Abstract
A growing body of literature reports the importance of non-prey food sources in boosting fitness of arthropod natural enemies, thus further contributing to their pest control efficacy. Although resources such as nectar, pollen, or honeydew have received a fair amount of research attention, little is known about the role of fruit juices. Under natural conditions, Tephritid fruit fly parasitoids enjoy ample access to fallen or damaged fruits and their saccharide-rich juices, and wasp fitness can potentially benefit in multiple ways from access to these resources. In this study, we compared the effect of fruit juice with other food resources on multiple fitness parameters in parasitoids that commonly forage on fallen, damaged fruits: the braconid Diachasmimorpha longicaudata and figitid Aganaspis pelleranoi. Parasitoids were subject to simple or combined diets of guava juice (Psidium guajava), honey and pollen, and their effect on wasp longevity, ovarian dynamics and (body) carbohydrate levels was assessed. For both species, adult longevity proved lowest on simple diets of water, guava juice, or pollen, while greatest longevity was attained on honey or combined diets. For D. longicaudata, egg load did not differ between the various diets, while A. pelleranoi egg load was higher for individuals that had access to honey or pollen, but did not differ between newly emerged wasps and those fed guava juice. In both parasitoid species, total sugars, fructose, and glycogen levels were highest in wasps fed with honey or combined diets and lowest under (simple) guava juice, pollen, or water diets. In conclusion, D. longicaudata and A. pelleranoi attained superior longevity and body nutrient levels with access to high-sucrose sugar sources, such as honey, but benefited comparatively little from access to guava juice. Our work hints the role of high-sucrose foods such as (extra-) floral nectar or artifical sugar sprays in boosting fitness of fruit fly parasitoids. We further discuss the relevance of these findings for fruit fly biological control, in crops such as guava.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many natural enemies, overall fitness, lifetime reproductive success, and ultimate pest control efficacy inherently depend upon their access to key resources, including non-prey foods (e.g., Tylianakis et al. 2004; Heimpel and Jervis 2005). Sugar-rich foods such as (extra-)floral nectar help satisfy energetic requirements of several parasitoids (e.g., Jervis et al. 1996; Wäckers 2005; Lundgren 2009). Access to nectar regularly augments longevity (Idris and Grafius 1995; Wyckhuys et al. 2008), fecundity (Baggen and Gurr 1998; Winkler et al. 2006), and overall activity of parasitic wasps (Takasu and Lewis 1994; Wäckers 1994). Similarly, resources such as pollen provide valuable nutrients for a myriad of natural enemy guilds (Majerus 1994; Canard 2001; Gilbert 1986) and benefit fitness of several parasitoid species (Hickman et al. 1995; Eijs et al. 1998). Next, honeydew is receiving growing attention as a food source for several predators and parasitoids (Wäckers et al. 2008; Dulaurent et al. 2011). However, one sugar-rich food that has received comparatively little attention is fruit pulp or juice (but see Eijs et al. 1998; Bautista et al. 2001; Sivinski et al. 2006; Hein and Dorn 2008; Yokoyama et al. 2011). Fruit juices are widely available in temperate and tropical fruit cropping systems and constitute major sources of multiple sugars (e.g., Brecht and Yahia 2009). In the meantime, countless natural enemies maintain an intimate connection with fruits, with some species even “swimming” in fruit pulp during host foraging (Ovruski 1994). Hence, fruit juices could be essential, yet under-studied, food resources for some of the natural enemies of high-profile pests, such as Tephritid fruit flies.
Fruit flies (Diptera: Tephritidae) are key pests of horticultural crops in the (sub-)tropics (White and Elson-Harris 1992; Aluja 1993). To control these pests, insect parasitoids have been used in many parts of the Americas (e.g., Ovruski et al. 2000; Montoya et al. 2009). Even though past efforts greatly promoted the mass release of exotic parasitoids, native species are increasingly incorporated in current biological control programs (Ovruski et al. 2000; Palenchar et al. 2009). The parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) has been released in many parts of the globe for control of Ceratitis capitata, Bactrocera dorsalis, and several Anastrepha species (Montoya et al. 2003; Wang and Messing 2004; Cancino et al. 2009). Unlike some other fruit fly parasitoids, D. longicaudata forages both on infested fruit on the tree and on damaged fruit on the ground (Purcell et al. 1994). This parasitoid attacks a range of fruit fly pests in guava (Psidium guajava) orchards, among others (e.g., Purcell et al. 1998). On the other hand, Aganaspis pelleranoi (Hymenoptera: Figitidae) is a native parasitoid in the Neotropics and attacks fruit fly species, such as the tephritids C. capitata and Anastrepha spp. and the lance fly Neosilba batesi (Wharton et al. 1998; Ovruski et al. 2000). In many parts of Latin America, A. pelleranoi is a key parasitoid of fruit flies on guava (Nuñez et al. 2004). This parasitoid commonly forages for hosts in fallen fruits (Ovruski et al. 2004), with female wasps penetrating the fruit through cracks and crevices and moving within the pulp in search of host larvae (Ovruski 1994). Similar to D. longicaudata, this parasitoid shows a favor for fruit juices from several fruit crops.
