Abstract
Tritrophic interactions play a pivotal role in maintaining a functional agroecosystem. After damaged by phytophagous insects, host plants release a blend of odorants called herbivore-induced plant volatiles (HIPVs) that are attractive to natural enemies including arthropod predators and, in particular, parasitoids. In the last three decades, the identities of HIPVs have been meticulously characterized in a variety of tritrophic systems by gas chromatography–mass spectrometry (GC–MS) analysis. A plethora of HIPV components have been physiologically screened by gas chromatography-electroantennogram detection (GC-EAD) and single sensillum recording (SSR). The effects of induced odorants on behavior of herbivores and parasitoids have been investigated using Y-tube olfactometer assays and wind tunnels in the laboratory and bait trap tests in the field. Given the potential utility of parasitic wasps for pest control, the understanding of olfactory mechanisms of how HIPVs are detected by herbivores and parasitic wasps could facilitate the exploitation of parasitoids as bio-control agents. As the advent of the genome sequencing and transcriptome analysis, a large repertoire of chemosensory protein genes including odorant receptors and odorant binding proteins has been identified in herbivores and parasitic wasps, providing an unprecedented opportunity to debunk the molecular basis of olfaction-based interactions. In this review, we will summarize the recent progresses in characterization of HIPVs, the studies of olfactory mechanisms underlying tritrophic interactions with a focus on parasitoids, Lepidopteran pests, and related host plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Retrospect of researches on herbivore-induced plant volatiles (HIPVs) in a tritrophic context
The theory of tritrophic interactions was envisioned by Price et al. (1980) to describe the mutual communications between three modalities: plants, herbivores, and the associated natural enemies including arthropod predators and parasitoids (Price et al. 1980). Since then, the effects of herbivore-induced plant volatiles (HIPV) have been extensively investigated for the attraction of predators and parasitoids of the inducing herbivores. Pioneering studies using lima bean plants–spider mites–carnivorous mites (Phaseolus lunatus [L.] Fabaceae-Tetranychus urticae Koch, Acari, Tetranychidae-Phytoseiulus persimilis Athiot, Mesostigmata, Phytoseiidae) (Dicke 1986, 1988), tomato–corn earworm–parasitoid wasps (Solanum lycopersicon [L.] Solanaceae-Helicoverpa zea Boddie, Lepidoptera, Noctuidae-Trichogramma pretiosum Riley, Hymenoptera, Trichogrammatidae) (Nordlund et al. 1985, 1987, 1988), and cotton–tobacco budworm–parasitoid wasps (Gossypium hirsutum [L.] Malvaceae-Heliothis virescens Fab, Lepidoptera, Noctuidae-Campoletis sonorensis Cameron, Hymenoptera, Ichneumonidae) (Vinson et al. 1984, 1987) provided substantial chemi-ecological underpinnings for indirect defenses of plants in tritrophic interactions. Two seminal works systematically identified the blend of volatiles emitted by herbivore-infested plants that actively recruit natural enemies of the herbivores, predatory mite P. persimilis (Dicke et al. 1990), and parasitoid Cotesia marginiventris Cresson (Hymenoptera, Braconidae) (Turlings et al. 1990b), providing the add-on chemical evidence for tritrophic interactions. From 1990 onward, numerous tritrophic systems have been studied in depth, unleashing an avalanche of reports about “infochemicals” that actively attract parasitoids to the host-infested plants (Turlings et al. 1995; Vet and Dicke 1992; Turlings and Tumlinson 1992; De Moraes et al. 1998; Kessler and Baldwin 2001; D’Alessandro and Turlings 2006; Hare 2011; Lucas-Barbosa et al. 2011; Clavijo McCormick et al. 2012; Aartsma et al. 2017; Turlings and Erb 2018). Meanwhile, the enhancement of performance of predators and parasitoids by host plants volatiles galvanized pest control researchers as an indirect defense approach of plants (Dicke and Sabelis 1987; Turlings et al. 1990a; Baldwin 1998; Karban et al. 1999; Paré and Tumlinson 1999; Thaler 1999; Loon et al. 2000; Kessler and Baldwin 2001, 2002; Dicke et al. 2003, 2009; Kessler et al. 2004; Arimura et al. 2005; Bruinsma and Dicke 2008; Heil 2008; Dicke and Baldwin 2010; Hare 2011; War et al. 2012; Dicke 2016).
Parallelly, the studies of the molecular pathways triggering plant defense systems to release HIPVs have been carried out (Kessler and Baldwin 2002; Dicke et al. 2009; Arimura et al. 2009; Wu and Baldwin 2009; Holopainen and Gershenzon 2010; Baldwin 2010; Erb et al. 2012; Aljbory and Chen 2018). Volicitin [N-(17-hydroxylinolenoyl)-l-glutamine], a fatty acid–amino acid conjugate, is one of important elicitors of plant resistance identified from the regurgitant of the beet armyworm Spodoptera exigua Hübner (Lepidoptera, Noctuidae) (Alborn et al. 1997; Turlings et al. 2000). Other elicitors encompassing enzymes (lipase, pectinase, β-glucosidase, and glucose oxidase), fatty acid–amino acid conjugates (FACs), peptides, esters, disulfooxy fatty acids, and debris of cell walls have been uncovered (Alborn et al. 1997, 2007; Doss et al. 2000; Kessler and Baldwin 2002; Schmelz et al. 2006; Erb et al. 2012; Aljbory and Chen 2018). The activation of nitric oxide and phytohormones, such as salicylic acids (Klessig et al. 2000; Bari and Jones 2009), jasmonic acid (Thaler et al. 2002; Schmelz et al. 2003a; Lou and Baldwin 2003; Lou et al. 2005; Bari and Jones 2009; Xin et al. 2012), and ethylene (O’Donnell et al. 1996; Kahl et al. 2000; Bari and Jones 2009; Lu et al. 2014), by the aforementioned elicitors results in the synthesis and emission of HIPVs. From late 1990s, field tests also provided ample evidence for the existence of tritrophic interactions, and the application of elicitors to enhance plant resistance in agriculture has been envisioned and discussed (Scutareanu et al. 1997; De Moraes et al. 1998; Thaler 1999; Kessler and Baldwin 2001; Poelman et al. 2009).
Moreover, the association between HIPVs and rewards, such as oviposition success, provides an unprecedented opportunity for studying associative learning using parasitoids as model organisms (Vet and Groenewold 1990; Vet and Dicke 1992; Turlings et al. 1993; Vet et al. 1995). Unambiguously, olfaction plays an essential role in fulfilling tritrophic interactions. However, the molecular dissection of neuronal substrates in the olfactory detection of HIPVs in herbivores and parasitoids are much lagged behind. In this review, we will summarize the progresses in investigations on the ethological significance and the olfactory detection of HIPVs with a focus on herbivorous insects and parasitoids.
The chemistry of HIPVs
The identities of HIPVs have been characterized for many host plant species, especially some crops, such as maize, cotton, and tobacco, by using gas chromatography-mass spectrometry (GC–MS) analysis (Table 1) (Turlings et al. 1990b, 2005; Loughrin et al. 1994, 1995; McCall et al. 1994; Röse et al. 1996; Yan et al. 2005; Yan and Wang 2006a). Different plant species and varieties release distinctive HIPV profiles after infestation by herbivores (Buttery et al. 1988; Chang et al. 1988; Loughrin et al. 1990; Turlings et al. 1990b, 1993, 1995; McCall et al. 1994; Yan et al. 2005; Röse and Tumlinson 2004; Addesso et al. 2010; Silva et al. 2017). HIPVs are typically composed by green leaf volatiles (GLVs), terpenoid, aliphatic and aromatic compounds. GLVs are a series of volatile components produced by green plants as a result of oxidative degradation of leaf lipids, encompassing a variety of 6-carbon alcohols, aldehydes, and esters (Dudareva 2004; Hassan et al. 2015). GLVs are not strictly HIPVs, since they are also constitutively released from healthy and mechanically damaged plants and the release is typically not an induced response (Loughrin et al. 1994; Holopainen 2004). Three kinds of GLVs, (Z)-3-hexenol, (Z)-3-hexenyl acetate, and (E)-2-hexenal, are shared in this group of HIPVs emitted by maize, cotton, and tobacco. Terpenoids are usually considered to be a major group of chemicals released from damaged plants. Tobacco infested by caterpillars emitted a different spectrum of terpenoids compared with the ones released from infested maize and cotton (McCall et al. 1994; Loughrin et al. 1995; Yan et al. 2005; Yan and Wang 2006a, b) (Table 1). The tremendous diversity and variability of terpenoids in the emissions released by different plant species and even different cultivars/varieties of the same plant species may serve as a hallmark for parasitoids to efficiently locate the appropriate host-infested plants (Turlings and Ton 2006; Mumm et al. 2008). For instance, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) is released from damaged plants of maize, cotton, lima bean, and pepper plants in large quantity (Turlings et al. 1993; Loughrin et al. 1995; Arimura et al. 2000; Yan and Wang 2006a; Addesso et al. 2010; Tamiru et al. 2015). Around one quarter of total amount of HIPVs released by maize Zea mays (L.) ‘Zhongdan-306’ (Poaceae) after infestation by the larvae of Helicoverpa armigera Hübner or Mythimna separata Walker (Lepidoptera, Noctuidae) is DMNT (Yan and Wang 2006a). However, DMNT is not found in the emissions of caterpillar-infested tobacco Nicotiana tabacum (L.) ‘K326’ (Solanaceae) (De Moraes et al. 2001). Moreover, β-pinene and linalool are the shared two terpenoids in HIPVs of different cultivars of maize, cotton, and tobacco damaged by noctuid caterpillars. However, they appear to have different valences to cause the behavioral changes as linalool is attractive to the generalist parasitoid Campoletis chlorideae Uchida (Hymenoptera, Ichneumonidae), but β-pinene is not (Loughrin et al. 1994; Yan et al. 2005; Yan and Wang 2006b). Interestingly, β-pinene is effective in orienting the specialist parasitoid, Microplitis croceipes Cresson (Hymenoptera, Braconidae), to infested cotton, G. hirsutum ‘max 9’ (Morawo and Fadamiro 2016). This suggests that sympatrically occurring parasitoid species indeed differentially read the same HIPV component to find their respective host larvae-infested plants.
The volatile blend of the same plant species, when infested by closely related herbivory species, usually shows a high degree of similarity in constituted components, but the proportions of the shared components have differences (Turlings et al.1993; Ngumbi et al. 2009). For instance, the composition of HIPVs is not qualitatively different between H. virescens-infested and S. exigua-infested cotton plants, but significant differences in the ratios of HIPV components have been discovered (Ngumbi et al. 2009). However, (Z)-jasmone, (E)-β-farnesene, and (E,E)-α-farnesene are released by cotton plants damaged by S. exigua (Loughrin et al. 1995) but not emitted by cotton plants infested by H. zea (McCall et al. 1994). Such differences may derive from the different cultivars of G. hirsutum ‘McNair 235’ and ‘Delta Pineland 90’ used in the two aforementioned studies. The HIPVs emitted from different cultivars or varieties of the same plant species could be quite distinctive in quality and quantity (Loughrin et al. 1995; Degen et al. 2004, 2012; Hare 2007; Schuman et al. 2009; Tamiru et al. 2011; Bruce 2014). For instance, the total emissions of HIPVs profoundly vary among 31 maize (Z. mays) inbred lines, with the 20-fold difference between the two extreme lines (Degen et al. 2004). On the other hand, the HIPV signatures are also exceedingly diversified. The emission of α-farnesene is detected in maize lines F2 and F7, but is completely absent in the HIPVs of other lines (Degen et al. 2004). Another great variety occurs in the ratios of HIPV components. Although (E)-β-caryophyllene is widely released by all maize lines, the ratios of this compound to the total emission vary by more than 40-fold between the two extremities (Degen et al. 2004). Similar phenomena are found in the different inbred lines of cotton (Loughrin et al. 1995) and rice (Lou et al. 2006). Terpinene is only detected in the HIPVs of cotton line TX2259 that has a propensity to release higher amount of HIPVs, including (Z)-3-hexenyl acetate, (E)-β-caryophyllene, (E)-β-ocimene, and myrcene (Loughrin et al. 1995). In addition, the proportions of (E)-β-caryophyllene range from 17 to 59% from different insect-damaged tobacco cultivars (Hare 2007). This enormous intraspecific variability of HIPV emissions provides great opportunities for plant breeders to cultivate new crop varieties with traits of attracting parasitoids to control herbivores.
