Abstract
Many arthropod predators and parasitoids exhibit either stage-specific or lifetime omnivory, in that they include extra-floral nectar, floral nectar, honeydew or pollen in their immature and/or adult diet. Access to these plant-derived foods can enhance pest suppression by increasing both the individual fitness and local density of natural enemies. Commercial products such as Amino-Feed®, Envirofeast®, and Pred-Feed® can be applied to crops to act as artificial-plant-derived foods. In laboratory and glasshouse experiments we examined the influence of carbohydrate and protein rich Amino-Feed UV® or Amino-Feed, respectively, on the fitness of a predatory nabid bug Nabis kinbergii Reuter (Hemiptera: Nabidae) and bollworm pupal parasitoid Ichneumon promissorius (Erichson) (Hymenoptera: Ichneumonidae). Under the chosen conditions, the provision of either wet or dry residues of Amino-Feed UV had no discernable effect on immediate or longer-term survival and immature development times of N. kinbergii. In contrast, the provision of honey, Amino-Feed plus extrafloral nectar, and extrafloral nectar alone had a marked effect on the longevity of I. promissorius, indicating that they were limited by at least carbohydrates as an energy source, but probably not protein. Compared with a water only diet, the provision of Amino-Feed plus extrafloral nectar increased the longevity of males and females of I. promissorius by 3.0- and 2.4-fold, respectively. Not only did female parasitoids live longer when provided food, but the total number of eggs laid and timing of deposition was affected by diet under the chosen conditions. Notably, females in the water and honey treatments deposited greater numbers of eggs earlier in the trial, but this trend was unable to be sustained over their lifetime. Egg numbers in these treatments subsequently fell below the levels achieved by females in the Amino-Feed plus extrafloral nectar and cotton extrafloral nectar only treatments. Furthermore, there were times when the inclusion of the Amino-Feed was beneficial compared with cotton extrafloral nectar only. Artificial food supplements and plant-derived foods are worthy of further investigation because they have potential to improve the ecosystem service of biological pest control in targeted agroecosystems by providing natural enemies with an alternative source of nutrition, particularly during periods of prey/host scarcity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many arthropod predators and parasitoids exhibit either stage-specific or lifetime omnivory, in that they include plant-derived foods, such as pollen, nectar, extra-floral nectar or honeydew, in their immature and/or adult diet (Hagen 1986; Coll and Guershon 2002; Wäckers and van Rijn 2005). Plant-derived foods can enhance pest suppression through improved nutrition, enhancing one or more measures of natural enemy fitness, such as longevity, fecundity or foraging behaviour (e.g., Georgiev 2005; Lee and Heimpel 2007; Wade and Wratten 2007). In this way, individual daily and/or lifetime attack rates may increase through expanded searching for location of suitable prey/host, and indirectly, through increasing rates of egg maturation and/or daily and lifetime reproductive output (Heimpel and Jervis 2005; Winkler et al. 2006). Secondly, natural enemies may be attracted to, and aggregate around, patches containing these foods (Lavandero et al. 2005), resulting in increased parasitism or predation by virtue of their greater localised density, rather than increased individual attack rates (in fact the latter may decline due to locally high densities).
Various habitat manipulation tactics have been proposed to boost the provision of plant-derived foods to natural enemies in both organic and conventional agroecosystems, (Landis et al. 2000; Zehnder et al. 2007). Although flowering non-crop “insectary” plants situated within or surrounding crop areas can provide natural enemies with plant-derived foods (e.g., Lavandero et al. 2005; Pontin et al. 2006), there are several drawbacks associated with this approach. These include lost income on land devoted to the non-crop, disruption to standard agronomic practices, and establishment and maintenance costs (particularly if plants become invasive weeds).
A potentially more refined and controlled habitat manipulation tactic that has been proposed involves the application of solutions rich in carbohydrates and/or proteins to crops to act as artificial supplemental food sources for natural enemies (e.g., Ben Saad and Bishop 1976; Mensah and Singleton 2002, 2003; Wade et al. 2008). The underlying rationale is to improve the synchrony of herbivore and natural enemy populations through time and space (Hagen 1986). In this context, it is reasonable to assume the extent of prey/host satiation will influence the requirement for food supplementation. However, the influence of artificial food supplements on the fitness of key species of natural enemies has seldom been investigated under controlled laboratory or glasshouse conditions (e.g., McEwen et al. 1996; Evans 2000). For instance, there is no published record of laboratory testing of the commercial products Amino-Feed, Envirofeast or Pred-Feed. This is remarkable, as these products are used by producers of arable crops in countries such as Australia and the United States, to increase the density of natural enemies (Mensah and Singleton 2002).