Several researches have shown the nutritional benefits of fruit juice or pulp for a limited set of parasitoid species. For example, Hyssopus pallidus, an ecto-parasitoid of coddling moth, experiences increased longevity and fecundity with access to apple juice (Hein and Dorn 2008). Similarly, D. longicaudata increased longevity and egg load when provided access to orange and peach juice (Sivinski et al. 2006). Lastly, Eijs et al. (1998) indicated that parasitoids of Drosophilidae increased longevity and fecundity through (adult) feeding on natural host substrates or honey. In contrast, other research showed no fitness benefits of access to (ripe) coffee juice or cut olive fruits for the parasitoids Fopius arisanus or Psyttalia humilis, respectively (Bautista et al. 2001; Yokoyama et al. 2011). Not only is little known about the comparative importance of distinct food resources for fitness of fruit fly parasitoids, but the few existing research endeavors have yielded starkly contrasting results. Lastly, it has been hypothesized that parasitoids that forage for hosts on fallen or damaged fruits rely more on fruit juice to sustain their fitness.
In this study, we investigated fitness implications of access to simple and combined diets of guava juice, (bee) pollen, and honey for the parasitoids A. pelleranoi and D. longicaudata. Under laboratory conditions, we assessed effects of the different diets on the following fitness currencies: longevity, egg load, and body nutrient levels. A profound understanding of the effect of these distinct food sources on performance of fruit fly parasitoids should further contribute to biological control of pests that are of global relevance.
Materials and methods
Insects
During January–July 2009, an A. pelleranoi laboratory colony was established by collecting adult parasitoids and (parasitized) Anastrepha striata (Diptera: Tephritidae) larvae from guava orchards in the townships of Velez (6°01′N, 73°41′W) and Barbosa (05°56′N, 73°37′W) in the territorial department of Santander (Colombia). Fruit fly larvae were transported to the laboratory and incubated at 25 ± 2°C, 70 ± 10% RH and 12:12 h l:D until parasitoid emergence. Adult D. longicaudata wasps were obtained from colonies at the Moscafrut Program facilities (Chiapas, México) (e.g., Montoya et al. 2000). Experimental colonies were kept at the Entomology Laboratory of the Universidad Jorge Tadeo Lozano (Chía, Colombia) and at the Biological Control Department in the Moscafrut Program.
Parasitoids were maintained on third instar Anastrepha obliqua, obtained from laboratory colonies at the Instituto Colombiano Agropecuario (ICA) in Mosquera and Ibague (Colombia) where they were reared on artificial diet (Artiaga-López et al. 2004). Fourteen-day old A. obliqua larvae were separated from artificial diet through washing with sterile distilled water. Larvae were subsequently placed in 10 cm diam. ring-type oviposition devices inside 30 cm × 30 cm × 30 cm fiberglass cages and exposed to 5–15-day-old adult parasitoids. Parasitoids were allowed to oviposit A. obliqua larvae exposed over the course of 24 h, after which parasitized larvae were extracted from oviposition devices and transferred to plastic trays with moistened vermiculite to permit pupation. Newly emerged parasitoids were transferred to a 30 cm × 30 cm × 30 cm plexiglass cage, allowed access to water and fed ad libitum with diluted honey. Parasitoid colonies were maintained in climate-controlled growth chambers at 25 ± 2°C, 70 ± 10% RH and 12:12 h l:D. Experimental trials were conducted under identical climatic conditions.
Longevity
Newly emerged, unmated wasps of either species were individualized within 90 ml cylindrical plastic containers. Each container was equipped with a 1 cm diam. ventilation opening and either one of the following food sources was provided: (1) honey, (2) pollen, (3) guava juice, (4) pollen, honey and guava juice, or (5) nothing. All wasps were allowed ample access to water.
Honey and sterile water solution (1:1, Natural Honey, Productos El Dorado S.A, Bogotá) was applied on separate cotton wicks that were inserted within the container. Approximately 100 mg of commercially available (honeybee) pollen was pulverized and brushed on moistened filter paper at the bottom of the container. Guavas were peeled and blended with sterile water. The resulting guava juice was applied on cotton balls that were placed in a 1 cm diam. opening in the lid. On a daily basis, food was replenished and wasp mortality in each container recorded. A total of 20 replicates were done for each diet, parasitoid species, and sex.
Carbohydrate metabolism (anthrone analysis)
Newly emerged, unmated parasitoid males and females were subject to the different diets, using the above procedures. For each diet, we collected a total of ten individuals at each of the following times: 2, 4, 6, 8, and 10 days. Each wasp was gently placed within a 1.5 ml microcentrifuge tube and frozen at—20°C. Additionally, twenty newly emerged wasps (10 females and 10 males) from each species were collected and frozen at −20°C. Wasps that died during the course of the experiment were not included in the experiment.
We determined the amounts of glycogen, total sugars, and fructose in each wasp using a series of biochemical tests (Olson et al. 2000; Fadamiro and Heimpel 2001; Lee et al. 2004). Procedures are similar to the ones in Wyckhuys et al. (2008). In brief, each wasp specimen was placed within a 1.5 ml Eppendorf tube, to which 50 μl of 2% sodium sulfate was added. Wasps were not surface-sterilized, possibly causing minor contamination from wasps walking in the different diets. Wasps were crushed using a glass capillary, which was subsequently washed with 250 μl of methanol:chloroform (2:1). Next, tubes were centrifuged at 13,000 r.p.m. for 3 min. The resulting supernatant was transferred to another microcentrifuge tube for fructose analysis, while the precipitate was used for analysis of glycogen.