The emission of HIPV components follows a diurnal rhythm (Loughrin et al. 1994; Turlings et al. 1995). The release of induced terpenes, such as (E)-β-farnesene, (E)-β-ocimene, and DMNT from S. exigua larvae-infested cotton plants, peaks during the late afternoon and wanes during the late night and morning (Loughrin et al. 1994). GLVs, such as (Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexenol do not follow this diurnal pattern and are released instantaneously after larval infestation (Loughrin et al. 1994; Turlings et al. 1995). Some terpenoids, like α-pinene and (E)-β-caryophyllene, are also released right after the larval infestation on cotton plants, but the larvae-infested corn does not release any kind of terpenoid instantly (Loughrin et al. 1994; Turlings et al. 1995). Those on-site released compounds could be synthesized beforehand and stored in resin ducts, glandular trichomes, and vacuoles, and released transiently from bursting storage structures (Turlings and Tumlinson 1992; Paré and Tumlinson 1997; Becker et al. 2015). The emissions of terpenoids, like DMNT, (E)-β-farnesene, (E)-β-ocimene, and (E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT) from larvae-infested cotton plants, are not detected within several hours after larval infestation, presumably because those terpenoids needs to be de novo synthesized in response to larval infestation, and then the emission starts to increase and persist to several days independent of larval infestation (Loughrin et al. 1994; Turlings et al. 1995). These emission patterns ensure the maximal protection for plants by extending the lifetime of HIPV presence.
The effects of HIPVs on the physiology of herbivores and parasitoids
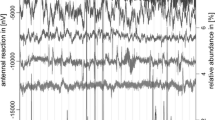
A wealth of bioactive HIPV components have been physiologically studied by EAD (electroantennogram detection), GC-EAD (gas chromatography-electroantennogram detection), and SSR (single sensillum recording). On the second tropical level, the antennal responses of Lepidoptera pests to HIPVs have been studied in depth. Female antennae of the Egyptian cotton leafworm moth, Spodoptera littoralis Boisduval (Lepidoptera, Noctuidae), show strong GC-EAD responses to HIPV components of cotton plants G. hirsutum ‘Delta Pineland 90’, β-myrcene, β-ocimene, (Z)-3-hexenyl acetate, linalool, and (Z)-jasmone, and to a less extent, α-pinene, β-pinene, (E)-hexenal, (Z)-3-hexenol, TMTT, and indole (Jönsson and Anderson 2002). In addition, following SSR recordings confirm the results of GC-EAD results (Jönsson and Anderson 2002). Later on, a similar work largely recapitulates those results (Zakir et al. 2013). Mated females of Helicoverpa assulta Guenée (Lepidoptera, Noctuidae) show strong GC-EAD responses to the components of headspace volatiles of tobacco flower N. tabacum ‘NC89,’ (E)-β-ocimene, octanal, (Z)-3-hexenyl acetate, (Z)-3-hexe-1-ol, nonanal, (Z)-3-hexenyl-2-methyl butyrate, linalool, and (E)-β-caryophyllene (Sun et al. 2012). The sensilla responsive to those chemicals have also been characterized by SSR (Sun et al. 2012). On the third trophic level, GC-EAD technique have been applied to compare the antennal responses of the specialist parasitoid, M. croceipes, and the generalist parasitoid, C. marginiventris, to cotton volatiles, G. hirsutum ‘max 9’, induced by the infestation of H. virescens and S. exigua (Ngumbi et al. 2009). The generalist, C. marginiventris bends the GC-EAD responses toward GLV components, e.g., (Z)-3-hexenal, (E)-2-hexenal, and (Z)-3-hexenal, while the specialist M. croceipes is predisposed to be more sensitive to terpenoid components, like linalool, DMNT, indole, (Z)-jasmone, α-farnesene, α-humulene (Ngumbi et al. 2009). These differences in the responsive pattern may foreshadow the distinct foraging strategies adopted by specialist and generalist parasitoids. In Microplitis mediator Haliday (Hymenoptera, Braconidae), a detailed GC-EAD analysis reveals that three principle HIPV components, namely DMNT, (Z)-3-hexenyl acetate and nonanal, emitted by H. armigera infested cotton plants, trigger the responses in antennae (Yu et al. 2010). The follow-up Y-tube olfactometer assays confirm the attractiveness of these three compounds to M. mediator (Yu et al. 2010). Finally, field cage studies indicate that the application of DMNT to the cotton plants dramatically augment the parasitism of H. armigera larvae by M. mediator (Yu et al. 2010). Although the local field potential recorded by EAD and SSR does not necessarily end up with behavioral changes (Wei and Kang 2006), it nevertheless provides valuable information for sifting out chemicals for behavioral tests.
The effects of HIPVs on the behavior of herbivores
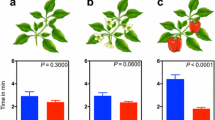
The effects of HIPVs to herbivores’ behavior seem to be bimodal. HIPVs either act as herbivore repellents (Landolt 1993; De Moraes et al. 2001; Signoretti et al. 2012; Reisenman et al. 2013) and/or work as attractants to herbivores (Anderson and Alborn 1999; Rojas 1999; Shiojiri and Takabayashi 2003; Sun et al. 2014). The recent progresses of HIPVs’ effects on the behavior of major herbivorous pests are summarized in Table 2. A large array of HIPV components have been ethologically characterized by, e.g., using oviposition bioassays, wind tunnels, Y-tube olfactometer assays, field bait traps. Linalool emitted from Nicotiana attenuata Torr. ex S. Watson (Solanaceae) upon infested by caterpillars of Manduca quinquemaculata Haworth (Lepidoptera, Sphingidae) significantly reduces the oviposition efficiency (Kessler and Baldwin 2001). Similarly, linalool emitted by damaged tea plants are repellent to Ectropis obliqua Prout (Lepidoptera, Geometridae) (Sun et al. 2014). Homoterpene DMNT significantly impairs the chemotaxis behavior of S. littoralis in wind tunnels (Hatano et al. 2015). Moreover, indole strongly repels both larvae and adults of S. littoralis (Veyrat et al. 2016). Conversion of (Z)-3-hexenyl acetate to (E)-2-hexenyl acetate trigged by the feeding of tobacco hornworm M. sexta on sacred Datura wrightii Regel (Solanaceae) plants changes the ratio of these compounds, which act as an alarm signal for the oviposition decision of Manduca moths (Allmann et al. 2013). Moreover, farnesene isomers released from damaged maize and cotton plants inhibits the oviposition of mated female H. assulta (Wu et al. 2018).
On the other hand, nerolidol and geranyl acetate have been shown to be attractive to H. assulta by using Y-tube olfactometer tests (Cui et al. 2018). Benzyl alcohol, (Z)-3-hexenyl hexanoate, and (Z)-3-hexenal emitted by infested tea plants (Camellia sinensis [L.] Kuntze, Theaceae) are attractive to E. obliqua (Sun et al. 2014). (Z)-jasmone, one of HIPV components released from herbivore damaged cotton and tobacco, is attractive to the caterpillar of H. armigera (Sun et al. 2018). Larvae of H. armigera are variably attracted by (Z)-3-hexenol, (Z)-3-hexenyl acetate, (Z)-3-hexenyl butyrate, caryophyllene, linalool, limonene, myrcene, ocimene, and (Z)-jasmone when tested separately by two-choice behavior assays in 9-cm disposable Petri dishes (Di et al. 2017). A blend of two HIPV components released from apple seedlings (Malus sp. [L.] Rosaceae), benzyl nitrile and acetic acid is attractive to the light brown apple moth, Epiphyas postvittana Walker, and the eye-spotted bud moth, Spilonota ocellana Denis & Schiffermüller (Lepidoptera, Tortricidae) (El-Sayed et al. 2016). Both (Z)-3-hexenyl acetate and TMTT are strong attractants to Aphis gossypii Glover (Hemiptera, Aphididae), while DMNT is not effective in eliciting of any behavioral modifications in the aphid (Hegde et al. 2011). The bimodal effects of some constitutes of HIPVs on the behavior of different herbivores, such as linalool that attracts larvae of H. armigera but reduces the oviposition rate of M. sexta, may reflect different survival strategies adopted by different insects. Swarming feeding could maximally exploit the nutrients of host plants, but also could dramatically increase the conspecific competitiveness (Prokopy and Roitberg 2001). Insects have to make a trade-off and adopt gregarious living or solitary living depending on the natural stress from their respective ecology niches. In this case, same chemical information that is used by gregarious insects as an aggregation signal could be employed by solitary insects as a dispersal signal. Moreover, different chemical dosages of HIPVs were used in different studies and different behavioral observations, like foraging, oviposition, and feeding preference, were investigated, which could lead to diverging conclusions and make the comparisons tenuous.
The effects of HIPVs on the behavior of parasitoids
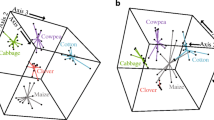
More than 30 parasitoid species have been shown to be attracted by HIPVs, including several representatives of Braconidae (Hymenoptera): Cotesia glomerate L. (Geervliet et al. 1996), Cotesia rubecula Marshall (Geervliet et al. 1996), Cotesia kariyai Watanabe (Ozawa et al. 2004), Cotesia marginiventris Cresson (D’Alessandro et al. 2009), Cotesia plutellae Kurdjumov (Shiojiri et al. 2000); Cotesia sesamiae Cameron (Tamiru et al. 2015), Diaeretiella rapae McIntosh (Cascone et al. 2018), Microplitis croceipes Cresson (Turlings et al. 1990b), Microplitis rufiventris Kokujev (Ngumbi et al. 2009), and Microplitis mediator Haliday (Yu et al. 2010); and Ichneumonidae (Hymenoptera): Campoletis sonorensis Cameron (Elzen et al. 1984), Campoletis chlorideae Uchida (Yan and Wang 2006a), Diadegma semiclausum Hellén (Houshyani et al. 2013), and Diadegma fenestrale Holmgren (Gols et al. 2012). Although host larvae-infested plants are largely attractive to parasitoids, the composition of HIPVs needed to elicit strong chemotaxis behavior of parasitoids seems to be variable. A blend of four volatiles [n-heptanal, α-pinene, sabinene, and (Z)-3-hexenyl acetate] released by Plutella xylostella (L.) (Lepidoptera, Plutellidae)-infested cabbage plants Brassica oleracea (L.) (var. capitata, cv. Shikidori) is necessary to elicit a strong chemotaxis behavior of the parasitoid, Cotesia vestalis (Haliday) (Hymenoptera, Braconidae), while none of those compounds alone shows attractiveness (Shiojiri et al. 2010). Moreover, attraction of parasitoids C. sonorensis and M. rufiventris is correlated with nonanal, α-pinene, (E)-β-ocimene, DMNT, (S)-linalool, and (E)-β-farnesene (Sobhy et al. 2018). On the other hand, single HIPV, linalool, acts as a potent attractant to a parasitoid Anagrus nilaparvatae Pang & Wang (Xiao et al. 2012) and C. sesamiae (Tamiru et al. 2015). (Z)-3-hexenol alone could effectively lure wasps Opius dissitus Muesebeck (Hymenoptera, Braconidae) towards lima bean plants damaged by the leafminer Liriomyza huidobrensis Blanchard (Diptera, Agromyzidae) (Wei et al. 2007). (Z)-jasmone acts as a strong attractant to C. chlorideae tested in both a Y-tubes olfactometer test and in a cage (Sun et al. 2018). Application of (Z)-jasmone to tobacco plants dramatically increases the parasitism rate of H. armigera by C. chlorideae (Sun et al. 2018). Several lines, including GC/MS quantification and forging behavior assays, suggest that DMNT is a potent attractant for certain species of parasitoids whose host larvae feed on cotton, maize, pepper, and cowpea. First, DMNT is an abundantly and promiscuously released HIPV component from various damaged plants, e.g., cotton (G. hirsutum) seedlings (McCall et al. 1994; Röse et al. 1996), maize (Z. mays) (Turlings and Tumlinson 1992; Yan and Wang 2006a), pepper (Capsicum annuum [L.] Solanaceae) (Addesso et al. 2010), and cowpea (Vigna unguiculata [L.] Walp, Fabaceae) (Turlings et al. 1993). Second, DMNT has been experimentally shown to be a strong attractant to several parasitoid species (Turlings et al. 1995; Kappers et al. 2005; D’Alessandro et al. 2009; Yu et al. 2010; Tamiru et al. 2011, 2015). DMNT alone shows the same attractiveness for M. mediator as does the blend of HIPVs (Yu et al. 2010). Most of conducted behavioral assays only compare the attraction of single HIPV component versus solvent, which efficiently identify the behavior-relevant components, but could inadvertently ignore the synergism between compounds (Hu et al. 2018). Additionally, the importance of identified compounds should be scrupulously reconsidered, because the attractiveness of blends has not been tested in parallel.