The present study examines the extent to which Amino-Feed UV or Amino-Feed, respectively, benefit a predator, the Pacific damsel bug Nabis kinbergii Reuter (Hemiptera: Nabidae) and a pupal endoparasitoid, the banded caterpillar parasite Ichneumon promissorius (Erichson) (Hymenoptera: Ichneumonidae). Both N. kinbergii and I. promissorius are important omnivorous natural enemies of various species of budworms and bollworms, Helicoverpa spp. (Lepidoptera: Noctuidae), which are major worldwide pests of arable crops such as cotton, soybean and sorghum (Johnson et al. 2000). Specifically, we examined how Amino-Feed UV or Amino-Feed, respectively affects (1) survival and development time of N. kinbergii in the laboratory; and (2) the longevity and fecundity of I. promissorius in the glasshouse. Additionally, two high carbohydrate–low protein diets were tested for I. promissorius as a comparison with Amino-Feed, which is high in both carbohydrates and proteins.
Materials and methods
The artificial food supplement used in Trial 1 was Amino-Feed UV, a commercially available liquid formulation with increased stability to ultra-violet light compared to standard Amino-Feed. Nonetheless, standard Amino-Feed was used in Trial 2 because this was the only formulation manufactured and available at the time. The two formulations varied only in the addition of 10% UV protectant, with Amino-Feed containing among other ingredients, 24.2% sugars (glucose, fructose and sucrose), 21.3% crude protein and amino acids and 1.8% nitrogen (product label data: Agrichem Manufacturing Industries, Loganholme, QLD, Australia).
Trial 1—survival and development of Nabis kinbergii in the laboratory
Insects and Amino-Feed UV
Nabis kinbergii nymphs were the progeny of field-collected adult bugs. The nymphs were supplied daily with a surfeit of cotton bollworm, Helicoverpa armigera (Hübner) eggs as prey and a water-soaked cotton dental roll for moisture upon eclosion and throughout the experimental period, but not during Amino-Feed UV application. As young nymphs rarely survive for more than a few days without arthropod prey (personal observation), bollworm eggs were provided in all treatments. Bollworm eggs supplied from the Queensland Department of Primary Industries and Forestry (QDPIF), Toowoomba Entomology Laboratory were replenished every 2–3 days.
Treatment details and product application
Newly eclosed (≤24 h) first-instar nymphs of N. kinbergii were provided with either wet and thereafter dried residues of Amino-Feed UV (“wet residue’’ treatment) or dry residues only (“dry residue” treatment). Water controls were included, both as wet and dry residue treatments. Thus, there were two treatment factors (product type and residue type), each with two levels, giving a total of four treatment combinations arranged in a factorial design. Each treatment was replicated 10 times. A total spray volume of 141.7 l per hectare was used in order to convert the dilution rate of Amino-Feed UV 3 l per hectare to 21.2 ml per litre. The sprayer used was a 500 ml plastic hand held atomiser with the nozzle adjusted to deliver medium-fine droplets in a hollow cone pattern. The inner surfaces of 10 ventilated 414 ml polystyrene cup-cages (see Grant and Shepard 1985, Fig. 3d) were sprayed with each treatment on 29 October 2001. In the wet residue treatments, 10 N. kinbergii nymphs were placed in each cup-cage prior to spraying. Conversely, in the dry residue treatments, the 10 N. kinbergii nymphs were introduced to each cage 1 h after spraying, with the spray having dried after 5 min. Insects were then transferred to a controlled environment room which averaged 24.5°C (range 23.1–25.4°C), 41.6% relative humidity (27.3–73.2%), and 15:9 h L:D photophase.
Assessments
The number of insects that remained alive was initially assessed after 2 days exposure to determine the immediate effects of treatment. To test for longer-term effects, individuals were transferred to ventilated 700 ml round plastic enclosures with a surfeit of prey and a moist dental roll, but free of Amino-Feed UV residues (to limit feeding time, as might happen in the field). In order to maintain a density of 10 insects per enclosure (a few had died during the initial exposure period), individuals from each treatment were assigned to new replicate cages with ca. 10 insects per enclosure. The number of insects alive and their life-stage was recorded daily over the next 5 days. Insect development time was calculated based on the number of days elapsed since the start of the trial until at least 50% of live insects in each enclosure had reached the third-instar (a nominal stage). The number of insects that remained alive when the majority had reached the third-instar was calculated. It was hypothesised that survival and development time would be enhanced in the Amino-Feed UV treatments, particularly in the wet residue treatment, where insects had access to the product before it dried as well as 2 days afterwards.