To assess body glycogen content, we added 1,000 μl of anthrone reagent (Product# A1631-25G, Sigma, Saint Louis, MO, USA) to the tube containing the precipitate. Next, the solution was mixed and heated to 90°C in a dry bath incubator for 15 min. After heating, tubes were placed in crushed ice for 15 min. Aliquots of 200 μl were pipetted in each of the 96-well plate, and absorbance was read at 620 nm using an ELISA reader (Sensident Scan, Merck, Germany). To determine fructose content (i.e., cold anthrone assay), we heated 200 μl of the supernatant in a dry bath incubator at 90°C for 30 min or until the liquid has evaporated. Tubes were placed in crushed ice for 15 min. Subsequently, we added 950 μl of anthrone reagent to each tube and heated them to 34°C for 1 h. The resulting solution was pipetted into 96-well plate and the absorbance was read at 620 nm. For total sugar assays (i.e., hot anthrone test), cold anthrone solutions were pippeted back into their respective Eppendorf tubes. Next, tubes were heated for 15 min at 90° C and cooled on crushed ice for 15 min. Again, samples were transferred into a 96-well plate and absorbance was read at 620 nm.
Standard curves were generated to convert absorbance readings into absolute amounts (mg) for each of the different sugars. For this purpose, we prepared glucose, fructose, and sucrose solutions at 1, 5, 10, 20, 30, 40, and 50 mg, with addition of 1 ml anthrone reagent. For glycogen (Product# G8876, Sigma, Saint Louis, MO, USA), solutions were prepared with 1, 5, 10, 25, 50, 75, and 100 mg, with addition of 1 ml anthrone reagent. Solutions were transferred to an ELISA plate and read at 620 nm. Per concentration, we ran a total of 3 replicates. Standard curves were obtained through linear regression.
Egg load
To examine the effect of food resources on parasitoid egg load, newly emerged females were individualized in a 90-ml plastic container and subjected to distinct diet regimes (as above). For each diet and species, a total of 10 females were collected at ages 2, 4, 6, 8, and 10 days. Females were dissected immediately, as such: the ovipositor was gently pulled using fine needles, until both the ovipositor and the ovaries became detached from the abdomen. The ovaries were subsequently placed on a Petri dish with a 1 ml of ringer solution (NaCl 6 g/l; C3H5NaO3 3.1 g/l; KCl 0.3 g/l; CaCl2·2H2O 0.2 g/l) and examined under a stereoscope. Ovaries were pressed with fine needles until eggs were expulsed, and fully matured eggs were counted for each of the wasps (e.g., Olson et al. 2000; Oskan 2007; Riddick 2007).
Statistical analysis
The effect of diet and sex on wasp longevity was analyzed using proportional hazard models (Lee et al. 2004; Fadamiro and Chen 2005). In the first analysis, we compared sugar levels present in newly emerged wasps (i.e., day 0) versus those at the end of their lives (or day 10), under the distinct diet regimes. Nutrient levels were compared using ANOVA, followed by a Tukey’s HSD post hoc test. In the second analysis, we assessed the effects of diet, age, and diet × age interaction on fructose, glycogen, and total sugars content, using multiple linear regression. Multiple regression was also used to determine the effect of diet regime upon egg load in both parasitoid species. Regression models were included with different diet types and age, and model coefficients were reported. Prior to analysis, we assessed normality and homoscedasticity of the data set and carried out logarithmic transformations, if necessary. Statistical analysis was done using absolute values instead absorbance values. All statistics were conducted using SPSS.
Results
Longevity
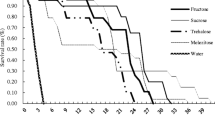
For D. longicaudata, greatest longevity was recorded for honey and complete (i.e., honey, pollen, guava juice) diet, with wasps attaining maximum longevity of 32 days (Fig. 1). Mean longevity of D. longicaudata with access to pollen and guava juice did not differ from that of wasps with sole access to water (Table 1). Proportional hazard models indicated a significant effect of diet on D. longicaudata longevity (X 2 = 4.966, df = 1, P = 0.026), while no effect was registered of wasp sex (X 2 = 1.80, df = 1, P = 0.18). Similarly, the interaction sex × diet did not affect longevity (X 2 = 0.007, df = 1, P = 0.934).
Similar results were found for A. pelleranoi, with wasps attaining maximum longevity of 57 days for honey and complete diet (Fig. 2). Longevity of A. pelleranoi with access to guava juice and pollen did not differ from that of wasps with sole access to water (Table 1). Significant effects on A. pelleranoi longevity were recorded for diet (X 2 = 14.652, df = 1, P < 0.001), sex (X 2 = 4.962, df = 1, P = 0.026), and a diet × sex interaction (X 2 = 13.401, df = 1, P < 0.001).
Carbohydrate metabolism
Both female and male D. longicaudata emerged with low total sugar levels, being 10.95 ± 21.02 μg, 12.07 ± 15.95 μg (mean ± SEM); respectively. With progressing parasitoid age, different patterns were recorded in total sugar levels depending upon diet (Fig. 3). Nevertheless, no significant regression was found between age and total sugar levels for different diets. Significant differences were recorded in endpoint total sugar levels in both female and male D. longicaudata (females F = 42.97, df = 5, P < 0.001; males F = 12.16, df = 5, P < 0.001). Endpoint total sugar levels were highest in wasps provided with access to honey, but did not differ between newly emerged wasps and those with access to water or guava juice (Table 2). Diet significantly affected total sugar levels in both males and females, when comparing diets of honey versus guava juice and honey versus pollen (Table 3). However, when comparing pollen versus guava juice diets, neither diet, age, nor a diet × age interaction affected total sugar levels in males. For females, when comparing pollen versus guava juice, diet significantly affected total sugar levels.