The components of HIPVs are not always attractive to parasitoids (Yan and Wang 2006a; Snoeren et al. 2010). For instance, parasitoid C. chlorideae has shown to be indifferent to (E)-2-hexenal and (Z)-3-hexenol even though they are one of major constitutes of HIPVs from M. separata-infested maize (Yan and Wang 2006a). Moreover, methyl salicylate has been reported to negatively affect the attraction of parasitoid D. semiclausum (Snoeren et al. 2010). This is seemingly counterintuitive since methyl salicylate is found in emissions of many pest-infested plants, such as tobacco (Yan et al. 2005), tomato (Ament 2004), and Lima bean (Dicke et al. 1990). However, the aversive components in HIPVs could be important as some parasitoids can take advantage of them to avoid non-host pests.
Genetically tractable plant, Arabidopsis thaliana (L.) Heynh. (Brassicaceae) not only has prodigiously contributed to the unraveling of signal transduction in indirect defense (Poecke et al. 2001; Poecke and Dicke 2004) but also has been demonstrated to be an ideal tool to study the modulation of behavior in parasitoids by HIPV components (Schnee et al. 2006; Houshyani et al. 2013; Zhang et al. 2013b). A maize terpene synthase gene, tps10, is responsible for the formation of several terpenoids, including (E)-α-bergamotene and (E)-β-farnesene, the major components of HIPVs of S. littoralis larvae-infested maize plants (Schnee et al. 2006). Overexpression of tps10 in A. thaliana results in an emission that is a mimicry of the HIPV blend of infested maize (Schnee et al. 2006). Unambiguously, the parasitoid C. marginiventris learns to exploit the tps10-expressing A. thaliana over the control, elegantly demonstrating the importance of terpenoids, in particular, (E)-α-bergamotene and (E)-β-farnesene, in the recruitment of the parasitoid C. marginiventris (Schnee et al. 2006). Moreover, A. thaliana that is genetically tailored to release higher amount of nerolidol outwits the control plants in terms of attracting the parasitoid D. semiclausum (Houshyani et al. 2013). Those examples clearly showcase the feasibility of genetic manipulation in studying correlations between HIPVs and the behavior of parasitoids.
Learning of parasitoids
Besides an innate preference of HIPVs, generalist parasitoids can leverage associative learning to distinguish most suitable host plant species from others after they have learned the association between host pests and plants (Turlings et al. 1990a; Geervliet et al. 1998; Steidle 1998; Peñaflor et al. 2011). Other than that, many specialist parasitoids also have a keen ability to learn to respond to HIPVs (Kaiser and Cardé 1992; Fukushima et al. 2001; Takasu and Lewis 2003; Peñaflor et al. 2011). For instance, the landing frequency of experienced females of the specialist parasitoid C. kariyai on the aphid-infested corn (Z. mays) is 60%, which is significantly higher than that of naïve females (12%), indicating initial host searching experiences greatly enhance the subsequent flight orientation to the herbivore-infested plants (Fukushima et al. 2001). Several lines of evidence indicate that the specialist parasitoids and the generalist parasitoid display different associative learning abilities (Vet and Dicke 1992; Simons et al. 1992; Geervliet et al. 1998; Bleeker et al. 2006; Ngumbi et al. 2012). The two closely related parasitoids, the generalist Cotesia glomerata (L.) (Hymenoptera, Braconidae) and the specialist C. rubecula, find their hosts by responding to HIPVs, but differ profoundly in olfactory learning: C. glomerata instantly changes its congenital preference for white cabbage Brassica oleracea (L.) convar. Capitate var. alba (Brassicaceae) towards brussels sprouts Brassica oleracea (L.) var. gemmifera (Brassicaceae) after a single oviposition experience, while the preference of C. rubecula for the cabbage remain unchanged even after 5 rounds of oviposition training (Geervliet et al. 1998). Moreover, one associative learning experience instills a strong and long-lasting memory in the generalist parasitoid, C. glomerata, whereas one oviposition experience only induces a short-lived memory trace in the specialist parasitoid, C. rubecula (Bleeker et al. 2006). Furthermore, a comparative study of learning abilities between the generalist C. marginiventris and the specialist M. croceipes to four behavior-related HIPV components, (E)-2-hexanal, α-pinene, (Z)-3-hexenyl butyrate, and (E,E)-α-farnesene, reveals that trained generalist parasitoid quickly associates all four odorants to sugar water, whereas the trained specialist only establishes the association of α-pinene and (E,E)-α-farnesene with the reward (Ngumbi et al. 2012). However, some caveats should be considered regarding the differential learning abilities of generalist and specialist parasitoids. Two congeneric parasitoid species, the specialist D. semiclausum and the generalist D. fenestrale, show similar behavior responses to HIPVs regardless of experience treatments (Gols et al. 2012). Taken together, both generalist and specialist parasitoids can locate their hosts with competitive efficiency and accuracy through associative learning irrespective of myriads of odorants released from non-host larvae-infested plants (Vet and Groenewold 1990; Turlings et al. 1993; Giunti et al. 2015). However, the associative learning seems to confer greater adaptive value to the generalist parasitoids than the specialist parasitoids (Vet and Groenewold 1990; Vet and Dicke 1992; Ngumbi et al. 2009).
The molecular basis of olfactory detection of HIPVs
Compared with the numerous reports on the identification and behavioral studies of HIPVs, the mechanisms underlying olfactory detection of HIPVs remain largely unexplored. However, the holistic view of insect olfactory signal transduction pathways has been obtained in fruit flies and mosquitos (Diptera) (Vosshall and Stocker 2007; Masse et al. 2009, Su et al. 2009; Ray 2015; Joseph and Carlson 2015). The detection of odorants in insects is orchestrated by a series of chemosensory proteins, including odorant binding proteins (OBPs), olfactory receptors (ORs), and odorant degrading enzymes (ODEs) (Vogt 2003; Leal 2013) (Fig. 1). OBPs bind to odorants, ferry the odorants across antennal lymph, and release them in the vicinity of ORs that are embedded in the membrane of olfactory receptor neurons (ORNs) (Su et al. 2009; Leal 2013) (Fig. 1). Insects OBPs are a class of small soluble proteins with a length of about 150 amino acids, and are mainly structured in 6 α-helix that are folded to form a hydrophobic binding pocket (Pelosi et al. 2006). Insect ORs are seven transmembrane proteins with an intracellular N terminus and extracellular C terminus, bearing no sequence similarities with their vertebrate counterparts (Clyne et al. 1999; Vosshall et al. 1999; Gao and Chess 1999; Benton et al. 2006). Insect ORs previously considered as a heterodimer (Sato et al. 2008; Wicher et al. 2008), but recent structure analysis favors a heterotetramer consisting of two subunits of tuning OR and two subunits of odorant receptor co-receptors (ORco) (Butterwick et al. 2018) (Fig. 1). Other than ORs, inotropic receptors (IRs) that are located to coeloconic ORNs are a special detector for amines and acids (Benton et al. 2009). Sensory neuron membrane protein 1 (SNMP1), a CD36-like protein (Rogers et al. 1997; Benton et al. 2007; Jin et al. 2008), along with pheromone binding proteins (PBPs) (Vogt and Riddiford 1981; Guo et al. 2012a) and pheromone receptors (PRs) (Nakagawa et al. 2005; Jiang et al. 2014; Yang et al. 2017), is located to the trichoid sensilla and essential for pheromone detection. A large array of basiconic sensilla are usually responsive to general odorants, including HIPVs. The concentrations of OBPs in the antennal lymph are staggeringly high (~ 10 mM) (Vogt et al. 1989). The numbers of OBP genes in a given species are lower that the numbers of ORs revealed by the numerous antennal transcriptome analysis (Zhang et al. 2015; Du et al. 2018). Those characteristics of OBPs imply the importance of OBPs for odorant detection. However, the competitive fluorescence binding assay reveals that OBPs equip some degree of odorant selectivity, but definitely cannot account for the specificity of ORNs. Instead, many lines of evidence support that ORs are major determinants of response patterns of ORNs (Hallem et al. 2004; Hallem and Carlson 2006). The chemical information generated by the ORs is relayed to the glomeruli located in antennal lobes where the olfactory information is preliminarily sorted and integrated (Galizia and Rössler 2010). The sorted information is further processed in mushroom bodies for associative learning and is decoded in the lateral horn for innate behavior (Su et al. 2009). Insects usually employ two coding paradigms to detect ecologically relevant odorants either by recruiting multiple ORN types (combinatorial coding) or by activating a single narrowly tuned ORN (labeled line) (Kaupp 2010). Highly relevant negatively acting odorants are tended to be sensed by a dedicated repulsion-inducing ORN type (Stensmyr et al. 2012).
Peripheral detection of odorants on the insect antennae. a Antennae are the major olfactory organ of insects and are decorated by the hair-like structure termed sensillum (Hansson and Stensmyr 2011). b The olfactory sensillum is multiporous and morphologically diverse. The dendrites of olfactory receptor neurons (ORNs) are bathed in the aqueous sensillum lymph. Odorant binding proteins (OBPs) are synthesized and secreted by support cells (Vogt 2003; Pelosi et al. 2006; Leal 2013). c Once upon diffusion into the sensillum through the pores, odorants are captured by OBPs and transferred to the vicinity of odorant receptors (ORs). The transient interaction between ligand and OR activates the channel which is formed by a predicted heterotetramer consisting of two subunits of tuning OR and two subunits of odorant receptor co-receptors (ORco) (Sato et al. 2008; Wicher et al. 2008; Butterwick et al. 2018)
Olfactory detection of HIVPs by herbivorous insects
The selectivity of OBPs has been widely tested by using competitive fluorescence binding assays. In Chilo suppressalis Walker (Lepidoptera, Crambidae), OBP8 displays high binding affinities to nerolidol, but also show somewhat equal bindings to non-HIPV compounds, such as β-Ionone, farnesol, and 2-hexanone (Yang et al. 2016). OBP1 of S. exigua shows higher binding capacities to (E)-β-caryophyllene over other tested odorants, such as farnesol and acetophenone (Liu et al. 2017). OBP6 of E. obliqua indiscriminately binds to a group of terpenoids, such as α-caryophyllene, α-terpinene, nerolidol, α-farnesene (Ma et al. 2018). Other reports about high selectivity of OBPs derive from the studies of OBP3 of two aphid species (Qiao et al. 2009; Vandermoten et al. 2011). OBP3 from the pea aphid Acyrthosiphon pisum (Harris) (Hemiptera, Aphididae) and the English grain aphid Sitobion avenae (Fabricius) (Homoptera, Aphididae) are highly conserved and both of OBPs show a specific binding affinity to (E)-β-farnesene, the alarm pheromone of aphid (Qiao et al. 2009; Vandermoten et al. 2011). Largely, OBPs bind to multiple odorants without appreciative discrimination. This fact complicates the conclusion on the roles of OBPs in detection of HIPVs.
Since ORs are the major determinants of response patterns of ORNs, functional characterizations of ORs are of paramount importance towards understanding the olfactory coding of odorants (Hallem et al. 2004). The recent deorphanized ORs are listed in Table 2. Using heterologous expression in Xenopus oocytes coupled with two-electrode voltage-clamp, many ORs tuning to salient HIPVs are deorphanized in herbivores. SexiOR3 in S. exigua has been reported to be narrowly tuned to E-β-farnesene (Liu et al. 2014). The counterpart of SexiOR3 in H. assulta, HassOR23 is also tuned to (E)-β-farnesene (Wu et al. 2018). OR12 conserved in H. armigera, H. assulta, and H. virescens shows strong responses to (−)-linalool, linalool, and (Z)-2-hexenyl acetate (Cao et al. 2016), whereas the OR12 from S. exigua is exclusively tuned to (Z)-3-hexenyl acetate (Zhang et al. 2013a). In H. assulta, OR40 has been shown to be a detector for nerolidol and geranyl acetate (Cui et al. 2018). In H. armigera, OR42 is tuned to both phenylacetaldehyde and (Z)-3-hexanyl acetate (Di et al. 2017). Although HarmOR60 shows detectable responses to many volatiles, the best ligand to it is (Z)-3-hexenol (Di et al. 2017). HarmOR41 is strongly activated by (Z)-jasmone, to a less extent, by benzaldehyde (Di et al. 2017). In Bombyx mori (L.) (Lepidoptera, Bombycidae), the detection of (Z)-jasmone is specially attributed to OR56 (Tanaka et al. 2009). However, it is not known whether (Z)-jasmone is constitutively or inductively released in mulberry leaves (Morus sp. [L.] Moraceae), the exclusive food of B. mori.