Statistical analyses
The effects of product type, residue type and their interaction on the immediate and longer-term survival and development time of N. kinbergii were analysed using separate generalized linear models (SAS GENMOD procedure, SAS release version 8.2) (McCullagh and Nelder 1989). Specifically, a Poisson model was used for the immediate and longer-term survival data because the data were essentially counts of survivors. A gamma model was used for the development time data because the data were continuous. Statistical tests were considered at an overall significance level of α = 0.05.
Trial 2—longevity and fecundity of I. promissorius in the glasshouse
Insects and plants
Adults of I. promissorius from a laboratory colony were reared on pupae of H. armigera. Potted cotton plants, Gossypium hirsutum L. (Malvaceae) cv. Delta Emerald were grown individually in the glasshouse using standard agronomic practices apart from no pesticide use. The plants were 60 days old and were flowering at the start of the trial, and produced copious amounts of extrafloral nectar from numerous foliar and bracteal nectaries throughout the trial. The provision of floral nectar and pollen from cotton flowers to I. promissorius was considered low because flowers bloom for less than 1 day, and contain little if any nectar. Furthermore, like most other parasitoid species, I. promissorius has not been observed to deliberately feed on pollen (Jervis et al. 1993).
Treatments and enclosures
Newly eclosed adult I. promissorius were provided with one of the following four treatments: (1) Amino-Feed plus cotton extrafloral nectar, (2) cotton extrafloral nectar, (3) honey, or (4) water only as a control. Treatments were replicated five times, with two newly emerged male-female pairs of I. promissorius in each, and arranged in a completely randomised design in the glasshouse. The cotton plants used in both extrafloral nectar treatments were enclosed individually by fine polyester gauze, supported by wooden rods inserted into the soil. Amino-Feed mixed with water at the rate of 10 ml per l was sprayed (equipment described earlier) onto the upper-leaf surface and reproductive structures (squares and flowers) of cotton plants at 0, 9, 23, 37 and 51 days after the trial commenced on 13 February 2001. The wasps were carefully removed from the enclosures prior to spraying to prevent them from escaping, and then returned before the spray had fully dried. For the honey and water treatments, wasps were enclosed in gauze cages as above, but in the absence of cotton plants. Undiluted honey (1–2 ml) was presented in a Petri dish lid. Honey was used as an artificial extrafloral nectar source because it contains fructose, glucose and sucrose as well as trace amounts of amino acids, which are the main components of floral and extrafloral nectar from most plants (Baker and Baker 1983). Water was provided in all treatments by way of a filled glass vial with a cotton wick protruding through a hole in the cap. Honey and water were replaced every other day.
Parasitoid survival was assessed daily until all had died. Starting on day 1, parasitoid fecundity under the different treatments was assessed every other day by placing two bollworm host pupae contained in a Petri dish lid in each enclosure for the proceeding 48 h. Assessments ceased once both females in each enclosure had died or 69 days after spraying (DAS). Although it is possible for individual female wasps to attack more than two hosts in 48 h, only two host pupae were provided during this period because of the low numbers available. Thus, realised fecundity was indicated by the total number of eggs deposited in a few hosts (individual female parasitoids will readily attack the same host more than once), rather than the number of hosts attacked. Generally only one egg is deposited on each oviposition event and if left to develop, only one adult parasitoid will finally emerge from each host (i.e., a solitary endoparasitoid).
The potentially parasitised pupae were examined under a stereo microscope (60×) to record the number of discernible blackened scars or “sting spots” present on the exoskeleton. The number of sting spots can be used as an indicator of attempted oviposition. Moreover, sting spots indicate deliberate acts of non-destructive host feeding, which reflects in part the nutritional status of parasitoids in the various treatments (though the latter type of spots is indistinguishable from those created by oviposition). Pupae were then dissected (60–300× magnification) to record the number of I. promissorius eggs they contained.
The numbers of sting spots and eggs deposited in both hosts were analysed on the basis of initial female numbers (i.e., five replicate cages at the start of the trial, each with two females), to produce measures of per capita reproductive output. An alternative approach not adopted was based on the number of female parasitoids alive at the start of each 2 days census period; however, this metric was highly sensitive to declining parasitoid counts. Temperature inside the glasshouse averaged 23.8°C (range 10.6–37.5°C) during the trial.
Statistical analyses
Survival analysis was used to examine the effect of diet on the longevity of I. promissorius, with each gender tested separately (SAS LIFETEST procedure). The product limit (Kaplan–Meier) method was used to estimate the survival distribution function in the analyses. The majority of wasps lived for more than a few days; therefore, the Log–Rank test statistic was used in favour of the Wilcoxon test statistic, because the former stresses late rather than early differences between survival times. The data were included as censored data in the survival analysis if individuals escaped from an enclosure between census periods. For multiple comparisons, all pair wise comparisons were tested with separate survival analyses. However, to account for any experiment-wide error, the alpha level was adjusted to designate significant differences (αnew = 1−(1−αoriginal)1/k = 0.0085, where αoriginal = 0.05, k = 6, pair wise comparisons for all diet combinations). This procedure, known as Šidák’s adjustment, is analogous to the use of a correction for means comparisons in analysis of variance (Šidák 1967).