Total sugar levels (±SE) in female Diachasmimorpha longicaudata that were newly emerged and under five diets over a period of 10 days (female emergence n = 10; honey n = 9, 9, 10, 9, 10; pollen n = 8, 10, 8; guava juice n = 9, 8, 9; honey + pollen + guava juice + water n = 10, 9, 8, 10, 9; water n = 8, 9, 10)
Newly emerged D. longicaudata had fructose levels that were close to zero. With access to different food sources, body fructose levels gradually incremented (Fig. 4). For example, honey-fed wasps showed fructose levels consistently higher to 1 μg (Table 2). No significant regression was found between age and fructose levels for different diets. Diet significantly affected body fructose levels, when comparing honey vs. guava juice and honey vs. pollen (Table 3). However, when comparing pollen vs. guava juice diets, neither diet, age, nor a diet × age interaction affected fructose levels in males. For females, when comparing pollen vs. guava juice, diet significantly affected fructose levels.
Fructose levels (±SE) in female Diachasmimorpha longicaudata that were newly emerged and under five diets over a period of 10 days (female emergence n = 10; honey n = 9, 9, 10, 9, 10; pollen n = 8, 10, 8; guava juice n = 9, 8, 9; honey + pollen + guava juice + water n = 10, 9, 8, 10, 9; water n = 8, 9, 10)
Glycogen levels in newly emerged male and female D. longicaudata were 65.37 ± 37.14 μg and 38.40 ± 16.72 μg, respectively. With access to different food sources, glycogen levels gradually incremented (Fig. 5), although age did not significantly affect glycogen levels for different diets. Endpoint glycogen levels in newly emerged wasps did not differ from those in water, pollen- and guava juice-fed wasps (females F = 22.40, df = 5, P < 0.001, males F = 5.16, df = 5, P < 0.001). Endpoint glycogen levels in honey-fed females and males were significantly higher than those in pollen- and guava juice-fed wasps (Table 2). Diet significantly affected glycogen levels, when comparing honey versus guava juice and honey versus pollen (Table 3). However, when comparing pollen versus guava juice diets in males, diet did not affect glycogen levels. For females, diet significantly affected glycogen levels.
Glycogen levels (±SE) in female Diachasmimorpha longicaudata that were newly emerged and under five diets over a period of 10 days (female emergence n = 10; honey n = 9, 9, 10, 9, 10; pollen n = 8, 10, 8; guava juice n = 9, 8, 9; honey + pollen + guava juice + water n = 10, 9, 8, 10, 9; water n = 8, 9, 10)
In A. pelleranoi, both females and males emerged with low total sugar levels (Females: 4.18 ± 3.93 μg; Males: 2.20 ± 2.16 μg). Different patterns were recorded in total sugar levels, depending upon diet (Fig. 6). Age did not affect total sugar levels for different diets. Endpoint total sugar levels were significantly higher in honey-fed wasps, while no differences were found between newly emerged wasps and those fed guava juice (females F = 29.12, df = 5, P < 0.001; males F = 31.36, df = 5, P < 0.001) (Table 2). Diet significantly affected total sugar levels, when comparing when honey vs. guava juice and honey vs. pollen (Table 4).
Fructose levels in newly emerged A. pelleranoi attained values close to zero. With access to the various food resources, body fructose levels gradually incremented (Fig. 7), although age did not have a significant effect on fructose levels for different diets. Endpoint fructose levels in newly emerged wasps did not differ from those fed with guava juice or with access to water (Table 2). Diet significantly affected fructose levels, when comparing honey versus guava juice and honey versus pollen (Table 4).
Fructose levels (±SE) in female (a) and male (b) Aganaspis pelleranoi that were newly emerged and under five diets over a period of 10 days (female emergence n = 10; honey n = 10, 9, 10; pollen n = 10, 10, 10; guava juice n = 10, 10, 9; honey + pollen + guava juice + water n = 10, 9, 10; water n = 9, 9, 9)
In newly emerged female and male A. pelleranoi, glycogen levels were 42.53 ± 24.20 μg and 41.01 ± 9.33 μg. Under certain diet regimes, glycogen levels rapidly increased (Fig. 8), although age did not affect these levels for different diets. Endpoint glycogen levels of honey-fed wasps were significantly higher than for those with access to guava juice, pollen, or water (females F = 9.26, df = 5, P < 0.001; males F = 11.93, df = 5, P < 0.001) (Table 2). However, endpoint levels did not differ between newly emerged wasps and those fed pollen, guava juice, or water (Fig. 8). Diet significantly affected glycogen levels, when comparing honey versus guava juice and honey versus pollen (Table 4). No effect was found of diet on glycogen levels in females, comparing guava juice versus pollen. For males though, significant effects were recorded (Table 4).
The relative importance of the different diets for each of the body nutrients is exemplified in Table 5. For both wasp species, the effect of honey and mixed diets on body nutrient levels was highly significant. Access to guava juice only had a small but significant effect on glycogen levels in males of either species. Lastly, regression models showed a significant effect of pollen on body sugar levels of D. longicaudata females and both sexes of A. pelleranoi (Table 5).
Egg load
Age significantly affected egg load in D. longicaudata (F = 42.83, df = 7, P < 0.001), with a minimum number of mature eggs found in newly emerged females (Fig. 9; Table 6). Similarly, in A. pelleranoi, age had a significant effect on maturation of eggs (F = 8.12, df = 5, P < 0,001) (Fig. 9; Table 6). Also, diet significantly affected egg load, when comparing honey versus guava juice and guava juice versus pollen.
