1 in the PPDA spectrum, which is also observed at 1615 cm−1 in the NbF5/PPDA product spectrum, can be attributed to -NH2 bending vibrations. The strong vibrational frequencies at 1514 cm−1 in the product spectrum and 1513 cm−1 in the PPDA spectrum are due to the C=C vibrations.19
ICP-OES and CHN Analyses
Based on the assumption that the precipitate has a similar stoichiometry to that of the blue complex, atoms of the starting materials were determined in the precipitate to confirm the spectrophotometric results. The Nb content of the precipitate, analyzed by ICP-OES, was found to be 26.6(3)%. The non-metals were determined using a CHNS microanalyzer which yielded C, N, H percentages of 32.6(4)%, 12.5(6)% and 4.34(8)% respectively.
19F-NMR Analysis
Quantitative fluoride analyses were performed using NaF as standard and the measurements were carried out at a fluoride peak of −128 ppm whose intensities changed proportionally with the fluoride concentration variation. A good calibration curve with R 2 = 0.9985 with a [F−] working range of 150–1000 mg/L and an average fluoride concentration of F = 18.8(9)% in the precipitate sample were obtained. Although the reproducibility of the fluoride results was good, the accuracy of these results may be doubtful due to the fact that quantitative 19F NMR is a new technique for fluoride analysis, and may require a complete method validation. This falls outside of the scope of this study.
Solid state 13C NMR Analysis
Two different solid state 13C-NMR experiments were performed on the isolated precipitate product. Experiments entailed the determination of all the carbons and the non-quaternary carbons. Since the signal intensities are proportional to the number of nuclei, which cause those signals, the 13C NMR peaks were integrated to obtain an estimate of the number of carbon atoms in the product. The integration of the 13C NMR peaks for the total number of carbons in the compound and the quaternary carbons indicated the presence of 15× C (expected 18× C) and 5× C (expected 6× C), respectively.
Prediction of Chemical Formula of the Nb PPDA Precipitate
The different elemental analyses of the final precipitation indicated the presence of Nb, C, H, N and fluoride ions with a molecular formula of precipitate predicted to be Nb2(C6H4N2H2)3F8 (theoretical %: Nb 28.24%, C 32.83%, H 3.06%, N 12.10% and F 23.10%). A Student’s t test indicated a significant difference between the experimental and theoretical percentages for F at the 95% confidence level. Attempts to quantify the fluoride content with a selective fluoride ion electrode, as an alternative to 19F-NMR technique, were unsuccessful due to a high acid concentration [H+], which negatively interfered with the fluoride measurements. The activity of the fluoride ion in solution decreases with decreasing pH values.20
The interpretation of all the results from the analyses of the white precipitate suggests a 1:1.5 Nb:PPDA ratio (Fig. 7), which is different from the 1:1 ratio that was obtained for the colored complex in solution. The color intensity of the colored complex also remained the same during the progress of the reaction, suggesting that the colored complex is not an intermediate in the final product formation or that the reaction from the colored complex to precipitate formation has a moderate equilibrium constant with some of the intermediate staying in solution. It is generally accepted that the estimation of the number of nuclei (carbons in this case) responsible for the signal intensity is not very accurate. The 13C NMR indicated approximately 17% less carbon content for both the total number of carbons and in the quaternary carbons compared to the predicted structure (Fig. 7).
Literature studies21–23 indicated that the reaction of MF5 (Ta or Nb) with an organic ligand can lead to the formation of adducts with general formulae [MF5(L)] and [MF4(L)2][MF6]. Levason et al.21 shows that a combination of MF5 with PMe3 or PPh3 in toluene, fluorobenzene or chlorobenzene solvents produces brown or purple solutions. Marchetti et al.24 attributes the formation of the colored solutions to the radical cation salt formation where MF5 acts both as an oxidizing agent (as it is converted into MF4) and fluoride acceptor (to afford [M2F11]− counterion). Levason et al.21 also obtained a white or cream precipitate from a 1:1 (molar ratio) reaction of (MF5 and PMe3) and (MF5 and PPh3) in anhydrous diethyl ether. They reported the formation of [MF5(PMe3)] and [MF5(PPh3)] as well as adducts with the formulae [MF4(PMe3)2][MF6] and [PPh3H][MF6] in excess PMe3 and CH2Cl2, respectively.21
Another study25 attributed the formation of the blue-colored solution (complex) to a possible oxidative deprotonation of the amino functional in PPDA. These researchers confirmed the existence of the [Mn](THF)-PPDA and [Mn](THF)-PPDA-[Mn](THF) with [Mn] = (η5-C5R5)(CO)2Mn(THF). The PPDA acts in the first compound as a one electron oxidation ligand to produce 1:1 and 2:1 metal:ligand compound in the second instance with the PPDA acting as a bridging ligand. In the second instance, both the -NH2 groups in the para-positions lose a H+ and an electron. These intensely blue- and red-colored compounds, also known as Wurster’s salts, are normally produced by one e− oxidation reaction, and have been known for more than a century.26 Thus, the PPDA ligand can act as a neutral, mono- as well as bivalent-anion, which complicates the possible type of compounds that may be formed with metal complexes such as NbF5.
According to this type of reaction, the deprotonation of the amine group results in the formation of PPDA− anion. This anion can then react with the NbF5 complex to produce an intensely colored intermediate complex. The reaction between metal fluoride and a negatively charged PPDA ligand can also occur through the displacement of a fluoride ion in the final product in order to maintain complex neutrality (precipitate formation) if the Nb maintains a formal oxidation state of +5. The oxidative deprotonation on both amino functional groups of this ligand would be expected to produce a 2:1 Nb:PPDA complex indicated in Eq. 4.