A one-way ANOVA (SAS MIXED procedure) was used to determine the effect of diet on lifetime (total) per capita fecundity of female I. promissorius. This was based on the cumulative number of eggs deposited in two hosts in 48 h over the lifespan of an average single live female parasitoid. In addition, the effect of diet on the total number of sting spots (i.e., host feeding and oviposition attempts) was analysed by one-way ANOVA over the same period. Furthermore, a series of repeated measures ANOVA tests were used to determine the effects of diet, census date, and diet × census date on the numbers of eggs deposited and sting spots by female I. promissorius in two hosts every 48 h. ANOVA tests were considered at an overall significance level of α = 0.05.
Results
Trial 1—survival and development of N. kinbergii in the laboratory
Immediate survival
There was no immediate effect of the treatments on survival of N. kinbergii at 2 DAS by product type (Poisson GLM: χ2 1 = 0.17, P = 0.6758), exposure manner (χ2 1 = 0.13, P = 0.7230) or the interaction of product type × exposure manner (χ2 1 = 0.21, P = 0.6443; Table 1).
Longer-term survival
Of the survivors that were transferred to residue free enclosures at 2 DAS, their longer-term survival under the various treatments approximately 4.5 DAS was not significantly affected by product type (χ2 1 = 0.57, P = 0.4504), exposure manner (χ2 1 = 0.25, P = 0.6106) or the interaction of product type × exposure manner (χ2 1 = 0.22, P = 0.6375). This retrospective calculation corresponded to when more than 50% of N. kinbergii that remained alive had reached the third-instar (Table 1).
Development time
The time elapsed from the start of the trial until the majority of surviving N. kinbergii nymphs had reached the third-instar under the various treatments was not significantly affected by product type (gamma GLM: χ2 1 = 0.27, P = 0.6040) or the interaction of product type × exposure manner (χ2 1 = 0.13, P = 0.7214). However, the rate of immature development was significantly affected by exposure manner (χ2 1 = 12.47, P = 0.0004). In the wet residue treatments (pooled across product types), N. kinbergii required 0.57 day or 14% longer to complete development of the first- and second-instars compared with the dry residue treatments (i.e., 4.7 ± 0.1 vs. 4.1 ± 0.1 day, respectively) (Table 1).
Trial 2—longevity and fecundity of I. promissorius in the glasshouse
Longevity
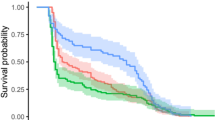
Overall, the longevity of I. promissorius males appeared shorter than females, though not statistically comparable. Survival curves differed significantly between the particular diets for both males (Log–Rank test: n = 40, χ2 3 = 29.47, P < 0.0001) and females of I. promissorius (n = 40, χ2 3 = 29.11, P < 0.0001). For both males and females, longevity was significantly shorter when provided with the water only treatment compared with the three other diets. While the exact placement of these other diets varied between sexes, importantly, among sexes these diets did not significantly differ from each other.
On average, the longevity of I. promissorius males provided with water only was 9.8 ± 0.3, honey was 27.8 ± 5.0, cotton extrafloral nectar was 31.8 ± 7.7 and Amino-Feed plus extrafloral nectar was 29.2 ± 5.2 days (Fig. 1a). The respective figures for females of I. promissorius were 27.6 ± 4.0 days, 51.6 ± 4.6, 43.1 ± 8.4 and 65.5 ± 6.1 days (Fig. 1b).
Survivorship curves of male (a) and female (b) Ichneumon promissorius under various treatment diets. Extrafloral nectar is EFN. Different letters denote a significantly different survivorship based on pair wise survival analysis comparisons, where the criterion for significant differences was the experiment-wide error, αnew = 1 − (1 − αoriginal)1/k = 0.0085
Lifetime fecundity
Diet had a significant effect on the total number of eggs, but not sting spots, produced by an average female of I. promissorius (one-way ANOVA, eggs: F 3,16 = 3.94, P = 0.0278; sting spots: F 3,16 = 1.13, P = 0.3671). Lifetime fecundity in the honey treatment was significantly greater than the cotton extrafloral nectar or water only treatments, but not the Amino-Feed plus extrafloral nectar treatment (Fig. 2).