Fruit flies Drosophila sp. Fallén (Diptera, Drosophilidae) empty neuron in which the endogenous OR22a is deleted has emerged as a faithful system to deorphanize ORs from other insect species (Hallem and Carlson 2006; Fouchier et al. 2017). A total of 24 ORs from the herbivorous pest S. littoralis were expressed in the ab3A neurons and the odorant detection spectrum against a panel of 51 volatiles was investigated (Fouchier et al. 2017). SlitOR4 was narrowly tuned to (±)-linalool, SlitOR17 to methyl salicylate, SlitOR27 to indole, and SlitOR28 to (Z)-3-hexenyl acetate. SlitOR24 and SlitOR36 were equally activated by three GLVs, (Z)-3-hexenol, (E)-2-hexenol, 1-hexanol. SlitOR29 was strongly activated by both (E)-β-ocimene and β-myrcene and, to a less extent, by DMNT and (Z)-3-hexenyl acetate (Fouchier et al. 2017). Although the best ligand to SlitOR3 was DMNT, the response was moderate (~ 90 spikes*s − 1) compared with the strong responses of other ORs to their best ligands (~ 200 spikes*s − 1) (Fouchier et al. 2017). DMNT is a potent herbivore deterrent for S. littoralis (Hatano et al. 2015). The activation of glomeruli by DMNT in antennal lobe is not detected by using optical calcium imaging, which leads to a conclusion that DMNT exerts its deterrent effects by attenuating the responses to (Z)-9-(11)-tetradecenyl acetate (the main sex pheromone component) and to (Z)-3-hexenyl acetate (a major plant volatile), but not by activation of dedicated ORNs (Hatano et al. 2015). This result is contradictory to the fact that SlitOR3 and SlitOR29 are tuned to DMNT, albeit with a moderate sensitivity (Fouchier et al. 2017). This incongruity may arise from different detection sensitivities of the two methods, as two-electrode voltage-clamp technique directly records the activation of ORs by ligands and the channel current is amplified preceding the conversion of acquired data, whereas optical calcium imaging indirectly monitors the fluctuations of cytosolic calcium concentrations by a calcium-sensitive dye (Wu et al. 2013, 2015).
Olfactory detection of HIPVs by parasitoids
Our knowledge of olfactory coding mechanism underlying parasitoid olfaction is very skimpy. Through genome sequencing and antennal transcriptome analysis, a slew of OBP genes has been identified in at least 10 parasitoid species, but most of them has yet functionally characterized (Vieira et al. 2012; Nishimura et al. 2012; Wang et al. 2014, 2015, 2018; Farias et al. 2015; Li et al. 2015; Zhou et al. 2015; Sheng et al. 2017; Liu et al. 2018). The binding patterns of seven OBPs of a solitary endoparasitoid M. mediator have been characterized (Zhang et al. 2011). All OBPs show somewhat binding affinities to a variety of odorants. MmedOBP4 shows binding to some aromatic compounds, like benzaldehyde and its derivatives (Zhang et al. 2011). The binding abilities of MmedOBP4 and MmedOBP6 are skewed to several terpenoids, like α-pinene, β-pinene, α-humulene, β-humulene, β-myrcene, nerolidol, limonene, and geraniol (Zhang et al. 2011), implying these two OBPs are involved in the olfactory detection of terpenoids.
Concurrently, a myriad of OR genes is found in multiple parasitoid species by genome sequencing or RNAseq (Robertson et al. 2010; Zhang et al. 2014; Sheng et al. 2017; Wang et al. 2017a, b; Liu et al. 2018; Sun et al. 2018). By mining the transcriptome of male and female antennae of C. chlorideae, 211 OR transcripts, with 95 being full length, were identified (Sun et al. 2018). The expression of OR14, OR52, OR53, OR60, OR62, OR63, and OR70 is female biased, implying that those ORs may be necessary for some female specific behaviors, such as the location of hosts (Sun et al. 2018). Among them, OR62 is exclusively expressed in female antennae. Indeed, OR62 co-expressed with ORco in Xenopus oocytes shows an exclusive response to (Z)-jasmone, a strong attractant to C. chlorideae (Sun et al. 2018) (Fig. 2). The (Z)-jasmone receptors in H. armigera and B. mori are OR41 and OR56, respectively. However, pairwise comparisons of the amino sequence of CchlOR62, HarmOR41, and BmorOR56 reveal the identities between them are merely 15%, suggesting ORs tuned to (Z)-jasmone in different insect species are evolutionarily divergent. Extensive RNAi screens coupled with EAG measurements and preference essays collectively demonstrates OR35 as a detector of oviposition attractants β-caryophyllene and (E)-α-farnesene in Anastatus japonicus Ashmed (Hymenoptera, Eupelmidae), the parasitic wasp of litchi pest Tessaratoma papillosa Stål (Heteroptera, Pentatomidae) (Wang et al. 2017b). However, most of OBPs and ORs in parasitoids remain uncharacterized, which is a major hindrance for the understanding of olfactory mechanisms of trophic interactions between host plants and parasitoids.
Schematic cascade of olfactory detection of (Z)-jasmone in female Campoletis chlorideae. (Z)-jasmone is released by cotton plants infested by the larvae of Helicoverpa armigera and attracts the female parasitoid C. chlorideae towards the infested plants. Female parasitoids detect (Z)-jasmone through OR62-expressing ORNs ensheathed in the antennal basiconic sensilla (Sun et al. 2018)
The numbers of OR genes found in different parasitoid species are strikingly variable, ranging from 21 OR genes found in pupal parasitoid of the oriental fruit fly, Spalangia endius Walker (Zhang et al. 2014) to 301 OR genes in the jewel wasp Nasonia vitripennis Walker (Hymenoptera, Pteromalidae) (Robertson et al. 2010), which may reflect the complexities of chemical space different parasitoids encounter. C. chlorideae is an endoparasitoid of the generalist herbivore H. armigera that can feed on more than 200 plants species across 60 families (Wang et al. 2004; Han et al. 2013). In nature, C. chlorideae need to faithfully and efficiently extract the most meaningful olfactory information against the bombardment of the myriads of non-relevant volatiles in order to locate its host larvae. The large number of OR repertoire may confer C. chlorideae with a strong associative learning ability expanding the odorant spectrum it can detect.
Detailed molecular dissection of neural mechanisms of learning in bee and fruit flies reveal that the response plasticity of peripheral olfactory systems and the temporary neural connections concertedly contribute to the orchestration of associative learning in insects (Hammer and Menzel 1995; McGuire et al. 2005; Busto et al. 2010). Repeated odorant stimulation with subthreshold concentrations sensitizes the ORNs, whereas repeated stimulations with high concentrations desensitize the ORs (Getahun et al. 2013; Guo et al. 2017). Moreover, the long-term olfactory stimulation increases the volume of corresponding glomeruli, presumably by enhancing synapse connections (Devaud et al. 2001; Das et al. 2011). The Kenyon cells in mushroom body are necessary for forming an olfactory memory (Heisenberg et al. 1985; McGuire 2001). The classical learning mutant rutabaga, which is deficient in the activity of a type I adenylate cyclase, shows a strikingly compromised associative olfactory memory (Levin et al. 1992; Han et al. 1992; McGuire 2001). However, the aforementioned mechanisms implicated in insect learning have not been verified in parasitoids, and only a few of works have been sporadically reported (Vet et al. 1990). In the larvae parasitoid of fruit fly, Leptopilina heterotoma Thomson (Hymenoptera, Figitidae), a correlation between the associative learning and the attenuation of ORNs sensitivities to the odors of food substrate has been elaborated (Vet et al. 1990). This downregulation of olfactory sensitivity may be attributed to olfactory receptor desensitization (Guo and Smith 2017). It seems like that the modulation on peripheral sensitivities, at least, can partially account for associate learning. However, this modulation has not been tested in other parasitoid species. It is worthwhile to check for the sensitivities of the peripheral olfactory detection before and after conditioning in various parasitoid wasps. Rewarding experiences, such as an encounter with hosts or an oviposition, are essential for establishing the associative learning (Turlings et al. 1991a; Geervliet et al. 1998; Tamò et al. 2006; Costa et al. 2010). This posits a neural connection between rewarding circuits and olfactory processing circuits.
Beyond tritrophic levels
In order to fully understand the tritrophic interactions between host plants, herbivores, and parasitoids, other factors cannot be overlooked. Hyperparasitoids comprise a major component of the fourth trophic level and attack parasitoids in the third trophic level. The hyperparasitoid Lysibia nana Gravenhorst (Hymenoptera, Ichneumonidae) is indifferent to the odorants derived from the pupae of primary parasitoids in the genus Cotesia (Hymenoptera: Braconidae), while is strongly attracted by HIPVs (Poelman et al. 2012). These results favor the hypothesis that hyperparasitoids eavesdrop the release of HIPVs and exploit them to locate the pupae of primary parasitoids (Kaplan 2012; Poelman et al. 2012). However, the prevalence of this phenomenon merits future examinations. Researchers therefore should be cautious when they consider seeking out HIPVs to augment the recruitment of parasitoids because the imprudent application of HIPVs could literally reduce the parasitism of herbivores by exposing primary parasitoids to hyperparasitoids.
Polydnaviruses (PDV) including the bracovirus (BV) and ichnovirus (IV) are co-opted by parasitoids to suppress host immune responses (Herniou et al. 2013). PDV is characterized as multi-segmented double-stranded DNA with heterogeneous sizes (Stoltz et al. 1984). PDV particles replicate exclusively in virogenic stroma within the calyx cell nuclei of female wasps and are injected into the Lepidoptera hosts where virus multiplies and hijacks host cells to accommodate the development of wasps (Strand and Burke 2015). Recently, PDV infection is reported to be a suppressor of β-glucosidase enzyme activity in parasitized caterpillar regurgitant, resulting in a modified composition of HIPV that reveals the location of the parasitoid C. glomerata to the hyperparasitoids L. nana (Zhu et al. 2018). In another work, PDV of the parasitoid M. croceipes suppresses the activity of glucose oxidase in caterpillar saliva, an elicitor of immune defense responses in plants (Tan et al. 2018). These two studies elegantly explain how PDV modify the composition of HIPV, which further manipulate the phenotypes of wasps and herbivores down the road. Other than that microbial symbionts living in the bodies of herbivores have been reported to affect the interactions between herbivores and higher tropic levels either by interfering with the release of HIPVs to reduce the recruitment of parasitoids (Dicke and Baldwin 2010; Frago et al. 2017) or producing new chemicals that are attractive to parasitoids (Adams and Six 2008; Boone et al. 2008). Taken together, symbionts and PDV add another degree of intricacy to the interactions between tropical levels.
The emission of HIPVs in nature is modulated by multiple biotic factors, such as plant genotypes (Loughrin et al. 1995; Degen et al. 2004; Schuman et al. 2009; Bruce 2014), pathogens (Rostás et al. 2006; Dicke 2016), herbivory duration (Loughrin et al. 1994), and feeding guilds (Rodriguez-Saona et al. 2003; Rasmann and Turlings 2007; Zhang et al. 2013b). For example, fungal infection causes a plummeted emission of HIPVs from S. littoralis-infested maize (Rostás et al. 2006). Moreover, simultaneous infestation of cotton by silverleaf whitefly Bemisia tabaci Gennadius (Hemiptera, Aleyrodidae) and beet armyworm S. exigua dramatically suppresses the emission of HIPVs compared to the emissions of HIPV when infested by S. exigua alone (Rodriguez-Saona et al. 2003). In addition to the biotic factors, a series of abiotic impacts, such as ambient temperatures, air and soil humidity, light, the levels of CO2 and ozone, and availability of nutrients, significantly regulate the emission dynamics of HIPVs (Schmelz et al. 2003b; Niinemets et al. 2004; Holopainen and Gershenzon 2010; Peñuelas and Staudt 2010; Holopainen and Blande 2013; Becker et al. 2015). Elevated ambient temperatures not only enhance the enzyme activities involved in synthesis of phytohormones, e.g., jasmonic acid, salicylic acid, and ethylene, but also change the physical structures of plants, such as the openness of stomatal aperture, resulting in an elevated emission of terpenoids (Niinemets et al. 2004; Gouinguene and Turlings 2002). Drought appears to increase the emission of HIPVs, most likely by boosting plant defense in the condition of water depletion (Gouinguené and Turlings 2002). The emission of HIPVs largely hinges on the light intensities, which coincides with the diurnal feeding rhythms of herbivores (Gouinguené and Turlings 2002). Moreover, elevated atmospheric CO2 concentrations have been reported to compromise the plant defense response to herbivory (Vuorinen et al. 2004). Recent findings indicate that increased CO2 concentrations enhance the salicylic acid pathway, and concomitantly suppress the jasmonic acid pathway, which would change the composition of HIPVs emitted from host plants, and in turn confound the foraging of herbivores and parasitoids (Guo et al. 2012b). O3 has been reported to hamper the emission of terpenoids (Himanen et al. 2009) and mediate the degradation of GLVs and terpenoids (Pinto et al. 2007). Taken together, those aforementioned abiotic factors exert tangible effects on the emission of HIPVs, and they should be considered when the interactions between different tropic levels are studied.