Discussion
Throughout the world, parasitic wasps have been widely used for biological control of a diversity of fruit fly pests (e.g., Ovruski et al. 2000), and associated mass rearing and release protocols have been broadly investigated. However, basic studies on parasitoid biology and ecology, all of which directly determine parasitoid efficacy, have received far less attention. Seminal work by Bautista et al. (2001), Sivinski et al. (2006), and Yokoyama et al. (2011) all point at the key role that food sources can play in boosting fitness of certain fruit fly parasitoids. Although ecological particularities of the two fruit fly parasitoids D. longicaudata and A. pelleranoi infer recurrent contact of adult wasps with fruit pulp or juice, our work showed that both parasitoids only receive minimal benefits from access to juice of one of their principal host plants, that is, P. guajava. Guava juice did not increase body nutrient levels, nor augment longevity for either species, while bringing about very modest increases in egg load only for A. pelleranoi. On the other hand, both parasitoids enjoyed substantial increments in all fitness parameters through consumption of high-carbohydrate food resources such as honey.
For both parasitoids, honey more than doubled adult longevity compared with wasps with access to water or guava juice. The short lifespan of D. longicaudata and A. pelleranoi on guava juice can possibly be attributed to the type and concentration of different sugars (e.g., Lee et al. 2004; Chen and Fadamiro 2006; Wu et al. 2008; Wyckhuys et al. 2008; Luo et al. 2010; Aung et al. 2010). Compared to honey, guava juice provides relatively small amounts of sugars, such as glucose (1.30–3.04%), fructose (1.75–3.53%), and sucrose (0.81–4.21%) (De Moreno et al. 1995). Although braconid wasps are thought to assimilate all different sugar types, their content in guava juice may be too low to cause clear increases in parasitoid longevity. As indicated by Sivinski et al. (2006) and Yokoyama et al. (2011), orange juice and pulp cause fruit fly parasitoids to attain a lifespan similar to that on honey, while feeding on peach pulp led to vast increases in longevity compared with water. In a similar fashion, apple juice greatly increased longevity of the H. pallidus (Hein and Dorn 2008). Possibly, guava juice provides less sugar than apple juice, peach pulp, or orange juice, but analyses may be needed to further corroborate such. Aside from providing relatively low amounts of sugars (as compared to honey), guava juice possibly also contains secondary compounds that interfere with herbivores or higher trophic levels. Many fruits contain phytochemicals such as phenolics, flavonoids, or carotenoids (e.g., Sun et al. 2002), some of which act in a repellent or even toxic fashion against certain insects (e.g. Ding et al. 2000). Given that guava has one of the highest contents of phenols (Patthamakanokporn et al. 2008), the nutritional benefit of access to saccharides in its juice could potentially be offset by those compounds. Lastly, low longevity on guava juice may also hint that D. longicaudata and A. pelleranoi are unable to metabolize P. guajava sugars.
Access to saccharide-rich foods such as honey led to vast increases in body nutrient levels for both parasitoids. Similar results were obtained by Olson et al. (2000), Fadamiro and Heimpel (2001) and Lee et al. (2004). No evidence was found for a mobilization of fructose and glycogen to total sugars (see Fadamiro and Heimpel 2001), nor for a sustained increase in body sugar levels with age, as in Diadegma insulare (Lee et al. 2004). Honey-fed wasps had consistently higher sugar levels than those with access to guava juice, thus further strengthening the assumption that guava provides comparatively little sugar to both parasitoids. Glycogen levels also considerably increased following honey feeding, which may hint at increased nutrient storage (Boggs 1997; Lee et al. 2004) and stayed relatively constant over time (e.g., Rivero and West 2002). Females of both parasitoids emerged with a limited number of mature eggs, as is standard in synovigenic wasps (Thompson 1999). Females of D. longicaudata emerged with 24.0 ± 14.6 mature eggs, similar amounts as in the ichneumonid D. insulare (Lee et al. 2004). In contrast, A. pelleranoi females emerged with 102.0 ± 16.4 mature eggs. Those initial eggs primarily matured using fat reserves acquired during the immature stage (Rivero and Casas 1999). Especially for A. pelleranoi, the large number of initial eggs reflects a substantial energy storage capacity in immatures and, for adults, an ability to oviposit a large number of eggs in a short period of time. For the parasitoid D. longicaudata, diet did not affect potential fecundity or egg load, while for A. pelleranoi, both honey and pollen diets significantly increased egg load in comparison with water or guava juice. For several insect parasitoids, sugar feeding has been reported to benefit egg maturation. For example, females of Ichneumon promissorius (Hymenoptera: Ichneumonidae) greatly increased egg load through consumption of honey (Wade et al. 2008). Alternative food sources such as orange juice augmented D. longicaudata egg load (Sivinski et al. 2006). In our study, guava juice did not benefit egg maturation of either fruit fly parasitoid. Although guava juice did not increase A. pelleranoi longevity nor boost parasitoid energy levels, it did, however, provide sufficient nutrients for a sustained (relatively low) fecundity over time. Contrastingly, ovarian dynamics for both species were similar under pollen and honey diets, as such hinting that pollen provides key nutrients for egg maturation in fruit fly parasitoids. Similar findings were made for different species of syrrphid flies (Irvin et al. 1999; Hickman et al. 1995) and the ichneumonid D. insulare (Lee et al. 2004). Our findings confirm that, even for fruit fly parasitoids with ample access to fruit juices, pollen may provide essential nutrients to parasitoids.