Mean (+SE) numbers of eggs laid and sting spots in hosts per female Ichneumon promissorius over its lifespan under various treatment diets. Extrafloral nectar is EFN. Stings spots are discernible scars on the pupal case that result from attempted oviposition and deliberate acts of host feeding. The numbers of eggs laid but not sting spots were not significantly affected by diet. Different letters of the same case denote significant differences based on Fisher’s LSD test (P < 0.05)
Age-specific fecundity
The number of eggs deposited by a single female I. promissorius in two hosts in 48 h was significantly affected by diet (repeated measures ANOVA on the basis of initial female numbers: F 3,16 = 4.78, P = 0.0145), census date (F 33,528 = 10.44, P < 0.0001) and the interaction of diet × census date (F 99,582 = 1.33, P = 0.0260) (Fig. 3a). Conversely, the number of stings was significantly affected by census date (F 33,582 = 10.55, P < 0.0001), but not diet (F 3,16 = 1.27, P = 0.3182) nor the interaction of diet × census date (F 99,582 = 1.27, P = 0.0540) (Fig. 3b).
Mean numbers of eggs laid (a) and sting spots (b) in hosts per female Ichneumon promissorius in 48 h periods over its lifespan under various treatment diets. Means are based on the initial number of females. Extrafloral nectar is EFN. Stings spots are discernible scars on the pupal case that result from attempted oviposition and deliberate acts of host feeding. The number of eggs laid was significantly affected by diet, census date and the interaction of diet × census date. Conversely, the number of stings was significantly affected by census date, but not diet nor the interaction of diet × census date. Standard error bars were omitted for clarity
Pooled across all 34 census dates, a single female of I. promissorius in the honey treatment produced significantly more eggs (4.1 ± 0.2 c eggs per female in 48 h) than those in cotton extrafloral nectar (2.3 ± 0.5 a) or water only (2.7 ± 0.5 ab) treatments, but not the Amino-Feed plus extrafloral nectar treatment (3.4 ± 0.3 bc). Indeed, overall fecundity in the Amino-Feed plus extrafloral nectar treatment was significantly greater than the cotton extrafloral nectar only treatment.
Numbers of eggs and sting spots varied over the duration of the trial. In general, numbers increased rapidly in the first 5 days, with a peak around days 9–25, followed by a steady decline (Fig. 3). Nonetheless, the pattern of egg lay was inconsistent across treatments over the trial (Fig. 3a). Notably, females in the water and honey treatments deposited the greatest number of eggs earlier in the trial, but this trend was unable to be sustained. For instance, egg counts at day 13 in the honey (11.3 ± 1.7 b eggs per female in 48 h) and water controls (8.4 ± 2.6 b) were significantly greater than the Amino-Feed plus extrafloral nectar (6.8 ± 1.8 a) and cotton extrafloral nectar only (5.5 ± 3.9 a). Egg counts in the honey and water controls subsequently fell below the levels achieved by females in the Amino-Feed plus extrafloral nectar and cotton extrafloral nectar only treatments. At day 39, for instance, the respective figures were 0.8 ± 0.7 a, 1.0 ± 1.2 a, 4.3 ± 1.4 b, and 2.3 ± 1.4 ab. Amino-Feed inclusion significantly improved egg counts at various periods during the trial, such as days 15 and 27. On these dates, the respective figures for Amino-Feed plus extrafloral nectar treatment versus cotton extrafloral nectar only were 7.0 ± 0.7 versus 4.2 ± 2.1 and 5.3 ± 1.0 versus 2.3 ± 1.5 (Fig. 3a).
Discussion
Under the chosen conditions, the provision of either wet or dry residues of Amino-Feed UV had no discernable effect on the survival and immature development time of N. kinbergii. Either (1) the nymphs did not respond to the wet or dry Amino-Feed UV residue, (2) the nymphs did respond but had difficulty feeding on the wet or dry Amino-Feed UV residue, or (3) the nymphs successfully fed but would not be nutritionally limited in the absence of Amino-Feed UV. On several informal occasions bugs were seen with probosces extended, apparently feeding on both wet and dry Amino-Feed UV residues, suggesting N. kinbergii were indeed using this material. The fact there was no effect on lifetable parameters indicates they were not nutritionally limited under the conditions tested. Thus, adequate nutrition was provided by the surfeit of bollworm eggs presented to N. kinbergii in all treatments, regardless of whether Amino-Feed UV was provided as wet or dry residues or not at all.