Future perspectives
Over the last three decades, enormous strides have been made in the identification of HIPVs and the disentanglement of intricate chemical interactions between tropical levels. However, in the next decade, there are several questions needed to be addressed. First, the effects of mixtures to the behavior of herbivores and parasitoids should be further addressed. To date, most of HIPVs have been individually examined against solvent control in behavior tests. As such, we cannot fully leverage the full advantage of the HIPV-mediated recruitment of parasitoids. Second, the transformation of our knowledge obtained in the lab into commercially accessible products for pest control needs to be expedited. Third, the olfactory mechanisms underlying HIPV perception remain largely unknown. ORs tuned to behavior-relevant HIPVs in herbivorous insects and parasitoids await functionally characterized. We expect to see that, in the next decade, the ORs tuned to ecology-relevant odorants will be deorphanized, and taking a step further, the coding atlas of ethologically relevant odorants in the insect brain will be unveiled. The correlations between modulations of the responsive sensitivity of ORs and learning in parasitoids await further studies. These interdisciplinary efforts will concretely expedite the development of an economic and practical way to exploit parasitoids as an arsenal to curb the occurrence of pests.
References
Aartsma Y, Bianchi FJJA, van der Werf W et al (2017) Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol 216:1054–1063. https://doi.org/10.1111/nph.14475
Adams AS, Six DL (2008) Detection of host habitat by parasitoids using cues associated with mycangial fungi of the mountain pine beetle, Dendroctonus ponderosae. Can Entomol 140:124–127. https://doi.org/10.4039/n07-018
Addesso KM, McAuslane HJ, Alborn HT (2010) Attraction of pepper weevil to volatiles from damaged pepper plants. Entomol Exp Appl 138:1–11. https://doi.org/10.1111/j.1570-7458.2010.01070.x
Alborn HT, Turlings TCJ, Jones TH et al (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949. https://doi.org/10.1126/science.276.5314.945
Alborn HT, Hansen TV, Jones TH et al (2007) Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci 104:12976–12981. https://doi.org/10.1073/pnas.0705947104
Aljbory Z, Chen M-S (2018) Indirect plant defense against insect herbivores: a review. Insect Sci 25:2–23. https://doi.org/10.1111/1744-7917.12436
Allmann S, Späthe A, Bisch-Knaden S et al (2013) Feeding-induced rearrangement of green leaf volatiles reduces moth oviposition. eLife. https://doi.org/10.7554/eLife.00421
Ament K (2004) Jasmonic Acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135:2025–2037. https://doi.org/10.1104/pp.104.048694
Anderson P, Alborn H (1999) Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol Exp Appl 92:45–51. https://doi.org/10.1046/j.1570-7458.1999.00523.x
Arimura G, Ozawa R, Shimoda T et al (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515. https://doi.org/10.1038/35020072
Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. Biochim Biophys Acta BBA - Mol Cell Biol Lipids 1734:91–111. https://doi.org/10.1016/j.bbalip.2005.03.001
Arimura G-i, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923. https://doi.org/10.1093/pcp/pcp030
Baldwin IT (1998) Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci 95:8113–8118. https://doi.org/10.1073/pnas.95.14.8113
Baldwin IT (2010) Plant volatiles. Curr Biol 20:R392–R397. https://doi.org/10.1016/j.cub.2010.02.052
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488. https://doi.org/10.1007/s11103-008-9435-0
Becker C, Desneux N, Monticelli L et al (2015) Effects of abiotic factors on HIPV-mediated interactions between plants and parasitoids. BioMed Res. https://doi.org/10.1155/2015/342982
Benton R, Sachse S, Michnick SW, Vosshall LB (2006) Atypical membrane topology and heteromeric function of drosophila odorant receptors in vivo. PLoS Biol 4:e20. https://doi.org/10.1371/journal.pbio.0040020
Benton R, Vannice KS, Vosshall LB (2007) An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450:289–293. https://doi.org/10.1038/nature06328
Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB (2009) Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136:149–162. https://doi.org/10.1016/j.cell.2008.12.001
Bleeker MAK, Smid HM, Steidle JLM et al (2006) Differences in memory dynamics between two closely related parasitoid wasp species. Anim Behav 71:1343–1350. https://doi.org/10.1016/j.anbehav.2005.09.016
Boone CK, Six DL, Zheng Y, Raffa KF (2008) Parasitoids and dipteran predators exploit volatiles from microbial symbionts to locate bark beetles. Environ Entomol 37:150–161
Bruce TJA (2014) Variation in plant responsiveness to defense elicitors caused by genotype and environment. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00349
Bruinsma M, Dicke M (2008) Herbivore-induced indirect defense: from induction mechanisms to community ecology. In: Schaller A (ed) Induced plant resistance to herbivory. Springer Netherlands, Dordrecht, pp 31–60
Busto GU, Cervantes-Sandoval I, Davis RL (2010) Olfactory learning in Drosophila. Physiology 25:338–346. https://doi.org/10.1152/physiol.00026.2010
Butterwick JA, Mármol J del, Kim KH et al (2018) Cryo-EM structure of the insect olfactory receptor Orco. Nature 560:447–452. https://doi.org/10.1038/s41586-018-0420-8
Buttery RG, Teranishi R, Ling LC et al (1988) Quantitative studies on origins of fresh tomato aroma volatiles. J Agric Food Chem 36:1247–1250. https://doi.org/10.1021/jf00084a030
Cao S, Liu Y, Guo M, Wang G (2016) A conserved odorant receptor tuned to floral volatiles in three Heliothinae Species. PLoS ONE 11:e0155029. https://doi.org/10.1371/journal.pone.0155029
Cascone P, Gols R, Fatouros NE et al (2018) The effect of rearing history and aphid density on volatile-mediated foraging behaviour of Diaeretiella rapae. Ecol Entomol. https://doi.org/10.1111/een.12704
Chang JF, Benedict JH, Payne TL, Camp BJ (1988) Volatile monoterpenes collected from the air surrounding flower buds of seven cotton genotypes. Crop Sci 28:685–688. https://doi.org/10.2135/cropsci1988.0011183X002800040026x
Clavijo McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310. https://doi.org/10.1016/j.tplants.2012.03.012
Clyne PJ, Warr CG, Freeman MR et al (1999) A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22:327–338. https://doi.org/10.1016/S0896-6273(00)81093-4
Costa A, Ricard I, Davison AC, Turlings TCJ (2010) Effects of rewarding and unrewarding experiences on the response to host-induced plant odors of the generalist parasitoid Cotesia marginiventris (Hymenoptera: Braconidae). J Insect Behav 23:303–318. https://doi.org/10.1007/s10905-010-9215-y
Cui W, Wang B, Guo M et al (2018) A receptor-neuron correlate for the detection of attractive plant volatiles in Helicoverpa assulta (Lepidoptera: Noctuidae). Insect Biochem Mol Biol 97:31–39. https://doi.org/10.1016/j.ibmb.2018.04.006
D’Alessandro M, Turlings TCJ (2006) Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131:24–32. https://doi.org/10.1039/B507589K
D’Alessandro M, Brunner V, Mérey G von, Turlings TCJ (2009) Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J Chem Ecol 35:999. https://doi.org/10.1007/s10886-009-9692-7
Das S, Sadanandappa MK, Dervan A et al (2011) Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci 108:E646–E654. https://doi.org/10.1073/pnas.1106411108
De Moraes CM, Lewis WJ, Paré PW et al (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573. https://doi.org/10.1038/31219
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580. https://doi.org/10.1038/35069058
Degen T, Dillmann C, Marion-Poll F, Turlings TCJ (2004) High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol 135:1928–1938. https://doi.org/10.1104/pp.104.039891
Degen T, Bakalovic N, Bergvinson D, Turlings TCJ (2012) Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore-induced volatile emissions. PLoS ONE 7:e47589. https://doi.org/10.1371/journal.pone.0047589
Devaud J-M, Acebes A, Ferrús A (2001) Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci 21:6274–6282. https://doi.org/10.1523/JNEUROSCI.21-16-06274.2001
Di C, Ning C, Huang L-Q, Wang C-Z (2017) Design of larval chemical attractants based on odorant response spectra of odorant receptors in the cotton bollworm. Insect Biochem Mol Biol 84:48–62. https://doi.org/10.1016/j.ibmb.2017.03.007
Dicke M (1986) Volatile spider-mite pheromone and host-plant kairomone, involved in spaced-out gregariousness in the spider mite Tetranychus urticae. Physiol Entomol 11:251–262. https://doi.org/10.1111/j.1365-3032.1986.tb00412.x
Dicke M (1988) Prey preference of the phytoseiid mite Typhlodromus pyri 1. Response to volatile kairomones. Exp Appl Acarol 4:1–13. https://doi.org/10.1007/BF01213837
Dicke M (2016) Plant phenotypic plasticity in the phytobiome: a volatile issue. Curr Opin Plant Biol 32:17–23. https://doi.org/10.1016/j.pbi.2016.05.004
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175. https://doi.org/10.1016/j.tplants.2009.12.002
Dicke M, Sabelis MW (1987) How plants obtain predatory mites as bodyguards. Neth J Zool 38:148–165. https://doi.org/10.1163/156854288X00111
Dicke M, Van Beek TA, Posthumus MA et al (1990) Isolation and identification of volatile kairomone that affects acarine predator prey interactions Involvement of host plant in its production. J Chem Ecol 16:381–396. https://doi.org/10.1007/BF01021772
Dicke M, van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4:27–42. https://doi.org/10.1078/1439-1791-00131
Dicke M, Loon JJA van, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol 5:317–324. https://doi.org/10.1038/nchembio.169
Doss RP, Oliver JE, Proebsting WM et al (2000) Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci 97:6218–6223. https://doi.org/10.1073/pnas.110054697
Du L, Zhao X, Liang X et al (2018) Identification of candidate chemosensory genes in Mythimna separata by transcriptomic analysis. BMC Genom. https://doi.org/10.1186/s12864-018-4898-0
Dudareva N (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902. https://doi.org/10.1104/pp.104.049981
El-Sayed AM, Knight AL, Byers JA et al (2016) Caterpillar-induced plant volatiles attract conspecific adults in nature. Sci Rep. https://doi.org/10.1038/srep37555
Elzen GW, Williams HJ, Vinson SB (1984) Isolation and identification of cotton synomones mediating searching behavior by parasitoid Campoletis sonorensis. J Chem Ecol 10:1251–1264. https://doi.org/10.1007/BF00988552
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259. https://doi.org/10.1016/j.tplants.2012.01.003
Farias LR, Schimmelpfeng PHC, Togawa RC et al (2015) Transcriptome-based identification of highly similar odorant-binding proteins among neotropical stink bugs and their egg parasitoid. PLoS ONE 10:e0132286. https://doi.org/10.1371/journal.pone.0132286
Fouchier A de, Walker WB, Montagné N et al (2017) Functional evolution of Lepidoptera olfactory receptors revealed by deorphanization of a moth repertoire. Nat Commun 8:15709. https://doi.org/10.1038/ncomms15709
Frago E, Mala M, Weldegergis BT et al (2017) Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat Commun 8:1860. https://doi.org/10.1038/s41467-017-01935-0
Fukushima J, Kainoh Y, Honda H, Takabayashi J (2001) Learning of host-infested plant volatiles in the larval parasitoid Cotesia kariyai. Entomol Exp Appl 99:341–346. https://doi.org/10.1046/j.1570-7458.2001.00833.x
Galizia CG, Rössler W (2010) Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol 55:399–420. https://doi.org/10.1146/annurev-ento-112408-085442
Gao Q, Chess A (1999) Identification of candidate drosophila olfactory receptors from genomic DNA sequence. Genomics 60:31–39. https://doi.org/10.1006/geno.1999.5894
Geervliet JBF, Vet LEM, Dicke M (1996) Innate responses of the parasitoids Cotesia glomerate and C. rubecula (Hymenoptera: Braconidae) to volatiles from different plant-herbivore complexes. J Insect Behav 9:525–538. https://doi.org/10.1007/BF02213877