Some of our findings, such as the possible positive effect of pollen on ovarian dynamics, could have been affected by experimental protocols. Concern may exist that applying pollen on wet filter paper can considerably change its nature. However, pollen is frequently consumed by parasitoid Hymenoptera when contaminating nectar, honeydew, and water sources (Jervis 1998). Also, other researchers have mixed pollen with other liquids such as honey or water and quantified its impact on key life history traits of parasitoids (Zhang et al. 2004; Geng et al. 2006). For example, mixing pollen with water improved longevity of Trichogramma brassicae over water alone (Zhang et al. 2004). Secondly, presence of pollen, guava juice, and honey in the same container (in combined diet treatments) not necessarily means that parasitoids actually fed on all food sources. Possibly, wasps may exhibit preference for some food source (e.g., Leius 1960), with observed fitness benefits reflecting the effect of this food. Follow-up research could help clarify whether A. pelleranoi or D. crawfordi exhibit feeding preferences for guava juice, honey, or pollen, and if (relatively) low-quality foods such as guava juice are avoided when having access to others.
Fruit juices may constitute valuable alternative food sources, as orange, peach, or apple juice all increase parasitoid fitness (e.g., Sivinski et al. 2006; Yokoyama et al. 2011), while wasps enjoy ample access to them during host foraging. However, our elucidation of the relatively poor effect of guava juice indicates that plant species vary considerably in their effect upon natural enemies (e.g., Ode 2006) and that not all fruit crops benefit fruit fly parasitoids to a similar extent. Parasitoid performance in fruit orchards will thus greatly depend upon the crop and the broader accessibility of alternative food sources such as (extra-)floral nectar within the orchard. The outspoken fitness benefit of high-saccharide food sources such as honey indicates that other non-prey foods could be of much greater importance for D. longicaudata and A. pelleranoi, in guava orchards. Consequently, research is urgently needed to assess whether flowering plants or plants with extra-floral nectaries provide much-needed sugars to foraging parasitoids (e.g., Lavandero et al. 2005; Berndt et al. 2006; Lee et al. 2006; Lee and Heimpel 2008). In a limited screening, Sivinski et al. (2006) showed that D. longicaudata only reaps marginal benefits from access to flowering plants, but more comprehensive research waits to be conducted. Also, one has to ensure that increased parasitoid fitness is readily translated into superior pest control efficacy. Along the same lines, it may be worthwhile to revisit the potential of (artificial) sugar sprays in attracting or boosting the performance of fruit fly parasitoids (see Canas and O’Neil 1998; Jacob and Evans 1998; Lundgren 2009). Even though Sivinski et al. (2006) saw little use for the rather elaborate scheme of sugar sprays, such activity may carry particular value in fruit crops that by themselves do not provide sufficient resources to natural enemies. In guava orchards, sugar sprays possibly could be important for parasitoids that receive few resources from flowering plants (Sivinski et al. 2006). Our works shows that habitat manipulation and artificial sugar sprays should merit additional research and may constitute valuable components of integrated pest management packages for fruit fly in smallholder crops (see also Bautista et al. 2001). In the meantime, our findings can help improve mass rearing and boost efficacy of augmentative release programs for both parasitoids.
References
Aluja M (1993) Manejo integrado de moscas de la fruta. Trillas, Mexico DF
Aung KSD, Takagi M, Ueno T (2010) Influence of food on the longevity and egg maturation of the egg parasitoid Ooencyrtus nezarae (Hymenoptera: Encyrtidae). J Fac Agr Kyushu Univ 55:79–81
Baggen LR, Gurr GM (1998) The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculetta (Lepidoptera: Gelechiidae). Biol Control 11:9–17
Bautista RC, Harris EJ, Vargas RI (2001) The fruit fly parasitoid Fopius arisanus: reproductive attributes of pre-released females and the use of added sugar as a potential food supplement in the field. Entomol Exp Appl 101:247–255
Berndt LA, Wratten SD, Scarratt SL (2006) The influence of floral resource subsidies on parasitism rates of leafrollers (Lepidoptera: Tortricidae) in New Zealand vineyards. Biol Control 37:50–55
Boggs CL (1997) Dynamics of reproductive allocation from juvenile and adult feeding: radiotracer studies. Ecology 78:192–202
Brecht JK, Yahia EM (2009) Postharvest Physiol. In: Litz RE (ed) Mango: botany, production and uses, 2nd edn. CAB International, Wallingord, pp 484–528
Canard M (2001) Présence en Grece continentale de Chrysoperla carnea (Stephens, 1836) sensu stricto (Neuroptera, Chrysopidae). Bull Soc Entomol Fr 106:416
Canas L, O’Neil R (1998) Applications of sugar solutions to maize, and the impact of natural enemies on fall armyworm. Int J Pest Manag 44:59–64
Cancino J, Ruiz L, Montoya P, Harris E (2009) Biological attributes of three introduced parasitoids as natural enemies of fruit flies, genus Anastrepha (Diptera: Tephritidae). J Appl Entomol 133:181–188
Chen L, Fadamiro HY (2006) Comparing the effects of five naturally occurring monosaccharide and oligosaccharide sugars on longevity and carbohydrate nutrient levels of a parasitic phorid fly, Pseudacteon tricuspis. Physiol Entomol 31:46–56