Whilst the addition of Amino-Feed UV had no discernable impact on N. kinbergii when a surfeit of prey was made available, the provision of artificial food supplements may be valuable during periods of prey scarcity or absence. This could buffer them against the unpredictability of spatial and temporal occurrence of food resources in patchy environments. Food resources can be quite variable for predators in ephemeral agricultural systems, such as during periods following planting, harvesting or spraying of insecticides (Coll and Guershon 2002). It is possible that Amino-Feed UV may be more important when either only plant material or nutritionally inferior (low quality) prey species are available, such as pea aphids, Acyrthosiphon pisum Harris, compared with nutritionally superior prey such as bollworms (Eubanks and Denno 2000). Though the ranking of prey species will depend on the predator species, for instance, A. pisum is nutritionally superior to H. armigera as prey for an aphidophagous ladybird beetle, regardless of sucrose provision (Evans 2000). In addition, life stages of N. kinbergii other than first-instar nymphs, such as adult females, may benefit more from Amino-Feed due to likely differences in their nutritional requirements. Indeed, these differences may be more pronounced in insect species that undergo complete metamorphosis (e.g., parasitoid wasps such as I. promissorius), which allows the immature and adult stages to consume different food types.
In contrast with N. kinbergii, the provision of Amino-Feed plus extrafloral nectar increased the longevity of males and females of I. promissorius by 3.0- and 2.4-fold, respectively, compared with the water only diet. This provides clear evidence for carbohydrate (i.e., energy) limitation in I. promissorius. However, the similar results achieved in the Amino-Feed plus extrafloral nectar diet, which is high carbohydrate–high protein, compared with honey only or extrafloral nectar only diets that are high carbohydrate–low protein, indicates that parasitoids were either not protein limited or did not feed on the Amino-Feed component. Wasps were seen lapping at the leaf surface, apparently feeding on dry Amino-Feed residues on several occasions, as well as at extrafloral nectaries and the cotton wick containing artificial extrafloral nectar. Indeed, the ability of insects to feed on dry (crystalline) sugar residues has been documented in numerous species, including the Ichneumonidae, presumably by dissolving the crystals in excreted saliva (Bartlett 1962; Wäckers 2000). Hence, it is likely that I. promissorius was able to imbibe the different foods, but there was no additive effect of Amino-Feed plus extrafloral nectar on longevity under the chosen conditions.
Not only did females of I. promissorius that received food as adults live an additional 15.5–37.9 days on an average, but their age-specific and lifetime fecundity was generally improved. The honey treatment was particularly beneficial earlier in the trial, but this trend was subsequently reversed, presumably as egg load became depleted. Interestingly, the temporal distribution of oviposition by I. promissorius in the water control followed a similar, but less pronounced trend. Furthermore, there were key times when the inclusion of the Amino-Feed was beneficial compared with cotton extrafloral nectar only. Most parasitoid species, including I. promissorius, have a synovigenic life history strategy, in that females emerge with a certain proportion of the egg complement that is immature, whilst leaving the remainder of oocytes to mature over their lifetime. Large parasitoids that tend towards the more extreme end of the synovigenic continuum, in particular, should be reliant on the provision of food resources as adults (Jervis et al. 2001).
In relation to the concept of egg- and time-limitation in insect evolution, it is generally accepted that increased longevity reduces the chances of female parasitoids becoming time-limited (i.e., where they have insufficient time to deposit all their available mature eggs), whereas increased potential fecundity reduces the chances of parasitoids becoming egg-limited. However, the occurrence and thus relative importance of each state of limitation remains largely unresolved. Empirical evidence for each state has been slow coming (reviewed by Heimpel and Rosenheim 1998), in part because of the difficulty in determining the age, realised lifetime fecundity and feeding history of field caught adult parasitoids.
Declining fecundity in our study demonstrates that I. promissorius, at least under the conditions tested, incurs egg-limitation later in life, though this can be partly ameliorated by access to plant-derived foods. This state of egg-limitation in I. promissorius should persist in the field, provided a reasonable source of adult food is available, although parasitoids would incur higher energetic costs associated with flying, crawling and digging in search of mates, food and host pupae (the latter are located in their underground chambers). Indeed, flying is an energetically costly activity (Hoferer et al. 2000) and unfed parasitoids exhaust their energy reserves at considerably faster rates under field conditions as compared with individuals kept in confined conditions (Steppuhn and Wäckers 2004). In large field cages, the consequence of energy depletion for the pupal parasitoid Diadegma semiclausum (Hellén) was a 105-fold reduction in parasitism rates (Winkler et al. 2006). Therefore, under conditions where females of I. promissorius have to fly extensively in search of hosts (e.g., when patches containing hosts are far apart or host density is low within a patch), and thus access to host-derived adult food becomes restricted, then the provision of artificial food supplements or plant-derived foods could be of greater importance. In addition, non-food mortality factors, such as predation (Heimpel et al. 1997) and weather conditions (Fink and Völkl 1995) are recognised as being important factors that limit lifetime reproductive success of parasitoids, and therefore, could shift the parasitoid population towards a state of time-limitation.