Geervliet JBF, Vreugdenhil AI, Dicke M, Vet LEM (1998) Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol Exp Appl 86:241–252. https://doi.org/10.1046/j.1570-7458.1998.00286.x
Getahun MN, Olsson SB, Lavista-Llanos S et al (2013) Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS ONE 8:e58889. https://doi.org/10.1371/journal.pone.0058889
Giunti G, Canale A, Messing RH et al (2015) Parasitoid learning: current knowledge and implications for biological control. Biol Control 90:208–219. https://doi.org/10.1016/j.biocontrol.2015.06.007
Gols R, Veenemans C, Potting RPJ et al (2012) Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim Behav 83:1231–1242. https://doi.org/10.1016/j.anbehav.2012.02.015
Gouinguene SP, Turlings TCJ (2002) The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol 129:1296–1307. https://doi.org/10.1104/pp.001941
Gregg PC, Socorro APD, Henderson GS (2010) Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust J Entomol 49:21–30. https://doi.org/10.1111/j.1440-6055.2009.00734.x
Guo H, Smith DP (2017) Odorant receptor desensitization in insects. J Exp Neurosci 11:117906951774860. https://doi.org/10.1177/1179069517748600
Guo H, Huang L-Q, Pelosi P, Wang C-Z (2012a) Three pheromone-binding proteins help segregation between two Helicoverpa species utilizing the same pheromone components. Insect Biochem Mol Biol 42:708–716. https://doi.org/10.1016/j.ibmb.2012.06.004
Guo H, Sun Y, Ren Q et al (2012b) Elevated CO2 reduces the resistance and tolerance of tomato plants to helicoverpa armigera by suppressing the JA signaling pathway. PLoS ONE 7:e41426. https://doi.org/10.1371/journal.pone.0041426
Guo H, Kunwar K, Smith D (2017) Odorant receptor sensitivity modulation in Drosophila. J Neurosci Off J Soc Neurosci 37:9465–9473. https://doi.org/10.1523/JNEUROSCI.1573-17.2017
Hallem EA, Carlson JR (2006) Coding of odors by a receptor repertoire. Cell 125:143–160. https://doi.org/10.1016/j.cell.2006.01.050
Hallem EA, Ho MG, Carlson JR (2004) The molecular basis of odor coding in the Drosophila antenna. Cell 117:965–979. https://doi.org/10.1016/j.cell.2004.05.012
Hammer M, Menzel R (1995) Learning and memory in the honeybee. J Neurosci 15:1617–1630. https://doi.org/10.1523/JNEUROSCI.15-03-01617.1995
Han P-L, Levin LR, Reed RR, Davis RL (1992) Preferential expression of the drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron 9:619–627. https://doi.org/10.1016/0896-6273(92)90026-A
Han L, Huang L, Wang C (2013) Host preference and suitability in the endoparasitoid Campoletis chlorideae is associated with its ability to suppress host immune responses. Ecol Entomol 38:173–182. https://doi.org/10.1111/een.12006
Hansson BS, Stensmyr MC (2011) Evolution of insect olfaction. Neuron 72:698–711. https://doi.org/10.1016/j.neuron.2011.11.003
Hare JD (2007) Variation in herbivore and methyl jasmonate-induced volatiles among genetic lines of Datura wrightii. J Chem Ecol 33:2028–2043. https://doi.org/10.1007/s10886-007-9375-1
Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56:161–180. https://doi.org/10.1146/annurev-ento-120709-144753
Hassan MNul, Zainal Z, Ismail I (2015) Green leaf volatiles: biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol J 13:727–739. https://doi.org/10.1111/pbi.12368
Hatano E, Saveer AM, Borrero-Echeverry F et al (2015) A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways. BMC Biol. https://doi.org/10.1186/s12915-015-0188-3
Hegde M, Oliveira JN, da Costa JG et al (2011) Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J Chem Ecol 37:741–750. https://doi.org/10.1007/s10886-011-9980-x
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61. https://doi.org/10.1111/j.1469-8137.2007.02330.x
Heisenberg M, Borst A, Wagner S, Byers D (1985) Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet 2:1–30. https://doi.org/10.3109/01677068509100140
Herniou EA, Huguet E, Thézé J et al (2013) When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Phil Trans R Soc B 368:20130051. https://doi.org/10.1098/rstb.2013.0051
Himanen SJ, Nerg A-M, Nissinen A et al (2009) Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol 181:174–186. https://doi.org/10.1111/j.1469-8137.2008.02646.x
Holopainen J (2004) Multiple functions of inducible plant volatiles. Trends Plant Sci 9:529–533. https://doi.org/10.1016/j.tplants.2004.09.006
Holopainen JK, Blande JD (2013) Where do herbivore-induced plant volatiles go? Front Plant Sci. https://doi.org/10.3389/fpls.2013.00185
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15:176–184. https://doi.org/10.1016/j.tplants.2010.01.006
Houshyani B, Assareh M, Busquets A et al (2013) Three-step pathway engineering results in more incidence rate and higher emission of nerolidol and improved attraction of Diadegma semiclausum. Metab Eng 15:88–97. https://doi.org/10.1016/j.ymben.2012.10.002
Hu L, Ye M, Erb M (2018) Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defense and resistance: synergistic defense enhancement by two volatiles. Plant Cell Environ. https://doi.org/10.1111/pce.13443
Jiang X-J, Guo H, Di C et al (2014) Sequence similarity and functional comparisons of pheromone receptor orthologs in two closely related Helicoverpa species. Insect Biochem Mol Biol 48:63–74. https://doi.org/10.1016/j.ibmb.2014.02.010
Jin X, Ha TS, Smith DP (2008) SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci 105:10996–11001. https://doi.org/10.1073/pnas.0803309105
Jönsson M, Anderson P (1999) Electrophysiological response to herbivore-induced host plant volatiles in the moth Spodoptera littoralis. Physiol Entomol 24:377–385. https://doi.org/10.1046/j.1365-3032.1999.00154.x
Jönsson M, Anderson P (2002) Electrophysiological response to herbivore-induced host plant volatiles in the moth Spodoptera littoralis. Physiol Entomol 24:377–385. https://doi.org/10.1046/j.1365-3032.1999.00154.x
Joseph RM, Carlson JR (2015) Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet 31:683–695. https://doi.org/10.1016/j.tig.2015.09.005
Kahl J, Siemens DH, Aerts RJ et al (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210:336–342. https://doi.org/10.1007/PL00008142
Kaiser L, Carde RT (1992) In-flight orientation to volatiles from the plant-host complex in Cotesia rubecula (Hym.: Braconidae): increased sensitivity through olfactory experience. Physiol Entomol 17:62–67. https://doi.org/10.1111/j.1365-3032.1992.tb00990.x
Kaplan I (2012) Trophic complexity and the adaptive value of damage-induced plant volatiles. PLoS Biol 10:e1001437. https://doi.org/10.1371/journal.pbio.1001437
Kappers IF, Aharoni A, Herpen TWJM van et al (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309:2070–2072. https://doi.org/10.1126/science.1116232
Karban R, Agrawal AA, Thaler JS, Adler LS (1999) Induced plant responses and information content about risk of herbivory. Trends Ecol Evol 14:443–447. https://doi.org/10.1016/S0169-5347(99)01678-X
Kaupp UB (2010) Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci 11:188–200. https://doi.org/10.1038/nrn2789
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144. https://doi.org/10.1126/science.291.5511.2141
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328. https://doi.org/10.1146/annurev.arplant.53.100301.135207
Kessler A, Halitschke R, Baldwin IT (2004) Silencing the jasmonate cascade: Induced plant defenses and insect populations. Science 305:665–668. https://doi.org/10.1126/science.1096931
Klessig DF, Durner J, Noad R et al (2000) Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci 97:8849–8855. https://doi.org/10.1073/pnas.97.16.8849
Landolt PJ (1993) Effects of host plant leaf damage on cabbage looper moth attraction and oviposition. Entomol Exp Appl 67:79–85. https://doi.org/10.1111/j.1570-7458.1993.tb01654.x
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58:373–391. https://doi.org/10.1146/annurev-ento-120811-153635
Levin LR, Han P-L, Hwang PM et al (1992) The Drosophila learning and memory gene rutabaga encodes a Ca2+ calmodulin-responsive adenylyl cyclase. Cell 68:479–489. https://doi.org/10.1016/0092-8674(92)90185-F
Li K, Yang X, Xu G et al (2015) Identification of putative odorant binding protein genes in Asecodes hispinarum, a parasitoid of coconut leaf beetle (Brontispa longissima) by antennal RNA-Seq analysis. Biochem Biophys Res Commun 467:514–520. https://doi.org/10.1016/j.bbrc.2015.10.008
Liu C, Liu Y, Guo M et al (2014) Narrow tuning of an odorant receptor to plant volatiles in Spodoptera exigua (Hübner): narrow tuning of an odorant receptor to volatiles. Insect Mol Biol 23:487–496. https://doi.org/10.1111/imb.12096
Liu N-Y, Zhu J-Y, Zhang T, Dong S-L (2017) Characterization of two odorant binding proteins in Spodoptera exigua reveals functional conservation and difference. Comp Biochem Physiol A Mol Integr Physiol 213:20–27. https://doi.org/10.1016/j.cbpa.2017.08.002
Liu J-B, Wu H, Yi J-Q et al (2018) Transcriptome characterization and gene expression analysis related to chemoreception in Trichogramma chilonis, an egg parasitoid. Gene 678:288–301. https://doi.org/10.1016/j.gene.2018.07.065
Loon JJA, Boer JG, Dicke M (2000) Parasitoid-plant mutualism: parasitoid attack of herbivore increases plant reproduction. Entomol Exp Appl 97:219–227. https://doi.org/10.1046/j.1570-7458.2000.00733.x
Lou Y-G, Baldwin IT (2003) Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proc Natl Acad Sci USA 100:14581–14586. https://doi.org/10.1073/pnas.2135348100
Lou Y-G, Du M-H, Turlings TCJ et al (2005) Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J Chem Ecol 31:1985–2002. https://doi.org/10.1007/s10886-005-6072-9
Lou Y-G, Hua X, Turlings TCJ et al (2006) Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the Field. J Chem Ecol 32:2375. https://doi.org/10.1007/s10886-006-9151-7
Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF (1990) Headspace compounds from flowers of Nicotiana tabacum and related species. J Agric Food Chem 38:455–460. https://doi.org/10.1021/jf00092a027
Loughrin JH, Manukian A, Heath RR et al (1994) Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Natl Acad Sci USA 91:11836–11840
Loughrin JH, Manukian A, Heath RR, Tumlinson JH (1995) Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol 21:1217–1227. https://doi.org/10.1007/BF02228321
Lu J, Li J, Ju H et al (2014) Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol Plant 7:1670–1682. https://doi.org/10.1093/mp/ssu085
Lucas-Barbosa D, van Loon JJA, Dicke M (2011) The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry 72:1647–1654. https://doi.org/10.1016/j.phytochem.2011.03.013
Ma L, Li Z, Zhang W et al (2018) The odorant binding protein 6 expressed in sensilla chaetica displays preferential binding affinity to host plants volatiles in Ectropis obliqua. Front Physiol. https://doi.org/10.3389/fphys.2018.00534
Masse NY, Turner GC, Jefferis GSXE (2009) Olfactory information processing in Drosophila. Curr Biol 19:R700–R713. https://doi.org/10.1016/j.cub.2009.06.026
McCall PJ, Turlings TCJ, Loughrin J et al (1994) Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L.) seedlings. J Chem Ecol 20:3039–3050. https://doi.org/10.1007/BF02033709
McGuire SE (2001) The role of Drosophila mushroom body signaling in olfactory memory. Science 293:1330–1333. https://doi.org/10.1126/science.1062622
McGuire SE, Deshazer M, Davis RL (2005) Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol 76:328–347. https://doi.org/10.1016/j.pneurobio.2005.09.003
Morawo T, Fadamiro H (2016) Identification of key plant-associated volatiles emitted by Heliothis virescens larvae that attract the parasitoid, Microplitis croceipes: implications for parasitoid perception of odor blends. J Chem Ecol 42:1112–1121. https://doi.org/10.1007/s10886-016-0779-7
Mumm R, Posthumus MA, Dicke M (2008) Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ 31:575–585. https://doi.org/10.1111/j.1365-3040.2008.01783.x
Nakagawa T, Sakurai T, Nishioka T, Touhara K (2005) Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307:1638–1642. https://doi.org/10.1126/science.1106267