De Moreno L, Marín M, De Rincón C, Sandoval L (1995) Determinación por HPLC de los azúcares en los frutos de guayaba (Psidium guajava L.) de una plantación comercial del Municipio Mara. Rev Fac Agron (LUZ) 12:467–483
Ding H, Lamb RJ, Ames N (2000) Inducible production of phenolic acids in wheat and antibiotic resistance to Sitodiplosis mosellana. J Chem Ecol 26:969–985
Dulaurent AM, Rossi JP, Deborde C, Moing A, Menassieu P, Jactel H (2011) Honeydew feeding increased the longevity of two egg parasitoids of the pine processionary moth. J Appl Entomol 135:184–194
Eijs IEM, Ellers J, Van Duinen GJ (1998) Feeding strategies in drosophilid parasitoids: the impact of natural food resources on energy reserves in females. Ecol Entomol 23:133–138
Fadamiro HY, Chen L (2005) Utilization of aphid honeydew and floral nectar by Pseudacteon tricuspis (Diptera: Phoridae), a parasitoid of imported fire ants, Solenopis spp. (Hymenoptera: Formicidae). Biol Control 34:73–82
Fadamiro HY, Heimpel GE (2001) Effects of partial sugar deprivation on lifespan and carbohydrate mobilization in the parasitoid Macrocentrus grandii (Hymenoptera: Braconidae). Ann Entomol Soc Am 94:909–916
Geng JH, Shen ZR, Song K, Zheng L (2006) Effect of pollen of regular cotton and transgenic Bt + CpTI cotton on the survival and reproduction of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae) in the laboratory. Environ Entomol 35:1661–1668
Gilbert FS (1986) Hoverflies. Cambridge University Press, Cambridge
Heimpel GE, Jervis MA (2005) An evaluation of the hypothesis that floral nectar improves biological control by parasitoids. In: Wäckers FL, van Rijn P, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 267–304
Hein S, Dorn S (2008) The parasitoid of a fruit moth caterpillar utilizes fruit components as nutrient source to increase its longevity and fertility. Biol Control 44:341–348
Hickman JM, Lövei GL, Wratten SD (1995) Pollen feeding by adults of the hoverfly Melanostoma fasciatum (Diptera: Syrphidae). N Z J Zool 22:387–392
Idris A, Grafius E (1995) Wildflowers as nectar sources for Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera: Yponomeutidae). Environ Entomol 24:1726–1735
Irvin NA, Wratten SD, Frampton CM, Bowie MH, Evans AM, Moar NT (1999) The phenology and pollen feeding of three hover fly (Diptera: Syrphidae) species in Canterbury, New Zealand. N Z J Zool 26:105–115
Jacob H, Evans E (1998) Effects of sugar spray and aphid honeydew on field populations of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ Entomol 27:1563–1568
Jervis MA (1998) Functional and evolutionary aspects of mouthpart structure in parasitoid wasps. Biol J Linnean Soc 63:461–493
Jervis MA, Kidd NAC, Heimpel GE (1996) Parasitoid adult feeding behaviour and biocontrol-a review. Biocon News Inf 17:11–22
Lavandero B, Wratten S, Shishehbor P, Worner S (2005) Enhancing the effectiveness of the parasitoid Diadegma semiclausum (Helen): movement after use of nectar in the field. Biol Control 34:152–158
Lee JC, Heimpel GE (2008) Floral resources impact longevity and oviposition rate of a parasitoid in the field. J Anim Ecol 77:565–572
Lee JC, Heimpel GE, Leibee GL (2004) Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol Exp Appl 111:189–199
Lee JC, Andow DA, Heimpel GE (2006) Influence of floral resources on sugar feeding and nutrient dynamics of a parasitoid in the field. Ecol Entomol 31:470–480
Leius K (1960) Attractiveness of different foods and flowers to the adults of some Hymenopterous parasites. Can Entomol 92:369–376
Lundgren J (2009) Relationships of natural enemies and non-prey foods. Springer International, Dordrecht
Luo S, Li J, Liu X, Lu Z, Pan W, Zhang Q (2010) Effects of six sugars on the longevity, fecundity and nutrient reserves of Microplitis mediator. Biol Control 52:51–57
Majerus MEN (1994) Ladybirds. The new naturalist series, vol 81. Harper Collins, London
Montoya P, Liedo P, Benrey B, Cancino J, Barrera JF, Sivinski J, Aluja M (2000) Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol Control 18:216–224
Montoya P, Benrey B, Barrera JF, Zenil M, Ruiz L, Liedo P (2003) Oviposition behavior and conspecific host discrimination in Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a fruit fly parasitoid. Biocon Sci Technol 13:683–690
Montoya P, Suárez A, López F, Cancino J (2009) Fopius arisanus oviposition in four Anastrepha fruit fly species of economic importance in Mexico. Biocontrol 54:437–444
Nuñez L, Gómez R, Guarín G, León G (2004) Moscas de las frutas (Díptera: Tephritidae) y parasitoides asociados con Psidium guajava L. y Coffea arabica L. en tres municipios de la Provincia de Vélez (Santander, Colombia) parte 2: identificación y evaluación de parasitoides del Orden Hymenoptera. Rev Corpoica 5:1
Ode PJ (2006) Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Ann Rev Entomol 51:163–185
Olson DM, Fadamiro H, Lundgren J, Heimpel G (2000) Effect of sugar feeding on carbohydrate and lipid metabolism in a parasitoid wasp. Physiol Entomol 25:17–26
Ovruski SM (1994) Comportamiento en la detección del huésped en Aganaspis pelleranoi (Brèthes) (Hymenoptera: Cynipoidea, Eucoilidae) parasitoide de larvas de Ceratitis capitata (Wied.) (Diptera: Tephritidae). Rev Soc Entomol Argentina 53:121–127
Ovruski SM, Aluja M, Sivinski J, Wharton R (2000) Hymenopteran parasitoids on fruit infesting Tephritidae (Diptera) in Latin America and the southern United States: diversity, distribution, taxonomic status and their use in fruit fly biological control. Int J Pest Manag 5:81–107