Host haemolymph from non-destructive feeding on bollworm pupae provided females in all treatments with additional source of nutrition for oogenesis and maturation (Godfray 1994). Informal observations revealed that males did not host feed, as is typical of parasitoids, but female wasps regularly fed upon host haemolymph that exuded from the site where the ovipositor was previously inserted. Indeed, the absence of host feeding by male parasitoids from either treatment could explain the 1.9-fold difference in longevity of males and females of I. promissorius (pooled treatment average, 24.7 vs. 47.0 days, respectively). Interestingly, the similar number of sting spots across the different diets suggests that host feeding satisfies only modest (not entire) requirements for parasitoid nutrition. Indeed, host feeding was predicted to be more frequent in the water control than the other treatments, but no evidence supported this hypothesis. The number of sting spots was consistently about 1.5 times higher than the number of eggs laid over the course of the wasps’ lifetime, rather than being independent of ovipositional activity. This suggests the process was likely the result of unsuccessful oviposition attempts rather than a deliberate acts of feeding. Indeed, females of another ichneumonid, Itoplectis conquisitor (Say), puncture the host pupa up to six times for each egg laid (Leius 1961). The present result for I. promissorius supports the view that under the chosen conditions, the addition of artificial food supplements and plant-derived foods with host-derived foods provides additional resources used for reproduction.
In conclusion, under these experimental conditions it was possible to alter the survival and reproduction of the parasitoid wasp I. promissorius by providing a source of artificial food supplements or plant-derived foods. Furthermore, there was evidence for a differential positive response to Amino-Feed plus extrafloral nectar for I. promissorius compared with extrafloral nectar only. However, no effect was observed for the nabid predator N. kinbergii. The dichotomous response for I. promissorius and N. kinbergii emphasises that entomologists and pest control advisors cannot assume that one food supplement will be suitable for all taxa. Nevertheless, artificial food supplements and plant-derived foods have potential to impact on the strength of food chain connections and top–down regulation of herbivores (Polis and Strong 1996). In the context of pest management, food supplements have potential to improve the ecosystem service of biological pest control in targeted agroecosystems by providing natural enemies with an alternative source of nutrition (e.g., Ben Saad and Bishop 1976; McEwen et al. 1996; Mensah and Singleton 2003). Artificial food supplements could also be used in combination with classical, inoculation and inundation biological control programmes to potentially improve the success of mass-released biological control agents in an approach dubbed “integrated biological control” (Gurr and Wratten 1999).
References
Baker HG, Baker I (1983) A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T (eds) The biology of nectaries. Columbia University Press, New York, pp 126–152
Bartlett BR (1962) The ingestion of dry sugars by adult entomophagous insects and the use of this feeding habit for measuring the moisture needs of parasites. J Econ Entomol 55:749–753
Ben Saad AA, Bishop GW (1976) Effect of artificial honeydews on insect communities in potato fields. Environ Entomol 5:453–457
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixing plant and prey diets. Ann Rev Entomol 47:267–297
Eubanks MD, Denno RF (2000) Healthy food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol Entomol 25:140–146
Evans EW (2000) Egg production in response to combined alternative foods by the predator Coccinella transversalis. Entomol Exp Appl 94:141–147
Fink U, Völkl W (1995) The effect of abiotic factors on foraging and oviposition success of the aphid parasitoid, Aphidius rosae. Oecologia 103:371–378
Georgiev G (2005) Bioecological characteristics of Bracon intercessor Nees (Hymenoptera: Braconidae) as a parasitoid of the poplar clearwing moth, Paranthrene tabaniformis (Rott.) (Lepidoptera: Sesiidae) in Bulgaria. J Pest Sci 78:161–165
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Grant JF, Shepard M (1985) Techniques for evaluating predators for control of insect pests. J Agric Entomol 2:99–116
Gurr GM, Wratten SD (1999) ‘Integrated biological control’: a proposal for enhancing success in biological control. Int J Pest Manage 45:81–84
Hagen KS (1986) Ecosystem analysis: plant cultivars (HPR), entomophagous species and food supplements. In: Boethel DJ, Eikenbary RD (eds) Interactions of plant resistance and parasitoids and predators of insects. Horwood, Chichester, pp 151–197