Ngumbi E, Chen L, Fadamiro HY (2009) Comparative GC-EAD responses of a specialist (Microplitis croceipes) and a generalist (Cotesia marginiventris) parasitoid to cotton volatiles induced by two caterpillar species. J Chem Ecol 35:1009–1020. https://doi.org/10.1007/s10886-009-9700-y
Ngumbi E, Jordan M, Fadamiro H (2012) Comparison of associative learning of host-related plant volatiles in two parasitoids with different degrees of host specificity, Cotesia marginiventris and Microplitis croceipes. Chemoecology 22:207–215. https://doi.org/10.1007/s00049-012-0106-x
Niinemets Ü, Loreto F, Reichstein M (2004) Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci 9:180–186. https://doi.org/10.1016/j.tplants.2004.02.006
Nishimura O, Brillada C, Yazawa S et al (2012) Transcriptome pyrosequencing of the parasitoid wasp Cotesia vestalis: genes involved in the antennal odorant-sensory system. PLoS ONE 7:e50664. https://doi.org/10.1371/journal.pone.0050664
Nordlund DA (1987) Plant produced allelochemics and their involvement in the host selection behaviour of parasitoids. In: Labeyrie V, Fabres G & Lachaise D (Eds) Insects–plants. Dr W. Junk, Dordrecht, pp. 103–107
Nordlund DA, Chalfant RB, Lewis WJ (1985) Response of Trichogramma pretiosum females to extracts of two plants attacked by Heliothis zea. Agric Ecosyst Environ 12:127–133. https://doi.org/10.1016/0167-8809(85)90073-8
Nordlund DA, Lewis WJ, Altieri MA (1988) Influence of plant produced allelochemicals on the host/prey selection behavior of entomophagous insects. In: Barbosa P (ed) Novel aspects of insect–plant interactions. D. K. Letourneau, New York, pp 65–90
O’Donnell PJ, Calvert C, Atzorn R et al (1996) Ethylene as a signal mediating the wound response of tomato Plants. Science 274:1914–1917. https://doi.org/10.1126/science.274.5294.1914
Ozawa R, Shiojiri K, Sabelis MW et al (2004) Corn plants treated with jasmonic acid attract more specialist parasitoids, thereby increasing parasitization of the common armyworm. J Chem Ecol 30:1797–1808. https://doi.org/10.1023/B:JOEC.0000042402.04012.c7
Paré PW, Tumlinson JH (1997) De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol 114:7
Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–332. https://doi.org/10.1104/pp.121.2.325
Pelosi P, Zhou J-J, Ban LP, Calvello M (2006) Soluble proteins in insect chemical communication. Cell Mol Life Sci 63:1658–1676. https://doi.org/10.1007/s00018-005-5607-0
Peñaflor MFGV, Erb M, Miranda LA et al (2011) Herbivore-induced plant volatiles can serve as host location cues for a generalist and a specialist egg parasitoid. J Chem Ecol 37:1304–1313. https://doi.org/10.1007/s10886-011-0047-9
Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15:133–144. https://doi.org/10.1016/j.tplants.2009.12.005
Pinto DM, Blande JD, Nykänen R et al (2007) Ozone degrades common herbivore-induced plant volatiles: does this affect herbivore prey location by predators and parasitoids? J Chem Ecol 33:683–694. https://doi.org/10.1007/s10886-007-9255-8
Poecke RMP van, Dicke M (2004) Indirect defence of plants against herbivores: using Arabidopsis thaliana as a model plant. Plant Biol 6:387–401. https://doi.org/10.1055/s-2004-820887
Poecke RMPV, Posthumus MA, Dicke M (2001) Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J Chem Ecol 27:1911–1928. https://doi.org/10.1023/A:1012213116515
Poelman EH, Oduor AMO, Broekgaarden C et al (2009) Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct Ecol 23:951–962. https://doi.org/10.1111/j.1365-2435.2009.01570.x
Poelman EH, Bruinsma M, Zhu F et al (2012) Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol 10:e1001435. https://doi.org/10.1371/journal.pbio.1001435
Price PW, Bouton CE, Gross P et al (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 11:41–65. https://doi.org/10.1146/annurev.es.11.110180.000353
Prokopy RJ, Roitberg BD (2001) Joining and a voidance in nonsocial insects. Annu Rev Entomol 46:631–665. https://doi.org/10.1146/annurev.ento.46.1.631
Qiao H, Tuccori E, He X et al (2009) Discrimination of alarm pheromone (E)-β-farnesene by aphid odorant-binding proteins. Insect Biochem Mol Biol 39:414–419. https://doi.org/10.1016/j.ibmb.2009.03.004
Rasmann S, Turlings TCJ (2007) Simultaneous feeding by aboveground and belowground herbivores attenuates plant-mediated attraction of their respective natural enemies. Ecol Lett 10:926–936. https://doi.org/10.1111/j.1461-0248.2007.01084.x
Ray A (2015) Reception of odors and repellents in mosquitoes. Curr Opin Neurobiol 34:158–164. https://doi.org/10.1016/j.conb.2015.06.014
Reisenman CE, Riffell JA, Duffy K et al (2013) Species-specific effects of herbivory on the oviposition behavior of the Moth Manduca sexta. J Chem Ecol 39:76–89. https://doi.org/10.1007/s10886-012-0228-1
Robertson HM, Gadau J, Wanner KW (2010) The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol Biol 19:121–136. https://doi.org/10.1111/j.1365-2583.2009.00979.x
Rodriguez-Saona C, Crafts-Brandner SJ, Cañas LA (2003) Volatile emissions triggered by multiple herbivore damage: beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 29:2539–2550. https://doi.org/10.1023/A:1026314102866
Rogers ME, Sun M, Lerner MR, Vogt RG (1997) Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J Biol Chem 272:14792–14799. https://doi.org/10.1074/jbc.272.23.14792
Rojas JC (1999) Influence of host plant damage on the host-finding behavior of Mamestra brassicae (Lepidoptera: Noctuidae). Environ Entomol 28:588–593. https://doi.org/10.1093/ee/28.4.588
Röse USR, Tumlinson JH (2004) Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta 218:824–832. https://doi.org/10.1007/s00425-003-1162-9
Röse U, Manukian A, Heath RR, Tumlinson JH (1996) Volatile semiochemicals released from undamaged cotton leaves (A systemic response of living plants to caterpillar damage). Plant Physiol 111:487–495. https://doi.org/10.1104/pp.111.2.487
Rostás M, Ton J, Mauch-Mani B, Turlings TCJ (2006) Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naïve parasitoids. J Chem Ecol 32:1897–1909. https://doi.org/10.1007/s10886-006-9147-3
Sato K, Pellegrino M, Nakagawa T et al (2008) Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452:1002–1006. https://doi.org/10.1038/nature06850
Schmelz EA, Alborn HT, Banchio E, Tumlinson JH (2003a) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673. https://doi.org/10.1007/s00425-002-0898-y
Schmelz EA, Alborn HT, Engelberth J, Tumlinson JH (2003b) Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol 133:295–306. https://doi.org/10.1104/pp.103.024174
Schmelz EA, Carroll MJ, LeClere S et al (2006) Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci 103:8894–8899. https://doi.org/10.1073/pnas.0602328103
Schnee C, Köllner TG, Held M et al (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci 103:1129–1134. https://doi.org/10.1073/pnas.0508027103
Schuman MC, Heinzel N, Gaquerel E et al (2009) Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. New Phytol 183:1134–1148. https://doi.org/10.1111/j.1469-8137.2009.02894.x
Scutareanu P, Drukker B, Bruin J et al (1997) Volatiles from psylla-infested pear trees and their possible involvement in attraction of anthocorid predators. J Chem Ecol 23:2241–2260. https://doi.org/10.1023/B:JOEC.0000006671.53045.16
Sheng S, Liao C-W, Zheng Y et al (2017) Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. Comp Biochem Physiol D 22:20–31. https://doi.org/10.1016/j.cbd.2017.01.002
Shiojiri K, Takabayashi J (2003) Effects of specialist parasitoids on oviposition preference of phytophagous insects: encounter–dilution effects in a tritrophic interaction. Ecol Entomol 28:573–578. https://doi.org/10.1046/j.1365-2311.2003.00539.x
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2000) Flight response of parasitoids toward plant-herbivore complexes:A comparative study of two parasitoid-herbivore systems on cabbage plants. Appl Entomol Zool 35:87–92. https://doi.org/10.1303/aez.2000.87
Shiojiri K, Ozawa R, Kugimiya S et al (2010) Herbivore-specific, density-dependent induction of plant volatiles: Honest or “Cry Wolf” signals? PLoS ONE 5:e12161. https://doi.org/10.1371/journal.pone.0012161
Signoretti AGC, Peñaflor MFGV, Moreira LSD et al (2012) Diurnal and nocturnal herbivore induction on maize elicit different innate response of the fall armyworm parasitoid, Campoletis flavicincta. J Pest Sci 85:101–107. https://doi.org/10.1007/s10340-011-0397-7
Silva DB, Weldegergis BT, Loon JJAV, Bueno VHP (2017) Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by either Tuta absoluta or Bemisia tabaci. J Chem Ecol 43:53–65. https://doi.org/10.1007/s10886-016-0807-7
Simons MTTP, Suverkropp BP, Vet LEM, Moed G de (1992) Comparison of learning in related generalist and specialist eucoilid parasitoids. Entomol Exp Appl 64:117–124. https://doi.org/10.1111/j.1570-7458.1992.tb01601.x
Snoeren TAL, Mumm R, Poelman EH et al (2010) The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J Chem Ecol 36:479–489. https://doi.org/10.1007/s10886-010-9787-1
Sobhy IS, Bruce TJ, Turlings TC (2018) Priming of cowpea volatile emissions with defense inducers enhances the plant’s attractiveness to parasitoids when attacked by caterpillars. Pest Manag Sci 74:966–977. https://doi.org/10.1002/ps.4796
Steidle JLM (1998) Learning pays off: influence of experience on host finding and parasitism in Lariophagus distinguendus. Ecol Entomol 23:451–456. https://doi.org/10.1046/j.1365-2311.1998.00144.x
Stensmyr MC, Dweck HKM, Farhan A et al (2012) A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151:1345–1357. https://doi.org/10.1016/j.cell.2012.09.046
Stoltz DB, Krell P, Summers MD, Vinson B (1984) Polydnaviridae—a proposed family of insect viruses with segmented, double-stranded, circular DNA genomes. Intervirology 21:1–4. https://doi.org/10.1159/000149497
Strand MR, Burke GR (2015) Polydnaviruses: from discovery to current insights. Virology 479–480:393–402. https://doi.org/10.1016/j.virol.2015.01.018
Su C-Y, Menuz K, Carlson JR (2009) Olfactory perception: receptors, cells, and circuits. Cell 139:45–59. https://doi.org/10.1016/j.cell.2009.09.015
Sun J-G, Huang L-Q, Wang C-Z (2012) Electrophysiological and behavioral responses of Helicoverpa assulta (Lepidoptera: Noctuidae) to tobacco volatiles. Arthropod-Plant Interact 6:375–384. https://doi.org/10.1007/s11829-012-9190-7
Sun X-L, Wang G-C, Gao Y et al (2014) Volatiles emitted from tea plants infested by Ectropis obliqua larvae are attractive to conspecific moths. J Chem Ecol 40:1080–1089. https://doi.org/10.1007/s10886-014-0502-5
Sun Y-L, Dong J-F, Ning C et al (2018) An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol Biol. https://doi.org/10.1111/imb.12523
Takasu K, Lewis WJ (2003) Learning of host searching cues by the larval parasitoid Microplitis croceipes. Entomol Exp Appl 108:77–86. https://doi.org/10.1046/j.1570-7458.2003.00070.x
Tamiru A, Bruce TJA, Woodcock CM et al (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by an herbivore. Ecol Lett 14:1075–1083. https://doi.org/10.1111/j.1461-0248.2011.01674.x
Tamiru A, Bruce TJA, Woodcock CM et al (2015) Chemical cues modulating electrophysiological and behavioural responses in the parasitic wasp Cotesia sesamiae. Can J Zool 93:281–287. https://doi.org/10.1139/cjz-2014-0266
Tamò C, Ricard I, Held M et al (2006) A comparison of naïve and conditioned responses of three generalist endoparasitoids of lepidopteran larvae to host-induced plant odours. Anim Biol 56:205–220. https://doi.org/10.1163/157075606777304177
Tan C-W, Peiffer M, Hoover K et al (2018) Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc Natl Acad Sci 115:5199–5204. https://doi.org/10.1073/pnas.1717934115
Tanaka K, Uda Y, Ono Y et al (2009) Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr Biol 19:881–890. https://doi.org/10.1016/j.cub.2009.04.035
Thaler JS (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399:686–688. https://doi.org/10.1038/21420
Thaler JS, Farag MA, Paré PW, Dicke M (2002) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5:764–774. https://doi.org/10.1046/j.1461-0248.2002.00388.x
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452. https://doi.org/10.1146/annurev-ento-020117-043507