Ovruski SM, Schliserman P, Aluja M (2004) Indigenous parasitoids (Hymenoptera) attacking Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in native and exotic host plants in Northwestern Argentina. Biol Control 29:43–57
Ozkan C (2007) Effect of food, light and host instar on the egg load of the synovigenic endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae). J Pest Sci 80:79–83
Palenchar J, Holler T, Moses-Rowley A, Mcgovern R, Sivinski J (2009) Evaluation of irradiated caribbean fruit fly (Diptera: Tephritidae) larvae for laboratory rearing of Doryctobracon areolatus (Hymenoptera: Braconidae). Florida Entomol 92:535–537
Patthamakanokporn O, Puwastein P, Nitihamyong A, Sirichakwal PP (2008) Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J Food Compos Anal 21:241–248
Purcell MF (1998) Contribution of biological control to integrated pest management of tephritid fruit flies in the tropic and subtropics. Integr Pest Manag Rev 3:63–83
Purcell MF, Jackson CG, Long JP, Batchelor MA (1994) Influence of guava ripening on parasitism of the Oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), by Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) and other parasitoids. Biol Control 4:396–403
Riddick EW (2007) Influence of honey and maternal age on egg load of lab-cultured Cotesia marginiventris. Biocontrol 52:613–618
Rivero A, Casas J (1999) Incorporating physiology into parasitoid behavioral ecology: the allocation of nutritional resources. Res Popul Ecol 41:39–45
Rivero A, West SA (2002) The physiological costs of being small in a parasitic wasp. Evol Ecol Res 4:407–420
Sivinski J, Aluja M, Holler T (2006) Food sources for adult Diachasmimorpha longicaudata, a parasitoid of tephritid fruit flies: effects on longevity and fecundity. Entomol Exp Appl 118:193–202
Sun J, Chu Y, Wu X, Liu RH (2002) Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem 50:7449–7454
Takasu K, Lewis WJ (1994) Importance of adult food sources to host searching of the larval parasitoid Microplitis croceipes. Biol Control 5:25–30
Thompson SN (1999) Nutrition and culture of entomophagous insects. Annu Rev Entomol 44:561–592
Tylianakis JM, Didham RK, Wratten SD (2004) Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85:658–666
Wäckers FL (1994) The effect of food deprivation on the innate visual and olfactory preferences in the parasitoid Cotesia rubecula. J Insect Physiol 40:641–649
Wäckers FL (2005) Suitability of (extra-) floral nectar, pollen, and honeydew as insect food sources. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University, Cambridge, pp 17–74
Wäckers FL, Van Rijn PCJ, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal? Biol Control 45:176–184
Wade MR, Hopkinson JE, Zalucki MP (2008) Influence of food supplementation on the fitness of two biological control agents: a predatory nabid bug and a bollworm pupal parasitoid. J Pest Sci 81:99–107
Wang XG, Messing RH (2004) Potential interactions between pupal and egg- or larval-pupal parasitoids of tephritid fruit flies. Environ Entomol 33:1313–1320
Wharton RA, Ovruski SM, Gilstrap FE (1998) Neotropical Eucoilidae (Cynipoidea) associated with fruit infesting Tephritidae, with new records from Argentina, Bolivia and Costa Rica. J Hymenop Res 7:102–115
White IM, Elson-Harris MM (1992) Fruit flies of economic significance: their identification and bionomics. CAB International, Wallingford
Winkler K, Wäckers FL, Bukovinszkine-Kiss G, van Lenteren JC (2006) Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl Ecol 7:133–140
Wu H, Meng L, Li B (2008) Effects of feeding frequency and sugar concentrations on lifetime reproductive success of Meteorus pulchricornis (Hymenoptera: Braconidae). Biol Control 45:353–359
Wyckhuys KAG, Strange-George JE, Kulhanek CA, Wäckers FL, Heimpel GE (2008) Sugar feeding by the aphid parasitoid Binodoxys communis: How does honeydew compare with other sugar sources? J Insect Physiol 54:481–491
Yokoyama VY, Rendon PA, Wang XG, Opp SB, Johnson MW, Daane KM (2011) Response of Psyttalia humilis (Hymenoptera: Braconidae) to olive fruit fly (Diptera: Tephritidae) and conditions in California olive orchards. Environ Entomol 40:315–323
Zhang G, Zimmermann O, Hassan SA (2004) Pollen as a source of food for egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocon Sci Technol 14:201–209
Acknowledgments
The authors thank Luz Stella Fuentes, Luis Alejandro Arias, Francisco López, Lina María Márquez, Carolina González and Sandra Herrera at the Centro de BioSistemas, Universidad Jorge Tadeo Lozano. We are also grateful to George Heimpel at the University of Minnesota, José Rengifo at the ICA Quarantine Treatment Laboratory, Armando Osorio at the Universidad del Tolima and Nubia Moreno at the Biotechnology Institute, Universidad Nacional de Colombia. In Mexico, Jose Manuel Gutíerrez Ruelas and Pablo Montoya facilitated shipments of biological material. This research was financed by the Colombian Ministry of Agriculture and Rural Development, with project grant MADR 2008L6772-3445 to KW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jonathan Lundgren.
Rights and permissions
About this article
Cite this article
Narváez, A., Cancino, J., Daza, N.C. et al. Effect of different dietary resources on longevity, carbohydrate metabolism, and ovarian dynamics in two fruit fly parasitoids. Arthropod-Plant Interactions 6, 361–374 (2012). https://doi.org/10.1007/s11829-012-9188-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9188-1