Alternatively, the precipitate could be a result of a second consecutive reaction in which the PPDA is coordinated to the Nb through a lone pair of electrons on the nitrogen of the amino group after the initial reaction of the PPDA ligand to produce a number of different metal:PPDA compounds as indicated by the following reactions (Eqs. 5 and 6):
or
The composition of the final product agrees, using a combination of the reactions proposed by Gross and Kaim26 as well as the quantification results obtained in this study. The characterization of the two products in this reaction does not give a clear indication on the type of reaction mechanism (Fig. 3) that is followed to produce the final isolated product, while its poor solubility points to some kind of polymeric structure.
Conclusion
This study demonstrated that the PPDA preferentially precipitates Nb from a 50% mixture of NbF5 and TaF5. Niobium recoveries in excess of 80% with only 4% Ta were obtained in the precipitate from the reaction of (Nb/Ta)F5 with PPDA in ethanol. The determination of Nb and Ta content in the supernatant solution by ICP-OES indicated that more than 95% of the initial Ta complex remained in solution. The PPDA reacted in a similar way with TaF5 and NbF5, but the precipitation as well as the selectivity was mainly governed by the initial concentrations of TaF5, NbF5 and PPDA. Accurate determination of the molecular or empirical formula of the isolated product was extremely difficult due to its nature. Consequently, only semi-quantitative analysis of the NbF5 and PPDA product was accomplished. Based on the quantitative results of a precipitate from the reaction between NbF5 and PPDA, a formula Nb2(C6H4N2H3)3F9 or Nb2(C6H4N2H2)3F8 for a 1:1.5 Nb:PPDA ratio was suggested due to the similarity of the elemental percentage in the unknown Nb precipitate compound. In conclusion, the reaction between PPDA and the metal pentafluorides selectively precipitated Nb in preference to Ta, and thereby successfully achieved the initial goal of separating the two metals from the same solution.
References
A. Angulyansky, The Chemistry of Tantalum and Niobium Fluoride Compounds (Amsterdam: Elsevier B.V, 2004).
D.R. Sadoway and S.N. Flengas, Metall. Trans. B 11B, 57 (1980).
M. Nete, W. Purcell, and J.T. Nel, J. Fluor. Chem. 165, 20 (2014).
J.W. Mellor, A Comprehensive Treatise on Inorganic and Theoretical Chemistry, Longmans (London: Green & Co. LTD, 1947).
J.R. Werning and K.B. Higbie, Ind. Eng. Chem. 46, 2491 (1954).
L.P. Varga, W.D. Wakley, L.S. Nicolson, M.L. Madden, and J. Patterson, Anal. Chem. 37, 1003 (1965).
W. Kock and P. Paschen, JOM 41, 33 (1989).
X. Wang, S. Zheng, H. Xu, and Y. Zhang, Hydrometallurgy 98, 219 (2009).
H.H. Htwe and K.T. Lwin, World Acad. Sci. Eng. Technol. 46, 133 (2008).
O.N. Grebneva, I.V. Kubrakova, T.F. Kudinova, and N.M. Kuzmin, Spectrochem. Acta B 52, 1151 (1997).
G.E.M. Hall and J.C. Pelchat, J. Anal. Atom. Spectrom. 5, 339 (1990).
M. Nete, W. Purcell, and J.T. Nel, Hydrometallurgy 149, 31 (2014).
M.J. Kabangu and P.L. Crouse, Hydrometallurgy 129–130, 151 (2012).
M.J. Ungerer, D.J. van der Westhuizen, G. Lachmann, and H.M. Krieg, Hydrometallurgy 144–145, 195 (2014).
F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry: A Comprehensive Text, 2nd ed. (New York: Wiley, 1968).
W. He, F. Du, Y. Wu, Y. Wang, X. Liu, H. Liu, and X. Zhao, J. Fluor. Chem. 127, 809 (2006).
D. Deng, P. Deng, X. Wang, and X. Hou, Spectrosc. Lett. 42, 334 (2009).
D.A. Skoog, J.F. Holler, D.M. West, and S.R. Crouch, Fundamentals of Analytical Chemistry, 8th ed. (Australia: Thomson Brooks/Cole, 2004).
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, 2nd ed. (New York: Wiley, 1970).
V. Sunitha and B. Muralidhara Reddy, Int. J. Curr. Res. Acad. Rev. 2, 159 (2014).
W. Levason, G. Reid, and W. Zhang, J. Fluorine Chem. 172, 62 (2015).
S.L. Benjamin, A. Hyslop, W. Levason, and G. Reid, J. Fluorine Chem. 137, 77 (2012).
W. Levason, M.E. Light, G. Reid, and W. Zhang, Dalton Trans. 43, 9557 (2014).
F. Marchetti, C. Pinzino, S. Zacchini, and G. Pampaloni, Angew. Chem. Int. 49, 5268 (2010).
J.W. Sibert, United States Patent US 6441164 B2 (Aug 27) (2002).
R. Gross and W. Kaim, Inorg. Chem. 26, 3596 (1987).
Acknowledgements
The authors thank the Research Fund of the University of the Free State, National Research Foundation of South Africa, Necsa and New Metals Development Network of the Advanced Metals Initiative of the Department of Science and Technology of South Africa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nete, M., Purcell, W. & Nel, J.T. Separation of Niobium and Tantalum Pentafluoride by Selective Precipitation Using p-Phenylenediamine. JOM 68, 2817–2823 (2016). https://doi.org/10.1007/s11837-016-2003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2003-1