Turlings TC, Ton J (2006) Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr Opin Plant Biol 9:421–427. https://doi.org/10.1016/j.pbi.2006.05.010
Turlings TC, Tumlinson JH (1992) Systemic release of chemical signals by herbivore-injured corn. Proc Natl Acad Sci 89:8399–8402. https://doi.org/10.1073/pnas.89.17.8399
Turlings TCJ, Scheepmaker JWA, Vet LEM et al (1990a) How contact foraging experiences affect preferences for host-related odors in the larval parasitoid Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae). J Chem Ecol 16:1577–1589. https://doi.org/10.1007/BF01014091
Turlings TCJ, Tumlinson JH, Lewis WJ (1990b) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250:1251–1253. https://doi.org/10.1126/science.250.4985.1251
Turlings TCJ, Tumlinson JH, Eller FJ, Lewis WJ (1991a) Larval-damaged plants: Source of volatile synomones that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol Exp Appl 58:75–82. https://doi.org/10.1111/j.1570-7458.1991.tb01454.x
Turlings TCJ, Tumlinson JH, Heath RR et al (1991b) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17:2235–2251. https://doi.org/10.1007/BF00988004
Turlings TCL, Wäckers FL, Vet LEM et al (1993) Learning of host-finding cues by Hymenopterous parasitoids. Insect Learn. https://doi.org/10.1007/978-1-4615-2814-2_3
Turlings TC, Loughrin JH, McCall PJ et al (1995) How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci 92:4169–4174. https://doi.org/10.1073/pnas.92.10.4169
Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH (2000) Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera Exigua: isolation and bioactivity. J Chem Ecol 26:189–202. https://doi.org/10.1023/A:1005449730052
Turlings TCJ, Jeanbourquin PM, Held M, Degen T (2005) Evaluating the induced-odour emission of a Bt maize and its attractiveness to parasitic wasps. Transgenic Res 14:807–816. https://doi.org/10.1007/s11248-005-0008-6
Vandermoten S, Francis F, Haubruge E, Leal WS (2011) Conserved odorant-binding proteins from aphids and eavesdropping predators. PLoS ONE 6:e23608. https://doi.org/10.1371/journal.pone.0023608
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172. https://doi.org/10.1146/annurev.en.37.010192.001041
Vet LEM, Groenewold AW (1990) Semiochemicals and learning in parasitoids. J Chem Ecol 16:3119–3135. https://doi.org/10.1007/BF00979615
Vet LEM, Jong RD, Giessen WV, Visser JH (1990) A learning-related variation in electroantennogram responses of a parasitic wasp. Physiol Entomol 15:243–247. https://doi.org/10.1111/j.1365-3032.1990.tb00512.x
Vet LEM, Lewis WJ, Cardé RT (1995) Parasitoid foraging and learning. Chem Ecol Insects 2:65–101. https://doi.org/10.1007/978-1-4615-1765-8_3
Veyrat N, Robert CAM, Turlings TCJ, Erb M (2016) Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J Ecol 104:591–600. https://doi.org/10.1111/1365-2745.12526
Vieira FG, Forêt S, He X et al (2012) Unique features of odorant-binding proteins of the parasitoid wasp Nasonia vitripennis revealed by genome annotation and comparative analyses. PLoS ONE 7:e43034. https://doi.org/10.1371/journal.pone.0043034
Vinson SB (1984) Parasitoid-host relationship. In: Bell WJ, Cardé RT (eds) Chemical ecology of insects. Sinauer Associates Inc., Sunderland, pp 111–124
Vinson SB, Elzen GW, Williams HJ (1987) The influence of volatile plant allelochemics on the third trophic level (parasitoids) and their herbivorous hosts. In: Labeyrie V, Fabres G, Lachaise D (eds) Insects–plants. W. Junk Publishers, Dordrecht, pp 109–114
Vogt RG (2003) 14 - Biochemical diversity of odor detection: OBPs, ODEs and SNMPs. In: Blomquist G, Vogt R (eds) Insect pheromone biochemistry and molecular biology. Academic Press, San Diego, pp 391–445
Vogt RG, Riddiford LM (1981) Pheromone binding and inactivation by moth antennae. Nature 293:161–163. https://doi.org/10.1038/293161a0
Vogt FG, Kiihne AC, Dubnau JT, Prestwich GD (1989) Expression of pheromone binding proteins during antenna development in the Gypsy Moth Lymantria dispar. J Neurosci 9:3332–3346. https://doi.org/10.1523/JNEUROSCI.09-09-03332.1989
Vosshall LB, Stocker RF (2007) Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30:505–533. https://doi.org/10.1146/annurev.neuro.30.051606.094306
Vosshall LB, Amrein H, Morozov PS et al (1999) A spatial map of olfactory receptor expression in the Drosophila Antenna. Cell 96:725–736. https://doi.org/10.1016/S0092-8674(00)80582-6
Vuorinen T, Nerg A-M, Ibrahim MA et al (2004) Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol 135:1984–1992. https://doi.org/10.1104/pp.104.047084
Wang C, Dong J, Tang D et al (2004) Host selection of Helicoverpa armigera and H. assulta and its inheritance. Prog Nat Sci 14:880–884. https://doi.org/10.1080/10020070412331344491
Wang J, Li D-Z, Min S-F et al (2014) Analysis of chemosensory gene families in the beetle Monochamus alternatus and its parasitoid Dastarcus helophoroides. Comp Biochem Physiol D 11:1–8. https://doi.org/10.1016/j.cbd.2014.05.001
Wang S-N, Peng Y, Lu Z-Y et al (2015) Identification and expression analysis of putative chemosensory receptor genes in Microplitis mediator by antennal transcriptome screening. Int J Biol Sci 11:737–751. https://doi.org/10.7150/ijbs.11786
Wang S-N, Shan S, Zheng Y et al (2017a) Gene structure and expression characteristic of a novel odorant receptor gene cluster in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). Insect Mol Biol 26:420–431. https://doi.org/10.1111/imb.12306
Wang Y, Chen Q, Guo J et al (2017b) Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus. Insect Biochem Mol Biol 89:58–70. https://doi.org/10.1016/j.ibmb.2017.09.001
Wang S-N, Shan S, Liu J-T et al (2018) Characterization of antennal chemosensilla and associated odorant binding as well as chemosensory proteins in the parasitoid wasp Microplitis mediator (Hymenoptera: Braconidae). Sci Rep. https://doi.org/10.1038/s41598-018-25996-3
War AR, Paulraj MG, Ahmad T et al (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. https://doi.org/10.4161/psb.21663
Wei J-N, Kang L (2006) Electrophysiological and behavioral responses of a parasitic wasp to plant volatiles induced by two leaf miner species. Chem Senses 31:467–477. https://doi.org/10.1093/chemse/bjj051
Wei J, Wang L, Zhu J et al (2007) Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2:e852. https://doi.org/10.1371/journal.pone.0000852
Wicher D, Schäfer R, Bauernfeind R et al (2008) Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452:1007–1011. https://doi.org/10.1038/nature06861
Wu J, Baldwin IT (2009) Herbivory-induced signalling in plants: perception and action. Plant Cell Environ 32:1161–1174. https://doi.org/10.1111/j.1365-3040.2009.01943.x
Wu H, Hou C, Huang L-Q, et al (2013) Peripheral coding of sex pheromone blends with reverse ratios in two Helicoverpa species. PLoS ONE 8:e70078. https://doi.org/10.1371/journal.pone.0070078
Wu H, Xu M, Hou C et al (2015) Specific olfactory neurons and glomeruli are associated to differences in behavioral responses to pheromone components between two Helicoverpa species. Front Behav Neurosci. https://doi.org/10.3389/fnbeh.2015.00206
Wu H, Li R-T, Dong J-F et al (2018) An odorant receptor and glomerulus responding to farnesene in Helicoverpa assulta (Lepidoptera: Noctuidae). Insect Biochem Mol Biol. https://doi.org/10.1016/j.ibmb.2018.11.006
Xiao Y, Wang Q, Erb M et al (2012) Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol Lett 15:1130–1139. https://doi.org/10.1111/j.1461-0248.2012.01835.x
Xin Z, Yu Z, Erb M et al (2012) The broad-leaf herbicide 2,4-dichlorophenoxyacetic acid turns rice into a living trap for a major insect pest and a parasitic wasp. New Phytol 194:498–510. https://doi.org/10.1111/j.1469-8137.2012.04057.x
Yan ZG, Wang CZ (2006a) Identification of Mythimna separata-induced maize volatile synomones that attract the parasitoid Campoletis chlorideae. J Appl Entomol 130:213–219. https://doi.org/10.1111/j.1439-0418.2006.01055.x
Yan Z-G, Wang C-Z (2006b) Similar attractiveness of maize volatiles induced by Helicoverpa armigera and Pseudaletia separata to the generalist parasitoid Campoletis chlorideae. Entomol Exp Appl 118:87–96. https://doi.org/10.1111/j.1570-7458.2006.00368.x
Yan Z, Yan Y, Wang C (2005) Attractiveness of tobacco volatiles induced by Helicoverpa armigera and Helicoverpa assulta to Campoletis chlorideae. Chin Sci Bull 50:1334–1341. https://doi.org/10.1360/982005-388
Yang K, Liu Y, Niu D-J et al (2016) Identification of novel odorant binding protein genes and functional characterization of OBP8 in Chilo suppressalis (Walker). Gene 591:425–432. https://doi.org/10.1016/j.gene.2016.06.052
Yang K, Huang L-Q, Ning C, Wang C-Z (2017) Two single-point mutations shift the ligand selectivity of a pheromone receptor between two closely related moth species. eLife 6:e29100. https://doi.org/10.7554/eLife.29100
Yu H, Zhang Y, Wyckhuys KAG et al (2010) Electrophysiological and behavioral responses of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ Entomol 39:600–609. https://doi.org/10.1603/EN09162
Zakir A, Bengtsson M, Sadek MM et al (2013) Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J Exp Biol 216:3257–3263. https://doi.org/10.1242/jeb.083188
Zhang S, Chen L-Z, Gu S-H et al (2011) Binding characterization of recombinant odorant-binding proteins from the parasitic Wasp, Microplitis mediator (Hymenoptera: Braconidae). J Chem Ecol 37:189–194. https://doi.org/10.1007/s10886-010-9902-3
Zhang J, Liu CC, Yan SW et al (2013a) An odorant receptor from the common cutworm (Spodoptera litura) exclusively tuned to the important plant volatile cis-3-Hexenyl acetate. Insect Mol Biol 22:424–432. https://doi.org/10.1111/imb.12033
Zhang P-J, Broekgaarden C, Zheng S-J et al (2013b) Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol 197:1291–1299. https://doi.org/10.1111/nph.12106
Zhang Y, Zheng Y, Li D, Fan Y (2014) Transcriptomics and identification of the chemoreceptor superfamily of the pupal parasitoid of the oriental fruit fly, Spalangia endius Walker (Hymenoptera: Pteromalidae). PLoS ONE 9:e87800. https://doi.org/10.1371/journal.pone.0087800
Zhang J, Wang B, Dong S et al (2015) Antennal transcriptome analysis and comparison of chemosensory gene families in two closely related noctuidae moths Helicoverpa armigera and H. assulta. PLoS ONE 10:e0117054. https://doi.org/10.1371/journal.pone.0117054
Zhou C-X, Min S-F, Yan-Long T, Wang M-Q (2015) Analysis of antennal transcriptome and odorant binding protein expression profiles of the recently identified parasitoid wasp, Sclerodermus sp. Comp Biochem Physiol D 16:10–19. https://doi.org/10.1016/j.cbd.2015.06.003
Zhu F, Cusumano A, Bloem J et al (2018) Symbiotic polydnavirus and venom reveal parasitoid to its hyperparasitoids. Proc Natl Acad Sci 115:5205–5210. https://doi.org/10.1073/pnas.1717904115
Acknowledgements
We are grateful to the members of Arthropod-Plant Interactions Editorial Committee for giving us the opportunity to write this review. We are indebted to Dr. Dagmar Voigt from Institute for Botany, Technische Universität, Dresden, Germany and the two anonymous reviewers for their valuable and insightful comments on the early version of this manuscript and we are thankful to our colleagues Dr. Yan-Lan Sun, Dr. Ke Yang, and Nan-Ji Jiang from Institute of Zoology, Chinese Academy of Sciences, Beijing, China for proofreading the manuscript. Our research was funded by the National Key R & D Program of China (Grant Number: 2017YFD020040), the National Natural Science Foundation of China (Grant No. 31471777), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant Number: XDB11010300).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dagmar Voigt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, H., Wang, CZ. The ethological significance and olfactory detection of herbivore-induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod-Plant Interactions 13, 161–179 (2019). https://doi.org/10.1007/s11829-019-09672-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-019-09672-5