Heimpel GE, Jervis MA (2005) Does floral nectar improve biological control by parasitoids? In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 267–304
Heimpel GE, Rosenheim JA (1998) Egg limitation in parasitoids: a review of the evidence and a case study. Biol Control 11:160–168
Heimpel GE, Rosenheim JA, Mangel M (1997) Predation on adult Aphytis parasitoids in the field. Oecologia 110:346–352
Hoferer S, Wäckers FL, Dorn S (2000) Measuring CO2 respiration rates in the parasitoid Cotesia glomerata. Mitteilungen der Deutschen Gesellschaft für allgemeine und angewandte Entomologie 12:555–558
Jervis MA, Kidd NAC, Fitton MG, Huddleston T, Dawah HA (1993) Flower-visiting by hymenopteran parasitoids. J Nat Hist 2:67–105
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NA (2001) Life-history strategies in parasitoid wasps: a comparative analysis of “ovigeny”. J Anim Ecol 70:442–458
Johnson M-L, Pearce S, Wade M, Davies A, Silberbauer L, Gregg P, Zalucki M (2000) Review of beneficials in cotton farming systems. Report to the Australian cotton research and development corporation, Narrabri, NSW, Australia, pp 86
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann Rev Entomol 45:175–202
Lavandero B, Wratten SD, Shishehbor P, Worner S (2005) Enhancing the effectiveness of the parasitoid Diadegma semiclausum (Helen): movement after use of nectar in the field. Biol Control 34:152–158
Lee JC, Heimpel GE (2007) Sugar feeding reduces short-term activity of a parasitoid wasp. Physiol Entomol 32:99–103
Leius K (1961) Influence of food on fecundity and longevity of adults of Itoplectis conquisitor (Say) (Hymenoptera: Ichneumonidae). Can Entomol 93:771–780
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman & Hall, London
McEwen PK, Jervis MA, Kidd NAC (1996) The influence of an artificial food supplement on larval and adult performance in the green lacewing Chrysoperla carnea (Stephens). Int J Pest Manage 42:25–27
Mensah R, Singleton A (2002) Use of food sprays in cotton systems: what do we know? In: Proceedings of the 11th Australian cotton conference, Brisbane, QLD, 13–15 August, pp 359–375
Mensah RK, Singleton A (2003) Optimum timing and placement of a supplementary food spray Envirofeast® for the establishment of predatory insects of Helicoverpa spp. in cotton systems in Australia. Int J Pest Manage 49:163–168
Polis GA, Strong DR (1996) Food web complexity and community dynamics. Am Nat 147:813–846
Pontin DR, Wade MR, Kehrli P, Wratten SD (2006) Attractiveness of single and multiple species flower patches to beneficial arthropods in agroecosystems. Ann Appl Biol 148:39–47
Šidák Z (1967) Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 62:626–633
Steppuhn A, Wäckers FL (2004) HPLC sugar analysis reveals the nutritional state and the feeding history of parasitoids. Funct Ecol 18:812–819
Wäckers FL (2000) Do oligosaccharides reduce the suitability of honeydew for predators and parasitoids? A further facet to the function of insect-synthesized honeydew sugars. Oikos 90:197–201
Wäckers FL, van Rijn PCJ (2005) Food for protection: an introduction. In: Wäckers FL, van Rijn PCJ, Bruin J (eds) Plant-provided food for carnivorous insects: a protective mutualism and its applications. Cambridge University Press, Cambridge, pp 1–14
Wade MR, Wratten SD (2007) Excised or intact inflorescences? Methodological effects on parasitoid wasp longevity. Biol Control 40:347–354
Wade MR, Zalucki MP, Wratten SD, Robinson KA (2008). Conservation biological control of arthropods using artificial food sprays: Current status and future challenges. Biol Control doi:10.1016/j.biocontrol.2007.10.024(in press)
Winkler K, Wäckers FL, Bukovinszkine-Kiss G, van Lenteren JC (2006) Nectar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl Ecol 7:133–140
Zehnder G, Gurr GM, Kuehne S, Wade MR, Wratten SD, Wyss E (2007) Arthropod pest management in organic crops. Ann Rev Entomol 52:57–80
Acknowledgments
We are grateful to Sue Maclean for providing the bollworm materials, Erin Conze of Agrichem for supplying the Amino-Feed UV, and Allan Lisle and Kerry Bell for statistical advice. Thanks to Nancy Schellhorn, Felix Wäckers and two anonymous reviewers for their valuable comments on an earlier version of the manuscript. Principal funding was provided by the Australian Cotton Research and Development Corporation (postgraduate research project UQ29C) and an in-kind contribution from the Australian Grains Research and Development Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Wade, M.R., Hopkinson, J.E. & Zalucki, M.P. Influence of food supplementation on the fitness of two biological control agents: a predatory nabid bug and a bollworm pupal parasitoid. J Pest Sci 81, 99–107 (2008). https://doi.org/10.1007/s10340-007-0191-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-007-0191-8